Effects of Extraction Solvents on the Total Phenolic Content, Total Flavonoid Content, and Antioxidant Activity in the Aerial Part of Root Vegetables
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Sample Extracts
2.3. Antioxidant Activity
2.3.1. DPPH Scavenging Assay
2.3.2. Ferric Reducing Antioxidant Power
2.3.3. Hydrogen Peroxide Scavenging Assay
2.4. Total Phenolic Content Determination
2.5. Total Flavonoids Determination
2.6. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Activity of the Aerial Part of Root Vegetables
3.2. Total Phenolic Content of the Aerial Part of Root Vegetables
3.3. Total Flavonoid Content of Aerial Part Wastes of Root Vegetables
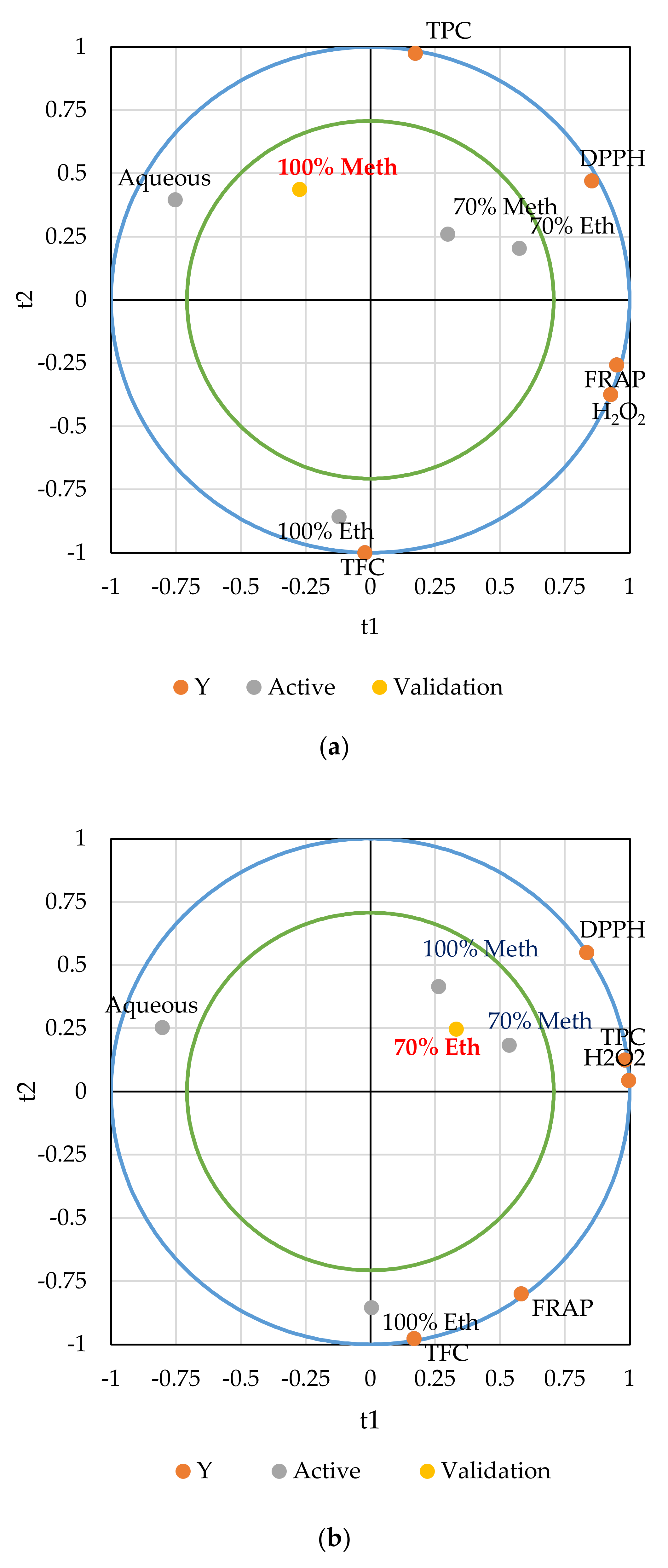
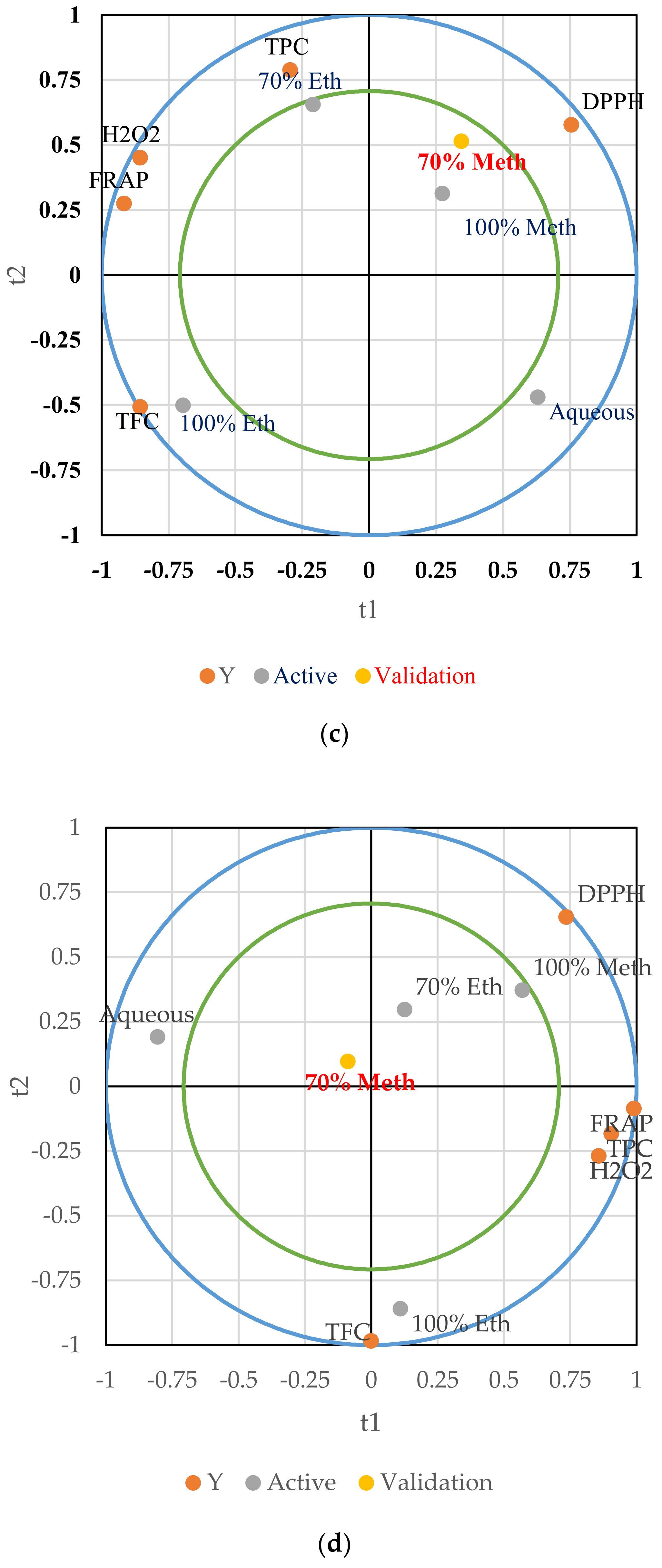
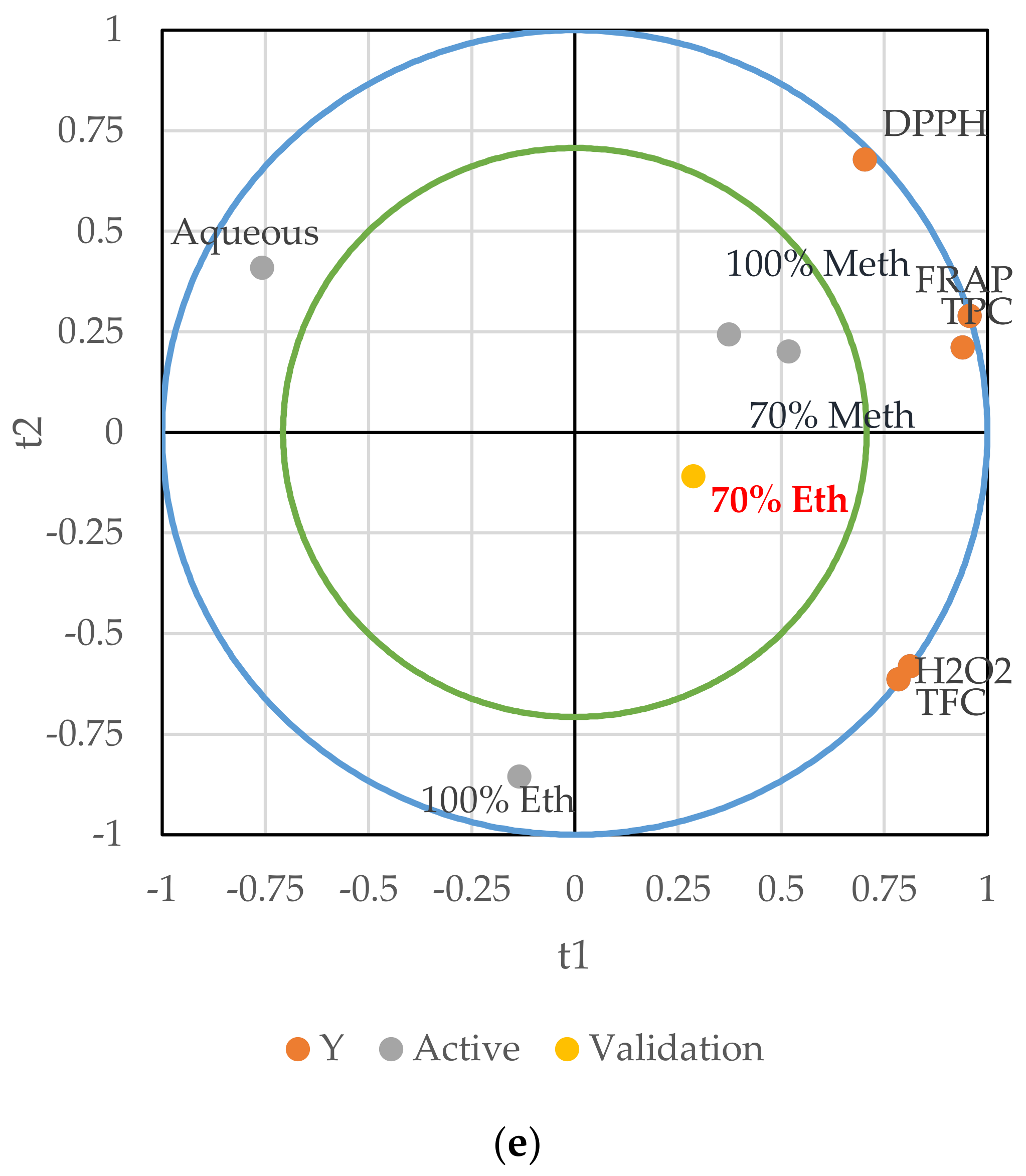
3.4. Partial Least Squares Regression Analysis (PLS)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ismail, A.; Marjan, Z.M.; Foong, C.W. Total antioxidant activity and phenolic content in selected vegetables. Food Chem. 2004, 87, 581–586. [Google Scholar] [CrossRef]
- Kahkonen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef] [PubMed]
- Vattem, D.; Shetty, K. Solid-state production of phenolic antioxidants from cranberry pomace by Rhizopus oligosporus. Food Biotechnol. 2002, 16, 189–210. [Google Scholar] [CrossRef]
- Negi, P.S.; Jayaprakasha, G.K.; Jena, B.S. Antioxidant and antimutagenic activities of pomegranate peel extracts. Food Chem. 2003, 80, 393–397. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef]
- Beevi, S.S.; Narasu, M.L.; Gowda, B.B. Polyphenolics profile, antioxidant and radical scavenging activity of leaves and stem of Raphanus sativus L. Plant Foods Hum. Nutr. 2010, 65, 8–17. [Google Scholar] [CrossRef]
- Co, M.; Fagerlund, A.; Engman, L.; Sunnerheim, K.; Sjoberg, P.J.R.; Turner, C. Extraction of antioxidants from Spruce (Picea abies) bark using ecofriendly solvents. Phytochem. Anal. 2012, 23, 1–11. [Google Scholar] [CrossRef]
- Boeing, J.S.; Barizão, É.O.; Silva, B.C.; Montanher, P.F.; de Cinque Almeida, V.; Visentainer, J.V. Evaluation of solvent effect on the extraction of phenolic compounds and antioxidant capacities from the berries: Application of principal component analysis. Chem. Cent. J. 2014, 8, 48. [Google Scholar] [CrossRef]
- Złotek, U.; Mikulska, S.; Nagajek, M.; Świeca, M. The effect of different solvents and number of extraction steps on the polyphenol content and antioxidant capacity of basil leaves (Ocimum basilicum L.) extracts. Saudi J. Biol. Sci. 2016, 23, 628–633. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542. [Google Scholar] [CrossRef]
- Chirinos, R.; Rogez, H.; Campos, D.; Pedreschi, R.; Larondelle, Y. Optimization of extraction conditions of antioxidant phenolic compounds from mashua (Tropaeolum tuberosum Ruíz & Pavón) tubers. Sep. Purif. Technol. 2007, 55, 217–225. [Google Scholar]
- Tabart, J.; Kevers, C.; Sipel, A.; Pincemail, J.; Defraigne, J.O.; Dommes, J. Optimisation of extraction of phenolics and antioxidants from black currant leaves and buds and of stability during storage. Food Chem. 2007, 105, 1268–1275. [Google Scholar] [CrossRef]
- Dhanani, T.; Shah, S.; Gajbhiye, N.A.; Kumar, S. Effect of extraction methods on yield, phytochemical constituents and antioxidant activity of Withania somnifera. Arab. J. Chem. 2017, 10, S1193–S1199. [Google Scholar] [CrossRef]
- Rababah, T.M.; Banat, F.; Rababah, A.; Ereifej, K.; Yang, W. Optimization of extraction conditions of total phenolics, antioxidant activities, and anthocyanin of oregano, thyme, terebinth, and pomegranate. J. Food Sci. 2010, 75, 626–632. [Google Scholar] [CrossRef]
- Michiels, J.A.; Kevers, C.; Pincemail, J.; Defraigne, J.O.; Dommes, J. Extraction conditions can greatly influence antioxidant capacity assays in plant food matrices. Food Chem. 2012, 130, 986–993. [Google Scholar] [CrossRef]
- Chang, S.T.; Wu, J.H.; Wang, S.Y.; Kang, P.; Yang, N.S.; Shyura, L.F. Antioxidant activity of extracts from Acacia confusa bark and heart wood. J. Agric. Food Chem. 2001, 49, 3420–3424. [Google Scholar] [CrossRef]
- Gulcin, I.; Oktay, M.; Kufre, I.; Vioglu, O.; Aslan, A. Determination of antioxidant activity of lichen (Cetraria islandica) Linn Ach. J. Ethnopharmacol. 2002, 79, 325–329. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Rao, L.J.; Sakariah, K.K. Antioxidant activities of flavidin in different in vitro model systems. Bioorg. Med. Chem. 2004, 12, 5141–5146. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Determination of Total Phenolics. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Ed.; John Wiley and Sons: New York, NY, USA, 2001; pp. I1.1.1–I1.1.8. [Google Scholar]
- Kim, D.O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 7th ed.; The Iowa State University Press: Ames, IA, USA, 1987; pp. 221–222. [Google Scholar]
- Tenenhaus, M.; Pagès, J.; Ambroisine, L.; Guinot, C. PLS methodology for studying relationships between hedonic judgments and product characteristics. Food Qual. Pref. 2005, 16, 315–325. [Google Scholar] [CrossRef]
- Alothman, M.; Bhat, R.; Karim, A.A. Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chem. 2009, 115, 785–788. [Google Scholar] [CrossRef]
- Zhu, K.X.; Lian, C.X.; Guo, X.N. Antioxidant activities and total phenolic contents of various extracts from defatted wheat germ. Food Chem. 2011, 126, 122–126. [Google Scholar] [CrossRef]
- Karadeniz, F.; Burdurlu, H.S.; Koca, N.; Soyer, Y. Antioxidant activity of selected fruits and vegetables grown in Turkey. Turk. J. Agric. For. 2005, 29, 297–303. [Google Scholar]
- Georgiev, V.; Weber, J.; Kneschke, E.; Denev, P.; Bley, T.; Pavlov, A. Antioxidant Activity and Phenolic Content of Betalain Extracts from Intact Plants and Hairy Root Cultures of the Red Beetroot Beta vulgaris cv. Detroit Dark Red. Plant Foods Hum. Nutr. 2010, 65, 105–111. [Google Scholar] [CrossRef]
- Holasová, M.; Fiedlerová, V. Comparison of antioxidant activity determination methods in fruit and vegetable juice. Chem. Listy 2011, 105, 766–772. [Google Scholar]
- Kaur, C.; Kapoor, H.C. Antioxidant activity and total phenolic content of some Asian vegetables. Int. J. Food Sci. Technol. 2002, 37, 153–161. [Google Scholar] [CrossRef]
- Barreira, J.C.; Ferreir, I.C.; Oliveira, M.B.P.; Pereira, J.A. Antioxidant activities of the extracts from chestnut flower, leaf, skins and fruit. Food Chem. 2008, 107, 1106–1113. [Google Scholar] [CrossRef]
- Munir, A.; Sultana, B.; Bashir, A.; Ghaffar, A.; Munir, B.; Shar, G.A.; Iqbal, M. Evaluation of Antioxidant Potential of Vegetables Waste. Pol. J. Environ. Stud. 2018, 27, 947–952. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef]
- Ku, C.S.; Mun, S.P. Antioxidant activities of ethanol extracts from seeds in fresh Bokbunja (Rubus coreanus Miq.) and wine processing waste. Bioresour. Technol. 2008, 99, 4503–4509. [Google Scholar] [CrossRef]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J. Chem. Technol. Metall. 2005, 40, 255–260. [Google Scholar]
| Extracts | Root Vegetables | ||||
|---|---|---|---|---|---|
| Onion | White Radish | Red Radish | Beet | Carrot | |
| Aqueous | 54.67 ± 0.95 c | 40.46 ± 0.87 b | 80.48 ± 0.98 b | 62.98 ± 0.19 c | 64.31 ± 0.19 c |
| 70% Methanol | 67.60 ± 0.52 b | 81.59 ± 1.42 a | 85.84 ± 1.64 a | 62.07 ± 1.66 c | 82.53 ± 0.47 a |
| 100% Methanol | 68.51 ± 0.90 b | 77.81 ± 1.64 a | 76.86 ± 0.94 c | 82.22 ± 0.98 a | 74.82 ± 1.19 b |
| 70% Ethanol | 75.99 ± 0.58 a | 81.90 ± 1.91 a | 81.43 ± 0.98 b | 72.14 ± 2.17 b | 82.22 ± 0.72 a |
| 100% Ethanol | 56.25 ± 1.19 c | 42.85 ± 1.16 b | 54.41 ± 0.81 d | 62.02 ± 2.29 c | 55.15 ± 1.42 d |
| F-test | ** | ** | ** | ** | ** |
| LSD 0.05 | 4.09 | 4.36 | 2.63 | 3.52 | 1.62 |
| Extracts | Root Vegetables | ||||
|---|---|---|---|---|---|
| Onion | White Radish | Red Radish | Beet | Carrot | |
| Aqueous | 0.41 ± 0.06 c | 1.65 ± 0.08 e | 3.44 ± 0.16 d | 4.34 ± 0.08 c | 7.13 ± 0.18 c |
| 70% Methanol | 1.40 ± 0.11 a | 4.23 ± 0.49 b | 4.26 ± 0.11 b | 9.66 ± 0.05 a | 10.18 ± 0.06 a |
| 100% Methanol | 0.45 ± 0.03 c | 2.65 ± 0.33 d | 3.99 ± 0.11 c | 9.68 ± 0.09 a | 9.88 ± 0.11 a |
| 70% Ethanol | 1.43 ± 0.08 a | 3.68 ± 0.26 c | 6.29 ± 0.05 a | 8.50 ± 0.91 b | 7.95 ± 0.03 b |
| 100% Ethanol | 1.20 ± 0.06 b | 5.36 ± 0.15 a | 6.13 ± 0.09 a | 8.49 ± 0.14 b | 7.74 ± 0.06 b |
| F-test | ** | ** | ** | ** | ** |
| LSD 0.05 | 0.15 | 0.53 | 0.19 | 0.17 | 0.18 |
| Extracts | Root Vegetables | ||||
|---|---|---|---|---|---|
| Onion | White Radish | Red Radish | Beet | Carrot | |
| Aqueous | 73.34 ± 0.04 d | 72.98 ± 0.09 d | 72.38 ± 0.98 d | 75.13 ± 0.08 c | 75.70 ± 0.19 c |
| 70% Methanol | 79.56 ± 0.13 b | 83.45 ± 1.06 a | 76.02 ± 1.04 c | 83.53 ± 1.12 a | 82.30 ± 0.13 a |
| 100% Methanol | 76.06 ± 0.20 c | 80.63 ± 1.04 b | 75.33 ± 0.94 c | 82.48 ± 1.10 b | 81.12 ± 0.19 b |
| 70% Ethanol | 81.22 ± 0.15 a | 80.22 ± 1.06 b | 82.38 ± 1.04 a | 84.14 ± 1.08 a | 82.85 ± 0.12 a |
| 100% Ethanol | 79.73 ± 0.12 b | 78.55 ± 1.16 c | 80.23 ± 1.10 b | 83.70 ± 1.10 a | 82.58 ± 1.13 a |
| F-test | ** | ** | ** | ** | ** |
| LSD 0.05 | 0.25 | 0.11 | 0.17 | 0.17 | 0.23 |
| Extracts | Root Vegetables | ||||
|---|---|---|---|---|---|
| Onion | White Radish | Red Radish | Beet | Carrot | |
| Aqueous | 11.01 ± 1.24 b | 12.31 ± 1.44 c | 18.99 ± 0.41 d | 20.78 ± 1.83 c | 9.59 ± 1.29 e |
| 70% Methanol | 11.97 ± 1.26 b | 30.17 ± 0.99 a | 36.37 ± 0.95 ab | 20.42 ± 1.83 c | 55.10 ± 1.58 b |
| 100% Methanol | 16.90 ± 0.65 a | 29.59 ± 0.36 a | 37.09 ± 0.36 a | 31.73 ± 0.95 a | 66.33 ± 1.49 a |
| 70% Ethanol | 10.90 ± 0.90 b | 28.83 ± 0.88 a | 33.24 ± 0.48 b | 24.23 ± 1.99 b | 47.44 ± 1.78 c |
| 100% Ethanol | 5.30 ± 0.71 c | 22.44 ± 0.71 b | 27.59 ± 0.80 c | 28.47 ± 1.17 a | 25.30 ± 1.56 d |
| F-test | ** | ** | ** | ** | ** |
| LSD 0.05 | 1.75 | 3.14 | 3.30 | 3.74 | 4.90 |
| Extracts | Root Vegetables | ||||
|---|---|---|---|---|---|
| Onion | White Radish | Red Radish | Beet | Carrot | |
| Aqueous | 3.71 ± 0.49 c | 4.78 ± 0.48 d | 11.17 ± 0.58 c | 14.64 ± 1.05 b | 15.75 ± 1.25 d |
| 70% Methanol | 4.78 ± 0.48 bc | 15.09 ± 1.14 b | 9.50 ± 0.71 d | 7.83 ± 0.72 c | 45.89 ± 1.47 b |
| 100% Methanol | 15.47 ± 0.87 a | 11.58 ± 0.72 c | 12.23 ± 0.93 c | 11.86 ± 1.05 b | 39.08 ± 0.72 c |
| 70% Ethanol | 5.47 ± 0.48 b | 15.33 ± 0.72 b | 16.03 ± 0.72 b | 8.25 ± 1.25 c | 48.25 ± 1.25 a |
| 100% Ethanol | 16.58 ± 0.72 a | 61.58 ± 1.91 a | 55.33 ± 0.72 a | 36.31 ± 1.58 a | 47.83 ± 0.24 ab |
| F-test | ** | ** | ** | ** | ** |
| LSD 0.05 | 1.12 | 1.98 | 1.77 | 2.81 | 2.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, E.A.; Abdalla, I.G.; Alfawaz, M.A.; Mohammed, M.A.; Al Maiman, S.A.; Osman, M.A.; Yagoub, A.E.A.; Hassan, A.B. Effects of Extraction Solvents on the Total Phenolic Content, Total Flavonoid Content, and Antioxidant Activity in the Aerial Part of Root Vegetables. Agriculture 2022, 12, 1820. https://doi.org/10.3390/agriculture12111820
Mohammed EA, Abdalla IG, Alfawaz MA, Mohammed MA, Al Maiman SA, Osman MA, Yagoub AEA, Hassan AB. Effects of Extraction Solvents on the Total Phenolic Content, Total Flavonoid Content, and Antioxidant Activity in the Aerial Part of Root Vegetables. Agriculture. 2022; 12(11):1820. https://doi.org/10.3390/agriculture12111820
Chicago/Turabian StyleMohammed, Eman A., Ismat G. Abdalla, Mohammed A. Alfawaz, Mohammed A. Mohammed, Salah A. Al Maiman, Magdi A. Osman, Abu ElGasim A. Yagoub, and Amro B. Hassan. 2022. "Effects of Extraction Solvents on the Total Phenolic Content, Total Flavonoid Content, and Antioxidant Activity in the Aerial Part of Root Vegetables" Agriculture 12, no. 11: 1820. https://doi.org/10.3390/agriculture12111820
APA StyleMohammed, E. A., Abdalla, I. G., Alfawaz, M. A., Mohammed, M. A., Al Maiman, S. A., Osman, M. A., Yagoub, A. E. A., & Hassan, A. B. (2022). Effects of Extraction Solvents on the Total Phenolic Content, Total Flavonoid Content, and Antioxidant Activity in the Aerial Part of Root Vegetables. Agriculture, 12(11), 1820. https://doi.org/10.3390/agriculture12111820





