Development and Validation of Pesticide Residues Determination Method in Fruits and Vegetables through Liquid and Gas Chromatography Tandem Mass Spectrometry (LC-MS/MS and GC-MS/MS) Employing Modified QuEChERS Method and a Centrifugal Vacuum Concentrator
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Reference Standards
2.2. Sample Collection
2.3. Equipment
2.4. Method Optimization—Selection of a Method Suitable for the Purpose for Each Type of Food
2.5. Sample Preparation
2.6. Liquid Chromatography with Tandem Mass Spectrometry (LC-MS/MS) Analysis
2.7. Gas Chromatography with Tandem Mass Spectrometry (GC-MS/MS) Analysis
2.8. Identification of Pesticides
2.9. Quantitative Determination of Pesticide Residues
2.10. Methods Validation
2.11. Quality Control
3. Results
3.1. Optimization Results
3.2. Results of Validation Requirements
3.2.1. Linearity
3.2.2. Repeatability
3.2.3. Reproducibility-Intermediate Precision
3.2.4. Sensitivity-LOQ
3.2.5. Specificity
3.2.6. Retention Time
3.2.7. Uncertainty
3.2.8. External Quality Control—Proficiency Testing
3.3. Pesticides That Satisfied the Validation Criteria
4. Discussion
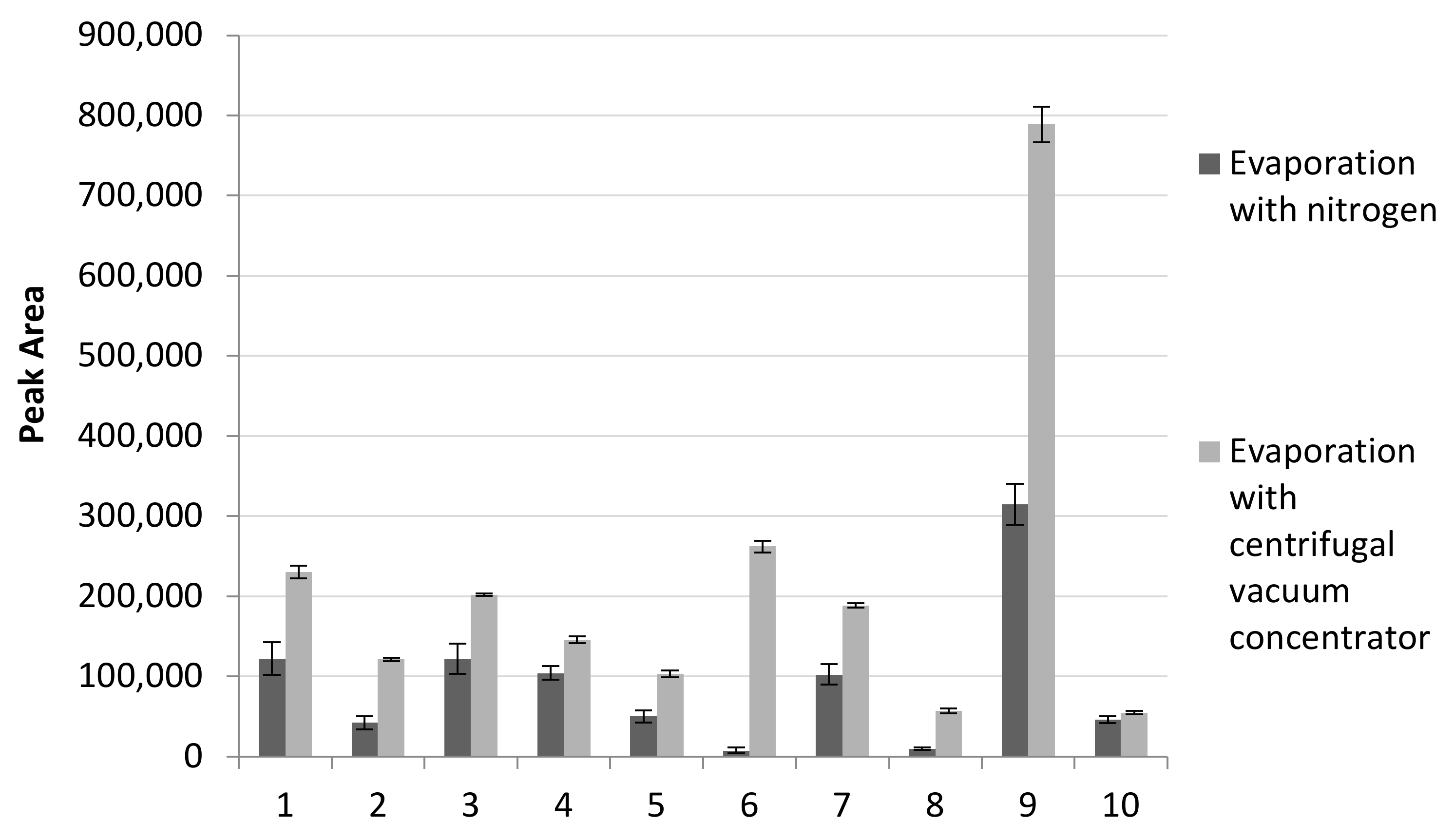
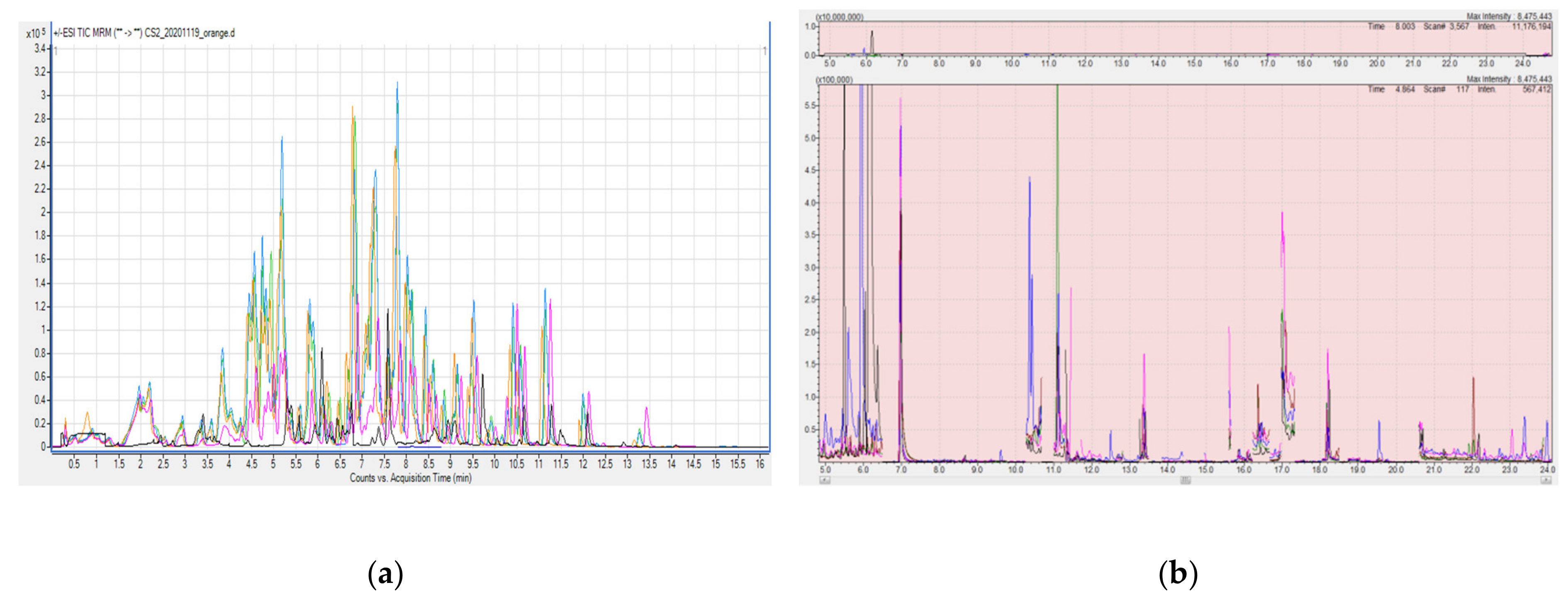
- A larger number of pesticides are detected. As presented in Table 4 we were able to determine many pesticides eluting at the beginning of the chromatographic analysis, which are more volatile and more sensitive to analysis, while when using nitrogen evaporation, we could not even detect them;
- According to the S/N values in Table 3, 154 pesticides achieved S/N ≥ 10 when using the centrifugal vacuum concentrator system. On the contrary, 104 pesticides using nitrogen evaporation satisfied this requirement;
- The sensitivity of the method increases, since in the majority of pesticides the S/N ratio is much higher when applying the centrifugal vacuum concentrator evaporation;
- The analysis time is reduced, especially in a larger number of samples. The evaporation with a centrifugal vacuum concentrator is performed in 30–60 min (depending on the substrate) and all the samples are evaporated at the same time because of the multiple positions of the centrifugal vials it has, while in nitrogen stream the evaporation time of a sample is longer than 40 min and there were only 12 positions to place the samples;
- The cost of analysis is reduced by not consuming nitrogen;
- The cost of analysis and the effort of the analyst are reduced from the non-use of plastic liners. These liners are used to supply nitrogen and need to be thoroughly rinsed with organic solvents, as well as they are usually deformed by excessive use due to their flexibility and need to be replaced with new ones;
- The evaporation with the centrifugal vacuum concentrator achieves greater repeatability and reproducibility in the majority of pesticides determined, in combination with the Multi-Tube Vortexer stirring: 1 < % RS ≤ 20 for N = 5 replicates and N = 10 replicates, as depicted in indicative Table 4.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abia, W.A.; Montgomery, H.; Nugent, A.P.; Elliott, C.T. Tropane alkaloid contamination of agricultural commodities and food products in relation to consumer health: Learnings from the 2019 Uganda food aid outbreak. Compr. Rev. Food Sci. Food Saf. 2021, 20, 501–525. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shukla, A.; Attri, K.; Kumar, M.; Kumar, P.; Suttee, A.; Singh, G.; Barnwal, R.; Singla, N. Global trends in pesticides: A looming threat and viable alternatives. Ecotoxicol. Environ. Saf. 2020, 201, 110812. [Google Scholar] [CrossRef] [PubMed]
- Greek Certification System, GCS (ΕΣΥΔ in Greek). Guidance Document on Method Validation and Quality Control Procedures for Pesticide Residues Laboratories; G-FYTOPROST: Athens, Greece, 2016. [Google Scholar]
- Valavanidis, A. Pesticide Residues in Fruit, Vegetables and Food. How Dangerous Are to Human Health? Studies of Pesticide Residues in Food in European Countries and in Greece, and Risk to Consumer’s Health. Ph.D. Thesis, Department of Chemistry, University of Athens, Athens, Greece, 2016. [Google Scholar]
- Carvalho, F. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Fenik, J.; Tankiewicz, M.; Biziuk, M. Properties and determination of pesticides in fruits and vegetables. Trends Anal. Chem. 2011, 30, 814–826. [Google Scholar] [CrossRef]
- Machado, I.; Gérez, N.; Pistón, M.; Heinzen, H.; Cesio, M. Determination of pesticide residues in globe artichoke leaves and fruits by GC–MS and LC–MS/MS using the same QuEChERS procedure. Food Chem. 2017, 227, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Anastassiades, M.; Lehotay, S.; Štajnbaher, D.; Schenck, F. Fast and Easy Multiresidue Method Employing Acetonitrile Extraction/Partitioning and “Dispersive Solid-Phase Extraction” for the Determination of Pesticide Residues in Produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Pu, J.; Wu, X.; Chen, B.; He, Y.; Zhang, Y.; Han, B. Evaluation of the matrix effect of pH value and sugar content on the analysis of pesticides in tropical fruits by UPLC-MS/MS. Microchem. J. 2021, 168, 106375. [Google Scholar] [CrossRef]
- Dąbrowski, Ł. Review of use of keepers in solvent evaporation procedure during the environmental sample analysis of some organic pollutants. TrAC Trends Anal. Chem. 2016, 80, 507–516. [Google Scholar] [CrossRef]
- Meyer, V.R. Example of gradient elution in normal-phase liquid chromatography. J. Chromatogr. A 1997, 768, 15–319. [Google Scholar] [CrossRef]
- EU Reference Laboratories for Residues of Pesticides. Available online: https://www.eurl-pesticides.eu/ (accessed on 30 January 2022).
- EU Reference Laboratories for Residues of Pesticides: Document No SANTE/12682/2019. Available online: https://www.eurl-pesticides.eu/userfiles/file/EurlALL/AqcGuidance_SANTE_2019_12682.pdf (accessed on 30 January 2022).
| Time (min) | %A | %B | Flow (mL/min) |
|---|---|---|---|
| 0.00 | 90 | 10 | 0.600 |
| 10.00 | 30 | 70 | 0.600 |
| 15.00 | 10 | 90 | 0.600 |
| 20.00 | 90 | 10 | 0.600 |
| 25.00 | 90 | 10 | 0.600 |
| Area | Height | |||
|---|---|---|---|---|
| Pesticide | Evaporation with Nitrogen | Evaporation with the Centrifugal Vacuum Concentrator | Evaporation with Nitrogen | Evaporation with the Centrifugal Vacuum Concentrator |
| Diphenylamine | 176,255 | 218,447 | 76,694 | 89,390 |
| Di-allate | 67,137 | 75,906 | 31,043 | 35,345 |
| Metolachlor | 1,087,219 | 1,359,908 | 449,229 | 554,375 |
| Phenothrin | 65,104 | 87,551 | 29,501 | 38,051 |
| Tetradifon | 85,394 | 113,522 | 37,779 | 48,653 |
| Permethrin | 101,182 | 146,641 | 45,546 | 60,044 |
| Flucythrinate | 72,328 | 103,736 | 30,843 | 43,334 |
| Area | S/N | |||
|---|---|---|---|---|
| Pesticide | Evaporation with Nitrogen | Evaporation with the Centrifugal Vacuum Concentrator | Evaporation with Nitrogen | Evaporation with the Centrifugal Vacuum Concentrator |
| Chlofentezine | 123,229 | 89,467 | 14.52 | 739.18 |
| Dichlorvos | 177,582 | 219,833 | 684.27 | 15,045.65 |
| Dichlobenil | 722,104 | 875,020 | 42,438.89 | 7625.23 |
| EPTC | 127,803 | 149,539 | 78.81 | 4504.1 |
| Propamocarb | 4011 | 4947 | 131.27 | 145.89 |
| Mevinphos | 369,434 | 522,548 | 20.3 | 3004.61 |
| Butylate | 341,677 | 419,314 | 26.13 | 7661.01 |
| Etridiazole | 169,289 | 200,476 | 135.82 | 3704.16 |
| Propham | 360,770 | 447,460 | 8.14 | 2005.92 |
| Methacrifos | 378,743 | 439,691 | 3407.33 | 28,314.14 |
| 2-Phenylphenol | 574,019 | 702,230 | 91.14 | 8280.63 |
| Molinate | 686,144 | 751,433 | 600.98 | 6.51 |
| Tecnazene | 68,057 | 81,796 | 1173.91 | 2238.88 |
| Propoxur | 291,132 | 436,214 | 36.42 | 1136.86 |
| Propachlor | 263,567 | 300,638 | 3,589,033.2 | 4,866,521.21 |
| Diphenylamine | 176,255 | 218,447 | 21.71 | 9,905,219.14 |
| Ethoprophos | 224,206 | 268,623 | 18.1 | 17.94 |
| Ethalfluralin | 45,195 | 54,101 | 1.56 | 1809.48 |
| Chlorpropham | 227,475 | 305,572 | 18.47 | 948.27 |
| Trifluralin | 128,909 | 153,185 | 4645.11 | 4945.71 |
| Benfluralin | 98,343 | 117,948 | 4543.55 | 5216.71 |
| Cadusafos | 520,550 | 646,087 | 1.14 | 33.58 |
| Di-allate | 187,758 | 220,277 | 2.9 | 33,846.33 |
| alpha-BHC | 594,646 | 684,202 | 4.15 | 816.71 |
| Hexachlorobenzene | 200,032 | 241,729 | 249.58 | 5582.26 |
| Dicloran | 66,456 | 95,659 | 14.96 | 486.46 |
| Simazine | 139,317 | 190,985 | 9.31 | 1052.16 |
| Dimethipin | 21,939 | 29,746 | 1.65 | 148.78 |
| Atrazine | 92,624 | 118,070 | 46 | 1094.27 |
| Chlorbufam | 32,982 | 44,504 | 133.65 | 610.67 |
| Clomazone | 311,198 | 394,187 | 89.12 | 2244.78 |
| beta-BHC | 190,185 | 287,292 | 0.88 | 72.71 |
| Quintozene | 35,719 | 39,349 | 31.49 | 585.41 |
| Dioxathion | 15,595 | 18,876 | 0.09 | 142.51 |
| gamma-BHC (Lindane) | 236,769 | 279,907 | 1451.52 | 1.64 |
| Terbufos | 322,969 | 366,145 | 457.9 | 4564.78 |
| Propyzamide | 576,662 | 712,115 | 540.99 | 293.59 |
| Diazinon | 81,040 | 96,787 | 2270.11 | 803.44 |
| Tefluthrin | 630,360 | 734,920 | 883.37 | 5745.48 |
| Tri-allate | 180,277 | 211,677 | 163.58 | 1092.95 |
| Pirimicarb | 891,731 | 1,185,571 | 20.05 | 145.46 |
| Formothion | 605 | 1030 | 0.25 | 0.31 |
| Dimethachlor | 260,765 | 315,437 | 43.25 | 4657.50 |
| Dimethenamid | 421,469 | 508,351 | 1077.21 | 12,123.16 |
| Acetochlor | 153,508 | 190,791 | 9,864,921.36 | 4,370,556.78 |
| Chlorpyrifos-methyl | 242,653 | 296,685 | 22.15 | 8587.13 |
| Vinclozolin | 66,822 | 79,772 | 1347 | 1011.08 |
| Parathion-methyl | 180,138 | 223,844 | 0.55 | 704.41 |
| Alachlor | 197,998 | 248,331 | 10.77 | 5817.62 |
| Tolclofos-methyl | 409,625 | 501,591 | 10.28 | 2741.49 |
| Heptachlor | 427,999 | 487,626 | 211.66 | 7530.75 |
| Fenchlorphos | 300,382 | 359,237 | 666.08 | 9595.41 |
| Pirimiphos-methyl | 279,950 | 335,424 | 912.75 | 2960.56 |
| Fenitrothion | 139,649 | 179,941 | 28.93 | 378.75 |
| Methiocarb | 42,976 | 74,071 | 77.29 | 145.03 |
| Ethofumesate | 235,529 | 282,317 | 43.25 | 1177.81 |
| Malathion | 362,917 | 491,875 | 2277.68 | 8416.23 |
| Metolachlor | 1,087,219 | 1,359,908 | 44.1 | 4331.05 |
| Chlorpyrifos | 349,564 | 449,808 | 132.05 | 13,353.84 |
| Thiobencarb | 130,079 | 170,180 | 17.25 | 1,470.02 |
| Fenthion | 273,955 | 332,114 | 33.15 | 1064.93 |
| Aldrin | 114,379 | 138,423 | 178.97 | 1685.69 |
| Chlorthal-dimethyl | 228,963 | 286,051 | 43.21 | 10,912.66 |
| Parathion | 63,696 | 84,970 | 0.58 | 112.16 |
| Triadimefon | 193,938 | 256,560 | 5.34 | 233.19 |
| Dicofol (DCBP) | 604,319 | 785,466 | 46.18 | 792.03 |
| Butralin | 34,777 | 47,759 | 255.2 | 696.38 |
| Pendimethalin | 56,722 | 75,396 | 513.04 | 750.50 |
| (E)-Chlorfenvinphos | 10,844 | 12,776 | 0.06 | 382.05 |
| Metazachlor | 265,284 | 333,915 | 198.15 | 2167.63 |
| Fipronil | 111,767 | 141,384 | 197.91 | 2542.64 |
| Penconazole | 321,349 | 404,029 | 430.00 | 3489.80 |
| Chlozolinate | 6203 | 7540 | 62.14 | 235.58 |
| (Z)-Chlorfenvinphos | 214,413 | 272,821 | 9.34 | 2617.19 |
| Heptachlor-exo-epoxide | 62,132 | 82,817 | 440.3 | 2067.10 |
| Mecarbam | 14,536 | 19,810 | 20.84 | 42.18 |
| Heptachlor-endo-epoxide | 14,155 | 17,919 | 0.64 | 133.98 |
| Quinalphos | 426,954 | 43,8189 | 28.47 | 6,624,899.58 |
| Procymidone | 154,130 | 190,749 | 240.30 | 4055.71 |
| Triflumizole | 121,005 | 144,913 | 12.43 | 737.31 |
| Zoxamide | 40,393 | 44,048 | 1.55 | 39.33 |
| Methoprene | 70,923 | 141,572 | 0.18 | 133,752.88 |
| Bromophos-ethyl | 231,527 | 286,039 | 275.71 | 3340.63 |
| Chlorbenside | 463,024 | 568,017 | 6593.54 | 4438.62 |
| cis-Chlordane | 126,575 | 146,610 | 1.13 | 2381.07 |
| trans-Chlordane | 114,358 | 146,610 | 1.23 | 2329.68 |
| o, p’-DDE | 4197 | 5014 | 5.53 | 16.00 |
| Paclobutrazol | 362,013 | 453,615 | 298.02 | 12,758.21 |
| Mepanipyrim | 1,051,066 | 1,410,620 | 11.97 | 6.05 |
| alpha-Endosulfan | 75,406 | 88,687 | 15.73 | 778.89 |
| Napropamide | 375,174 | 518,589 | 6.77 | 1,437,868.74 |
| Chlorfenson | 724,840 | 936,452 | 3.25 | 9476.61 |
| Fludioxonil | 324,924 | 436,468 | 120.05 | 3168.12 |
| Fenamiphos | 90,109 | 114,318 | 134.09 | 659.52 |
| Tricyclazole | 277,480 | 423,024 | 24.19 | 1134.98 |
| Profenofos | 54,981 | 72,622 | 2714.59 | 1334.41 |
| Oxadiazon | 199,906 | 226,688 | 541.4 | 3759.60 |
| p, p’-DDE | 561,385 | 631,455 | 87.06 | 6112.72 |
| Myclobutanil | 393,777 | 501,559 | 364.84 | 2266.38 |
| Iprovalicarb | 82,867 | 117,512 | 1.55 | 151.70 |
| Oxyfluorfen | 27,241 | 35,081 | 60.89 | 312.61 |
| Flusilazole | 161,763 | 202,572 | 186.58 | 7755.73 |
| Dieldrin | 60,014 | 70,904 | 5.76 | 981.42 |
| Endrin | 67,017 | 81,552 | 0.78 | 319.75 |
| Bupirimate | 163,604 | 203,339 | 206.19 | 2165.78 |
| o, p’-DDD | 51,093 | 70,606 | 4.19 | 55.95 |
| Carboxin | 546,214 | 432,900 | 1806.28 | 205.68 |
| Chlorfenapyr | 20,921 | 24,793 | 96.99 | 334.43 |
| Aramite | 55,845 | 67,213 | 3.84 | 1547.28 |
| Chlorobenzilate | 845,151 | 990,529 | 41.43 | 4577.30 |
| beta-Endosulfan | 38,920 | 50,948 | - | 723.86 |
| Diniconazole | 264,523 | 340,574 | 210.68 | 592.68 |
| Oxadixyl | 353,274 | 411,206 | 111.07 | 1464.63 |
| Ethion | 305,498 | 366,263 | 2.41 | 8.55 |
| o,p’-DDT | 990,310 | 1,163,918 | 3060.36 | 35,409,580.79 |
| p,p’-DDD | 1,071,418 | 1,274,806 | 3751.03 | 41,936,916.5 |
| Mepronil | 1,857,251 | 2,318,169 | 2.84 | 2106.24 |
| Triazophos | 220,306 | 290,282 | 3.03 | 149.63 |
| Carfentrazone-ethyl | 158,046 | 190,676 | 21.90 | 2069.87 |
| Benalaxyl | 317,509 | 403,508 | 2.67 | 410.06 |
| Endosulfan sulfate | 115,936 | 143,804 | 102.75 | 534.16 |
| Pyraflufen-ethyl | 95,959 | 113,102 | 145.1 | 3103.57 |
| p,p’-DDT | 812,240 | 904,674 | 194.72 | 3419.61 |
| Diclofop-methyl | 172,404 | 212,563 | 164.80 | 5155.66 |
| Propargite | 320,216 | 452,987 | 0.61 | 43.36 |
| Piperonyl butoxide | 480,861 | 599,925 | 61.22 | 26,179.65 |
| Resmethrin (Bioresmethrin) | 131,735 | 159,161 | 6.47 | 43.89 |
| Bromuconazole | 198,976 | 247,694 | 1.09 | 10,877,233.91 |
| Tetramethrin | 136,449 | 188,587 | 0.34 | 1031.21 |
| Bifenazate | 141,256 | 180,715 | 1429.42 | 2221.02 |
| Methoxychlor | 644,484 | 786,627 | 641.65 | 4325.77 |
| Etoxazole | 18,946 | 25,235 | 133.13 | 1083.36 |
| Fenpropathrin | 76,639 | 105,178 | 99.15 | 1100.56 |
| Fenamidone | 120,860 | 161,456 | 10.70 | 2927.11 |
| Tebufenpyrad | 451,206 | 578,838 | 263.05 | 7435.66 |
| Tetradifon | 85,394 | 113,522 | 22.41 | 1,416,336.27 |
| Phenothrin | 206,647 | 261,617 | 3.20 | 1961.73 |
| Phosalone | 191,137 | 291,508 | 0.07 | 492.00 |
| Triticonazole | 107,791 | 143,226 | 59.74 | 238.20 |
| Pyriproxyfen | 356,159 | 491,117 | 1.86 | 6.24 |
| Cyhalofop-butyl | 450,839 | 610,550 | 99.31 | 2014.65 |
| Acrinathrin | 8522 | 15,309 | 0.23 | 93.69 |
| Amitraz | 156,383 | 117,684 | 17.29 | 8369.99 |
| Pyrazophos | 318,943 | 411,239 | 58.66 | 619.68 |
| Fenarimol | 178,882 | 228,637 | 122.9 | 10,901.89 |
| Spirodiclofen | 13,423 | 17,581 | 26.36 | 167.34 |
| Permethrin | 101,182 | 146,641 | 0.20 | 3,075,240.78 |
| Bitertanol | 49,979 | 92,576 | 0.06 | 301.76 |
| Dioxathion | 64,501 | 82,770 | 1.73 | 1,709.66 |
| Pyridaben | 720,297 | 931,972 | 26.88 | 33,742.89 |
| Cyfluthrin | 39,079 | 57,440 | 0.41 | 0.46 |
| Cypermethrin | 103,363 | 143,311 | 2.60 | 2.55 |
| Etofenprox | 1,681,402 | 2,165,175 | 63.92 | 30,980.56 |
| Pyridalyl | 207,703 | 288,298 | 37.68 | 2819.59 |
| Flucythrinate | 72,328 | 103,736 | 0.75 | 3,956,346.4 |
| Flumioxazin | 25,724 | 34,851 | 17.37 | 261.49 |
| Deltamethrin | 34,854 | 28,161 | 1.10 | 585.19 |
| Indoxacarb | 13,754 | 29,080 | 4.71 | 39.56 |
| Dimethomorph | 174,667 | 224,810 | 1.10 | 2657.37 |
| Cinidon-ethyl | 147,692 | 201,824 | 134.31 | 895.71 |
| Propaquizafop | 9962 | 13,506 | 316.77 | 486.44 |
| %RSD (Ν = 5) | ||
|---|---|---|
| Pesticide | Evaporation with Nitrogen | Evaporation with the Centrifugal Vacuum Concentrator |
| Methacrifos | 11.4 | 4.5 |
| Propoxur | - | 3.5 |
| Chlorpyrifos methyl | 5.9 | 1.6 |
| Heptachlor | 5.9 | 0.7 |
| Pirimiphos methyl | 4.1 | 2.7 |
| Fenitrothion | - | 3.5 |
| Malathion | - | 2.5 |
| Chlorpyrifos | 3.6 | 1.4 |
| Butralin | - | 3.8 |
| Dieldrin | 5.1 | 4.9 |
| o, p’-DDD | - | 5.0 |
| o, p’-DDT | 7.7 | 2.6 |
| p, p’-DDT | 5.3 | 1.9 |
| Methoxychlor | 6.5 | 1.0 |
| Tebufenpyrad | - | 4.9 |
| Phenothrin | 13.0 | 4.9 |
| Phenothrin | 3.0 | 2.4 |
| Permethrin | 7.3 | 6.8 |
| Cypermethrin | 3.8 | 2.3 |
| Indoxacarb | - | 5.5 |
| LC-MS/MS | GC-MS/MS | |||||
|---|---|---|---|---|---|---|
| Commodity | Pesticide | Assigned Value (mg/kg) | z-Score | Pesticide | Assigned Value (mg/kg) | z-Score |
| Apple purée | Pendimethalin | 0.041 | 0.7 | Flucythrinate | 0.042 | 0.0 |
| Profenofos | 0.119 | −0.5 | HCB | 0.051 | −0.7 | |
| Proquinazid | 0.083 | −0.2 | Oxyfluorfen | 0.051 | 0.4 | |
| Tebufenpyrad | 0.056 | −0.3 | Pendimethalin | 0.041 | −0.6 | |
| Procymidone | 0.077 | −0.6 | ||||
| Profenofos | 0.119 | −0.5 | ||||
| Tebufenpyrad | 0.059 | −0.3 | ||||
| Lime | Ethion | 0.043 | −1.4 | 2-Phenylphenol | 0.092 | 1.0 |
| Myclobutanil | 0.123 | −0.2 | trans-Chlordane | 0.056 | 1.5 | |
| Phosalone | 0.051 | −1.7 | Chlorpyrifos (ethyl) | 0.107 | −1.0 | |
| Imazalil | 0.169 | −2.3 | Diazinon | 0.061 | −0.2 | |
| Ethion | 0.043 | −0.5 | ||||
| Myclobutanil | 0.123 | 0.1 | ||||
| Phosalone | 0.051 | −0.2 | ||||
| Lettuce | Piperonyl butoxide | 0.054 | 0.4 | Piperonyl butoxide | 0.054 | 0.4 |
| Fluopicolide | 0.042 | −0.1 | Chlorpropham | 0.031 | −1.5 | |
| Oxadixyl | 0.029 | −0.7 | Chlorpyrifos-ethyl | 0.039 | −1.4 | |
| Pencycuron | 0.025 | −0.5 | Deltamethrin | 0.024 | −0.8 | |
| Propamocarb | 0.028 | −0.9 | Fludioxonil | 0.027 | −0.7 | |
| Propyzamide | 0.087 | 0.8 | gamma-HCH | 0.069 | −0.3 | |
| Pirimicarb | 0.062 | −0.9 | Oxadixyl | 0.029 | −0.5 | |
| Procymidone | 0.060 | −0.9 | ||||
| Propamocarb | 0.028 | −1.4 | ||||
| Propyzamide | 0.087 | −0.6 | ||||
| Pirimicarb | 0.062 | −0.8 | ||||
| Vinclozolin | 0.094 | −0.9 | ||||
| Commodity | Total Pesticides LC-MS/MS | Total Pesticides GC-MS/MS | Common Pesticides LC&GC | Overall Pesticides Determined |
| Apple | 136 | 140 | 58 | 218 |
| Orange | 136 | 152 | 66 | 222 |
| Onion | 131 | 159 | 80 | 210 |
| Lettuce | 136 | 127 | 53 | 210 |
| Tomato | 129 | 147 | 64 | 212 |
| LC-MS/MS |
| Common pesticides in 5 commodities |
| Acephate, Acetochlor, Alachlor, Ametoctradin, Atrazine, Azinphos-ethyl (Guthion ethyl), Benalaxyl, Bitertanol, Bromuconazole, Bupirimate, Butralin, Cadusafos, Carbetamide, Carbofuran, Carboxin, Carfentrazone-ethyl, Chloridazon (Pyrazon), Chloroxuron, Chromafenozide, Clofentezin, Clomazone, Cyazofamid, Cymoxanil (Curzate), Desmedipham, Dichlorvos, Dimethenamide (SAN 582H), Dimethomorph(E), Diniconazole, Dodemorph, EPTC, Ethirimol, Ethoprophos, Etoxazole, Fenamidone, Fenamiphos, Fenamiphos–sulfone, Fenamiphos-sulfoxide, Fenarimol, Fenazaquin, Fenhexamid, Fenthion sulfoxide (Mesulfenfos), Fenthion-oxon-sulfoxide, Fipronil, Flufenoxuron, Fluometuron, Fluopicolid, Flurtamone, Flusilazole, Forchlorfenuron, Imazalil (Enilconazole), Iprovalicarb, Isoxaben, Lenacil, Mepanipyrim, Mepronil, Metaflumizone, Metamitron, Methabenzthiazuron, Methiocarb sulfone, Methiocarb sulfoxide, Metolachlor, Metribuzin, Mevinphos (Phosdrin), Molinate, Monocrotophos (Azodrin), Monolinuron (Phenylurea), Monuron, Myclobutanil, Novaluron, Oxadixyl, Oxamyl, Oxycarboxin, Penconazole, Pencycuron, Pendimethalin (Penoxalin), Phenmedipham, Phosphamidon, Pirimicarb, Pirimiphos-methyl, Propargite, Propham, Propoxur, Propyzamide (Pronamide), Proquinazid, Prosulfocarb, Pyrazophos, Pyridaben, Pyriproxyfen, Rotenone, Simazine, Spirodiclofen, Spiromesifen, Spirotetramat, Tepp-A, Thiabendazole, Thiodicarb, Triadimefon, Tri-allate, Triazophos, Trichlorfon (DEP), Tricyclazole, Triflumizole |
| GC-MS/MS |
| Common pesticides in 5 commodities |
| 2-Phenylphenol, Acetochlor, Alachlor, Aldrin, alpha-Endosulfan, Atrazine, Benalaxyl, beta-BHC, Bitertanol, Bromophos-ethyl, Bromuconazole, Bupirimate, Butralin, Butylate, Cadusafos, Carboxin, Carfrentrazone-ethyl, Chlorbenside, Chlorbufam, Chlorfenapyr, Chlorfenson, Chlorobenzilate, Chlorpropham, Chlorpyrifos, Chlorpyrifos-methyl, Chlorthal-dimethyl, Cinidon-ethyl, Clomazone, Cyhalofop-butyl, Di-allate, Dichlobenil, Diclofop-methyl, Dicloran, Dicofol (DCBP), Dimethachlor, Dimethenamide, Dimethomorph, Diniconazole, Dioxathion, Dioxathion, Diphenylamine, E-Chlorfenvinphos, Endosulfan sulfate, Endrin, EPTC, Ethion, Ethofumosate, Ethoprophos, Etofenprox, Etoxazole, Fenamidone, Fenamiphos, Fenarimol, Fenchlorphos, Fenitrothion, Fenpropathrin, Fenthion, Fipronil, Fludioxonil, Flusilazole, Heptachlor, Heptachlor-endo-epoxide, Heptachlor-exo-epoxide, Iprovalicarb, Mepronil, Metazachlor, Methoxychlor, Metolachlor, Molinate, Myclobutanil, Napropamide, o,p’-DDD, o,p’-DDT, Oxadiazon, Oxadixyl, p,p’-DDE, p,p’-DDT, Paclobutrazol, Parathion-methyl,, Penconazole, Permethrin, Phenothrin, Piperonyl butoxide, Pirimicarb, Pirimiphos-methyl, Procymidone, Propachlor, Propaquizafop, Propargite, Pyrazophos, Pyridaben, Pyriproxyfen, Quinalphos, Simazine, Spirodiclofen, Tebufenpyrad, Tecnazene, Tefluthrin, Terbufos, Tetradifon, Thiobencarb, Tolclofos-methyl, Tri-allate, Triadimefon, Triazophos, Tricyclazole, Triflumizole, Triticonazole, Vinclozolin, Z-Chlorfenvinphos, Zoxamide |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romniou, S.E.; Nana, K.; Dasenaki, M.; Komaitis, E.; Proestos, C. Development and Validation of Pesticide Residues Determination Method in Fruits and Vegetables through Liquid and Gas Chromatography Tandem Mass Spectrometry (LC-MS/MS and GC-MS/MS) Employing Modified QuEChERS Method and a Centrifugal Vacuum Concentrator. Agriculture 2022, 12, 1936. https://doi.org/10.3390/agriculture12111936
Romniou SE, Nana K, Dasenaki M, Komaitis E, Proestos C. Development and Validation of Pesticide Residues Determination Method in Fruits and Vegetables through Liquid and Gas Chromatography Tandem Mass Spectrometry (LC-MS/MS and GC-MS/MS) Employing Modified QuEChERS Method and a Centrifugal Vacuum Concentrator. Agriculture. 2022; 12(11):1936. https://doi.org/10.3390/agriculture12111936
Chicago/Turabian StyleRomniou, Styliani E., Konstantina Nana, Marilena Dasenaki, Efstratios Komaitis, and Charalampos Proestos. 2022. "Development and Validation of Pesticide Residues Determination Method in Fruits and Vegetables through Liquid and Gas Chromatography Tandem Mass Spectrometry (LC-MS/MS and GC-MS/MS) Employing Modified QuEChERS Method and a Centrifugal Vacuum Concentrator" Agriculture 12, no. 11: 1936. https://doi.org/10.3390/agriculture12111936
APA StyleRomniou, S. E., Nana, K., Dasenaki, M., Komaitis, E., & Proestos, C. (2022). Development and Validation of Pesticide Residues Determination Method in Fruits and Vegetables through Liquid and Gas Chromatography Tandem Mass Spectrometry (LC-MS/MS and GC-MS/MS) Employing Modified QuEChERS Method and a Centrifugal Vacuum Concentrator. Agriculture, 12(11), 1936. https://doi.org/10.3390/agriculture12111936








