Effect of Growth Regulators on In Vitro Micropropagation of Stahlianthus thorelii Gagnep
Abstract
1. Introduction
2. Materials and Methods
Experimental Layout
3. Results
3.1. Shoot Induction In Vitro and Explant Viability
3.2. Effect of Type and Concentration of Cytokinin and Auxin on In Vitro Shoot Multiplication
3.3. The Impact of NAA and IBA on In Vitro Root Development
3.4. Acclimatization of Tissue Cultured Plantlets
4. Discussion
4.1. Shoot Induction In Vitro and Explant Viability
4.2. Effect of Type and Concentration of Cytokinin and Auxin on In Vitro Shoot Multiplication
4.3. The Impact of NAA and IBA on In Vitro Root Development
4.4. Acclimatization of Tissue Cultured Plantlets
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BAP | 6-Benzyl Amino Purine |
| NAA | α-Naphthalene Acetic Acid |
| IBA | Indol-3-Butyric Acid |
| MS | Murashige and Skoog |
References
- Chi, V.V. Tu Dien Cay Thuoc Viet Nam-Vietnamese Herbal Medicine Dictionary; Medical Publishing House: Hanoi, Vietnam, 2012; pp. 769–770. [Google Scholar]
- Yu, J.G.; Chen, Y.H.; Fang, H.J. Studies on the medicinal plants of Chinese Zingiberaceae. Acta Pharm. Sin. 1983, 18, 839–842. [Google Scholar]
- Binh, N. Flora of Vietnam, ZingiberaceaeLindl, Han Noi; House for Science and Technology: Ha Noi, Vietnam, 2017; Volume 21, pp. 306–318. [Google Scholar]
- Chi, V.V. Common Botanical Dictionary; Science and Technology: Hanoi, Vietnam, 2004; pp. 243–567. [Google Scholar]
- Loi, D.T. Vietnamese Medicinal Plants and Herbs; Medicine Publisher House: Hanoi, Vietnam, 2004; p. 256. [Google Scholar]
- Hung, D.N.; Phuong, V.T.; Thuy, B.T. Research on propagating Panax pseudoginseng by stem cuttings in Hoang Su Phi district, Ha Giang province. J. Sci. Technol. 2013, 108, 135–139. [Google Scholar]
- Van Quang, L.; Luyen, P.T.; Hung, B.K. Research some techniques to propagate Stahlianthus thorelii Gagnep by tubers. Vietnam J. For. Sci. 2018, 18, 40–49. [Google Scholar]
- Sunitibala, H.; Damayanti, M.; Sharma, G.J. In vitro propagation and rhizome formation in Curcuma. Cytobios 2001, 409, 71–82. [Google Scholar]
- Loc, N.H.; Duc, D.T.; Kwon, T.H.; Yang, M.S. Micropropagation of zedoary (Curcuma zedoaria)—Avaluable medicinal plant. Plant Cell Tissue 2005, 81, 119–122. [Google Scholar] [CrossRef]
- Kambaska, K.B.; Santilata, S. Effect of plant growth regulator on micropropagtion of ginger (Zingiber officinale Rosc.) cv-Suprava and Suruchi. J. Agric. Technol. 2009, 5, 271–280. [Google Scholar]
- Azhar, S.Z.A.; Ghani, K.A.; Yusuf, N.A. In vitro induction of adventitious root from shootbud of Boesenbergia rotunda (Zingiberaceae): Effect of plant growth regulators. Sci. Int. 2018, 30, 147–151. [Google Scholar]
- Sathyagowri, S.; Seran, T.H. In vitro plant regeneration of ginger (Zingiber officinale Rosc.) with emphasis on initial culture. Int. J. Med. Aromat. Plants 2011, 1, 195–202. [Google Scholar]
- Van Quang, L.; Hai, T.N.; Son, H.L. Morphological characteristics and anotomical structure of Stahlianthus thorelii Gagnep growing in Bavi distric, Hanoi city. Vietnam J. For. Sci. 2020, 2, 52–58. [Google Scholar]
- Huong, B.T.; Bac, N.T.; Gioi, D.H. In vitro Plant Regenration of red turmeric (Curcuma longa L.) from Tubers. J. For. Sci. Technol. 2020, 2, 3–9. [Google Scholar]
- Skoog, F.; Miller, C. Chemical regulation of growth and organ formation in plant tissue cultured in vitro. Symp. Soc. Exp. Biol. 1957, 1, 118–131. [Google Scholar]
- Phillips, I. Apical dominance. Annu. Rev. Plant Physiol. 1975, 1, 341–367. [Google Scholar] [CrossRef]
- Gan, S.; Amasino, R. Cytokinins in plant senescence: From spay and pray to clone and play. Bioessays 1996, 181, 557–565. [Google Scholar] [CrossRef]
- Sachs, T.; Thimann, K.V. Release of lateral buds from apical dominance. Nature 1964, 201, 939–940. [Google Scholar] [CrossRef]
- Sachs, T.; Thimann, K.V. The role of auxins and cytokinins in the release of buds from dominance. Am. J. Bot. 1967, 54, 136–144. [Google Scholar] [CrossRef]
- Kalousek, P.; Buchtova, D.; Balla, J.; Reinoehl, V.; Prochazka, S. Cytokinins and polar transport of auxin in axillary pea buds. Acta Univ. Agric. Silv. Mend. Brun. 2010, 58, 79–87. [Google Scholar] [CrossRef]
- Dun, E.A.; de Saint Germain, A.; Rameau, C.; Beveridge, C.A. Antagonistic action of strigolactone and cytokinin in bud outgrowth control. Plant Physiol. 2012, 158, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Mok, D.W.S.; Mok, M.C. Cytokinins: Chemistry, Activity and Function; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Nayak, S.; Parida, R.; Mohanty, S. Evaluation of genetic fidelity of invitro propagated greater Galangal (Alpinia galanga L.) using DNA based. Int. J. Plant Anim. Environ. Sci. 2011, 3, 124–133. [Google Scholar]
- Behera, K.K.; Pani, D.; Shahoo, S. Effect of plant growth regulator on in vitro multiplucation of turmeric (Curcumar longa L. cv. Ranga). Int. J. Biol. Technol. 2010, 1, 16–23. [Google Scholar]
- Bhattacharya, M. In vitro regeneration of pathogen free Kaempferia galanga L. A rare medicinal plant. Res. Plan Biol. 2013, 3, 24–30. [Google Scholar]
- Senarath, R.M.U.S.; Karunarathna, B.M.A.C.; Senarath, W.T.P.S.K.; Jimmy, G.C. In Vitro Propagation of Kaempferia Galanga (Zingiberaceae) and Comparison of Larvicidal Activity and Phytochemical Identities of Rhizomes of Tissue Cultured and Naturally Grown Plants. J. Appl. Biotechnol. Bioeng. 2017, 2, 00040. [Google Scholar]
- Goyal, A.K.; Ganguly, K.; Mishra, T.; Sen, A. In vitro multiplication of Curcuma longa Linn.—An important. NBU J. Plant Sci. 2010, 4, 21–24. [Google Scholar] [CrossRef]
- Naz, S.; Ilyas, S.; Javad Sand Ali, A. In vitro clonal multiplication and acclimatization of different varieties of turmeric (Curcuma longa L.). Pak. J. Bot. 2009, 41, 2807–2816. [Google Scholar]
- Ongaro, V.; Leyser, O. Hormonal control of shoot branching. Exp. Bot. 2008, 59, 67–74. [Google Scholar] [CrossRef]
- Müller, D.; Leyser, O. Auxin, cytokinin and the control of shoot branching. Ann. Bot. 2011, 107, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Leduc, N.; Roman, H.; Barbier, F.; Péron, T.; Huché-Thélier, L.; Lothier, J.; Demotes-Mainard, S.; Sakr, S. Light signaling in bud outgrowth and branching in plants. Plants 2014, 3, 223–250. [Google Scholar] [CrossRef]
- Barbier, F.F.; Lunn, J.E.; Beveridge, C.A. Ready, steady, go! A sugar hit starts the race to shoot branching. Curr. Opin. Plant Biol. 2015, 25, 39–45. [Google Scholar] [PubMed]
- Rameau, C.; Bertheloot, J.; Leduc, N.; Andrieu, B.; Foucher, F.; Sakr, S. Multiple pathways regulate shoot branching. Front. Plant Sci. 2015, 5, 741. [Google Scholar] [CrossRef] [PubMed]
- Schaller, G.E.; Bishopp, A.; Kieber, J.J. The Yin-Yang of Hormones: Cytokinin and Auxin Interactions in Plant Development. Plant Cell 2015, 27, 44–63. [Google Scholar] [CrossRef] [PubMed]
- Nordstrom, A.; Tarkowski, P.; Tarkowska, D.; Norbaek, R.; Astot, C.; Dolezal, K.; Sandberg, G. Auxin regulation of cytokinin biosynthesis in Arabidopsis thaliana: A factor of potential importance for auxin-cytokinin-regulated development. Proc. Natl. Acad. Sci. USA 2004, 102, 8039–8044. [Google Scholar] [CrossRef]
- Chatfield, S.P.; Stirnberg, P.; Forde, B.G.; Leyser, O. The hormonal regulation of axillary bud growth in Arabidopsis. Plant J. 2000, 24, 159–169. [Google Scholar] [CrossRef]
- Waldie, T.; Leyser, O. Cytokinin targets Auxin transport to promote shoot branching. Plant Physiol. 2018, 177, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Hamirah, M.N.; Sani, H.B.; Boyce, P.C.; Sim, S.L. Micropropagation of red ginger (Zingiber montanum Koenig), a medicinal plant. Proc. Asia Pacific Conf. Plant Tissue Agribiotechnol. 2007, 17, 17–21. [Google Scholar]
- Khairudin, N.A.; Haida, Z.; Hakiman, M. In Vitro Shoot and Root Induction of Kaempferia parviflora (Zingiberaceae) Rhizome Using 6-Benzylaminopurine. J. Trop. Plant Physiol. 2020, 2, 23–32. [Google Scholar]
- Mongkolsawat, W.; Saensouk, S.; Maknoi, C.; Saensouk, P. In vitro propagation of Stahlianthus campanulatus Ku ntze, a rare plant species from Thailand. J. Anim. Plant Sci. 2018, 85, 85–91. [Google Scholar]
- Abdelmageed, H.A.; Faridah, Q.Z.; Norhana, F.M.A.; Julia, A.A. Micropropagation of Etlingera elatior (Zingiberaceae) by using axillary bud explants. J. Med. Plants Res. 2011, 5, 4465–4469. [Google Scholar]
- Faridah, Q.Z.; Abdelmageed, A.H.A.; Julia, A.A. Efficient in vitroregeneration of Zingiber zerumbet Smith (a valuable medicinal plant) plantlets fromrhizome bud explants. Afr. J. Biotechnol. 2011, 10, 9303–9308. [Google Scholar] [CrossRef]
- Rahman, M.M.; Amin, M.N.; Jahan, H.S.; Ahmed, R. In vitro Regeneration of Plantlets of Curcuma longa Linn. A Valuable Spice Plant in Bangladesh. Asian J. Plant Sci. 2004, 3, 306–309. [Google Scholar] [CrossRef]
- Rahayu, S.; Adil, W.H. The effce of BAP and Thidiazuron on in vitrogrowth of Java Turmeric (Curcuma xanthorrhiza Roxb). ARPN J. Agric. Biol. Sci. 2012, 7, 2006–2012. [Google Scholar]
- Abbas, M.S.; Taha, H.S.; Aly, U.I.; El-Shabrawi, H.M.; Gaber, E.S.I. In vitro propagation of ginger (Zingiber officinale Rosco). J. Genet. Eng. Biotechnol. 2011, 9, 165–172. [Google Scholar] [CrossRef]
- Abbas, M.; Aly, U.; Taha, H.; Gaber, E. In vitro Production of Microrhizomes In Ginger (Zingiber officinale Rosco). J. Microbiol. Biotechnol. Food Sci. 2014, 10, 142–148. [Google Scholar] [CrossRef]
- Dharmasiri Dharmasiri, S.; Weijers, D.; Lechner, E.; Yamada, M.; Hobbie, L.; Ehrismann, J.S.; Jurgens, G.; Estelle, M. Plant development is regulated by a family of auxin receptor F box proteins. Dev. Cell 2005, 10, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.H.; Li, J.L.; Zhang, X.Q.; Yang, W.Y.; Wan, Y.; Ma, Y.M.; Zhu, Y.Q.; Peng, Y.; Huang, L.K. Effect of Naphthalene acetic acid on adventitious roodevelopmen and associated physiological changes in stem cutting of Hemarthria compressa. PLoS ONE 2014, 9, e90700. [Google Scholar] [CrossRef]
- Muday, G.K.; Haworth, P. Tomato root growth, gravitropism, and lateral development: Correlation with auxin transport. Plant Physiol. Biochem. 1994, 32, 193–203. [Google Scholar]
- Zeadan, S.M.; Macleod, R.D. Some effects of indol-3-yl-acetic acid on lateral root development in attached and excised roots of Pisum sativum L. Ann. Bot. 1984, 54, 759–766. [Google Scholar] [CrossRef]
- Hurren, N.G.; Zeadan, S.M.; Macleod, R.D. Lateral root development in attached and excised roots of Zea mays L. cultivated in the presence or absence of indol-3-yl acetic acid. Ann. Bot. 1988, 61, 573–579. [Google Scholar] [CrossRef]
- Lloret, P.G.; Pulgarín, A. Effect of naphthaleneacetic acid on the formation of lateral roots in the adventitious root of Allium cepa: Number and arrangement of laterals along the parent root. Can. J. Bot. 1992, 70, 1891–1896. [Google Scholar] [CrossRef]
- Gaspar, T.H.; Kevers, C.; Faivre-Rampant, O.; Crèvecour, M.; Penel, C.L.; Greppin, H.; Dommes, J. Changing concepts in plant hormone action. In Vitro Cell. Dev. Biol. Plant 2003, 39, 85–106. [Google Scholar]
- Evans, M.L.; Ishikawa, H.; Estelle, M.A. Responses of Arabidopsis roots to auxin studied with high temporal resolution: Comparison of wild type and auxin-response mutants. Planta 1994, 194, 215–222. [Google Scholar] [CrossRef]
- Muday, G.K.; DeLong, A. Polar auxin transport: Controlling where and how much. Trends Plant Sci. 2001, 6, 535–542. [Google Scholar] [CrossRef]
- Hartmann, H.T.; Kester, D.E.; Davies, F.T.; Geneve, R.L. Plant Propagation: Principles and Practices, 8th ed.; Prentice Hall of India Pvt. Ltd.: Boston, MA, USA, 2011. [Google Scholar]
- Seran, T.H.; Hirimburegama, K.; Gunasekare, M.T.K. Encapsulation of embryonic axes of Camellia sinensis (L.) O. Kuntze (tea) and subsequent in vitro germination. J. Hortic. Sci. 2005, 80, 154–158. [Google Scholar]

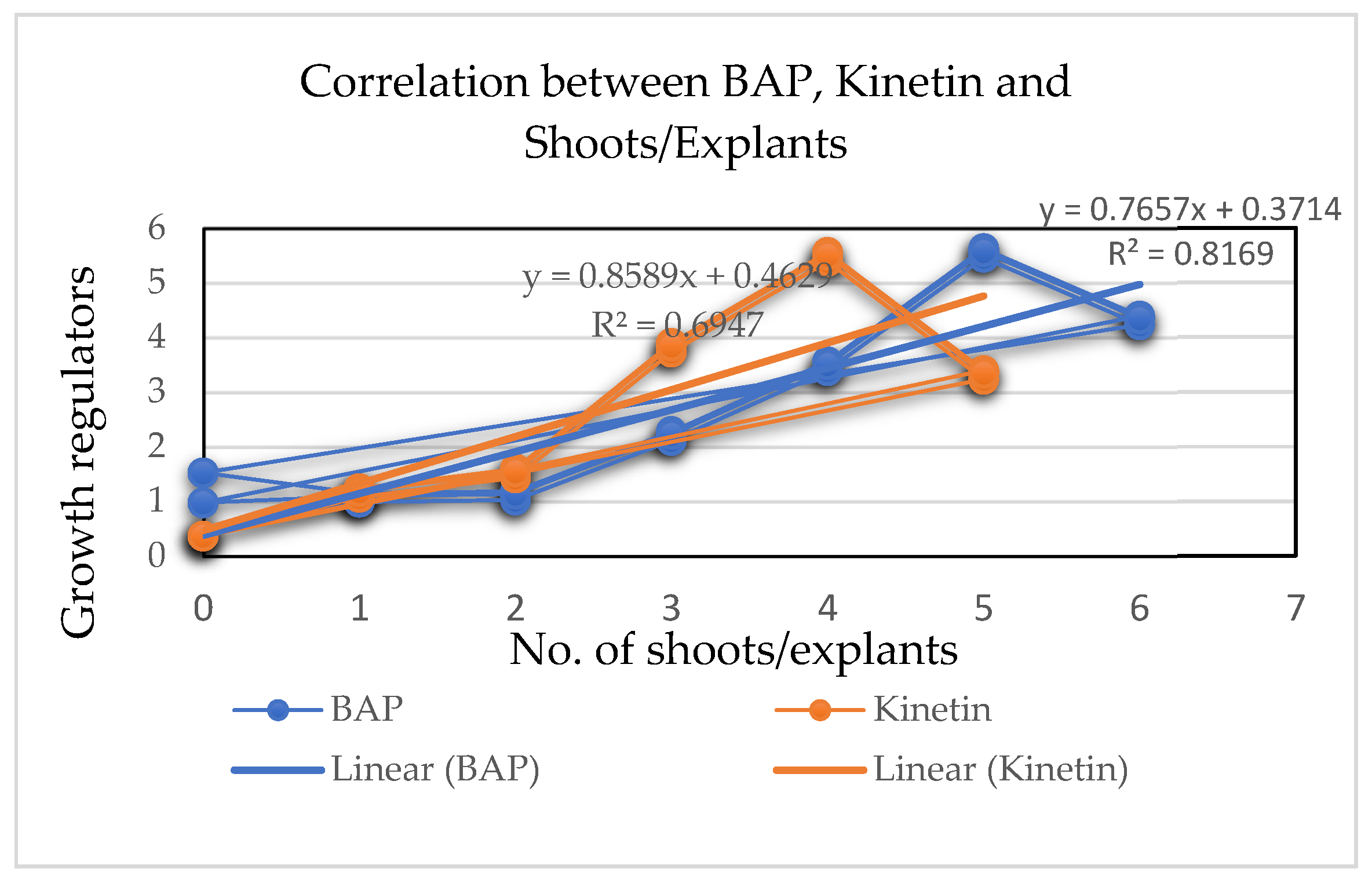

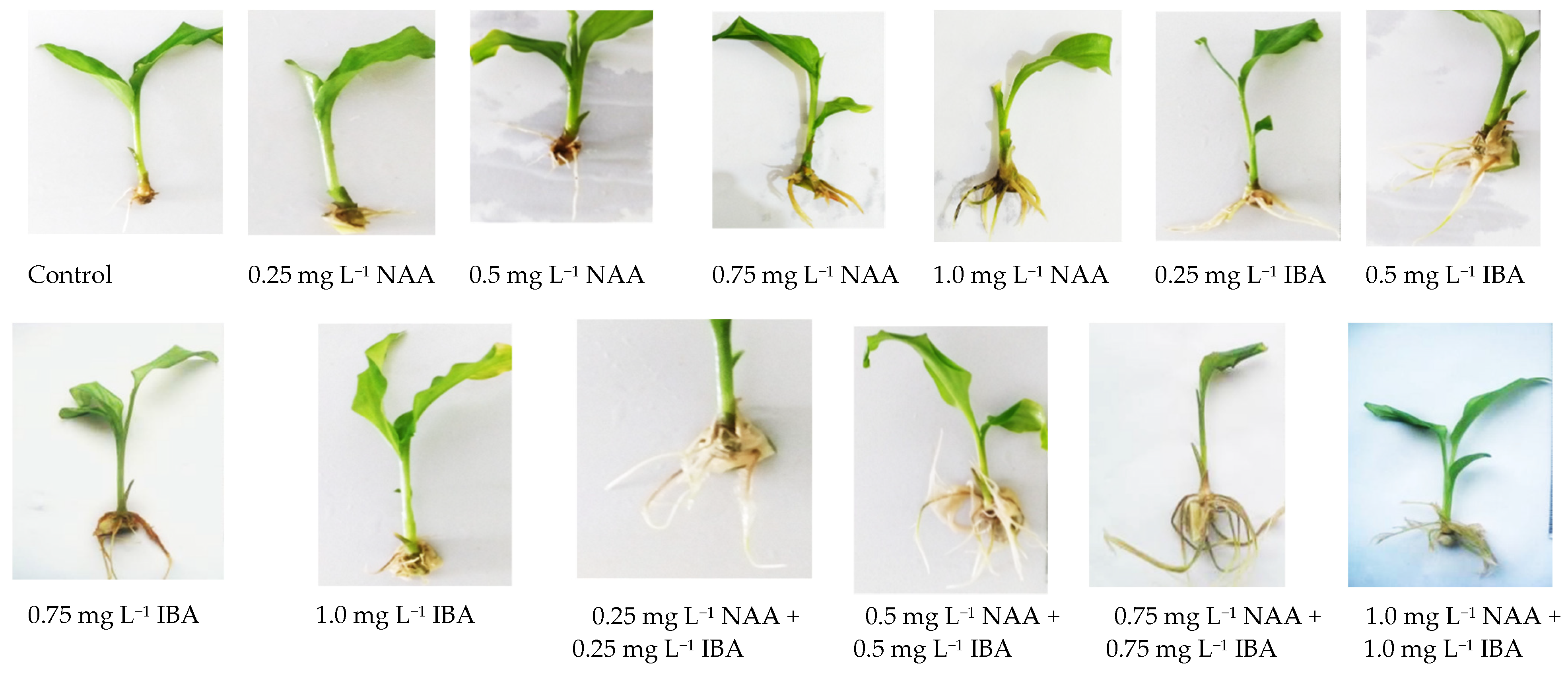

| Growth Regulators | Explants Inoculated | Shoots Initiated | Shoots/Explants (mean ± S.E.) |
|---|---|---|---|
| Basal medium (control) | 44 | 42 | 0.95 ± 0.46 g |
| BAP (mg L−1) | |||
| 1.0 | 44 | 47 | 1.07 ± 0.75 g |
| 2.0 | 44 | 49 | 1.11 ± 0.56 g |
| 3.0 | 44 | 97 | 2.20 ± 0.30 e |
| 4.0 | 44 | 153 | 3.48 ± 0.71 d |
| 5.0 | 44 | 244 | 5.45 ± 0.59 a |
| 6.0 | 44 | 190 | 4.32 ± 0.87 b |
| Kinetin (mg L−1) | |||
| 1.0 | 44 | 51 | 1.16 ± 0.51 g |
| 2.0 | 44 | 67 | 1.52 ± 0.71 f |
| 3.0 | 44 | 168 | 3.82 ± 0.61 c |
| 4.0 | 44 | 241 | 5.48 ± 0.87 a |
| 5.0 | 44 | 146 | 3.32 ± 0.73 d |
| CV% | 6.5 |
| Growth Regulators | No. of Shoots/Explants (mean ± S.E.) | Height of Shoots (cm) (mean ± S.E.) | Shoot Quality | |
|---|---|---|---|---|
| BAP (mg L−1) | NAA (mg L−1) | |||
| 0.0 | 0.0 | 0.67 ± 0.17 k | 0.76 ± 0.37 f | + |
| 1.0 | Absent | 2.45 ± 0.50 hi | 4.79 ± 1.11 e | + |
| 2.0 | 4.31 ± 0.45 e | 4.68 ± 1.06 e | ++ | |
| 3.0 | 4.74 ± 0.92 d | 5.04 ± 1.12 de | ++ | |
| 4.0 | 3.97 ± 1.18 ef | 5.52 ± 1.05 d | ++ | |
| Absent | 0.25 | 2.34 ± 0.48 i | 6.19 ± 1.48 c | + |
| 0.5 | 2.78 ± 0.86 gh | 6.91 ± 1.19 ab | + | |
| 0.75 | 2.51 ± 0.75 gh | 7.03 ± 1.23 a | + | |
| 1.0 | 2.85 ± 1.07 g | 6.98 ± 1.19 a | + | |
| 1.0 | 0.25 | 6.06 ± 0.87 c | 6.42 ± 1.02 bc | ++ |
| 2.0 | 0.5 | 7.54 ± 0.79 a | 6.81 ± 1.11 ab | ++ |
| 3.0 | 0.75 | 7.01 ± 0.71 b | 6.84 ± 1.42 ab | ++ |
| 4.0 | 1.0 | 6.43 ± 0.53 c | 6.76 ± 1.12 ab | ++ |
| CV% | 5.20 | 5.60 | ||
| Growth Regulators | Rooting Rate (%) | No. of Roots/Shoots (mean ± S.E.) | Root Length (cm) (mean ± S.E.) | |
|---|---|---|---|---|
| NAA (mg L−1) | IBA (mg L−1) | |||
| 0.0 | 0.0 | 80 | 1.61 ± 0.4 k | 2.63 ± 101 e |
| 0.25 | Absent | 100 | 15.26 ± 1.9 g | 8.48 ± 1.25 a |
| 0.5 | 100 | 18.60 ± 1.1 e | 6.42 ± 2.10 d | |
| 0.75 | 100 | 14.25 ± 1.5 h | 7.59 ± 1.45 b | |
| 1.0 | 100 | 10.75 ± 1.2 i | 7.03 ± 1.80 bcd | |
| Absent | 0.25 | 100 | 18.78 ± 1.7 e | 7.11 ± 1.32 bc |
| 0.5 | 100 | 19.76 ± 1.9 d | 7.06 ± 1.58 bcd | |
| 0.75 | 100 | 17.22 ± 1.8 f | 8.72 ± 1.41 a | |
| 1.0 | 100 | 14.62 ± 1.7 gh | 7.31 ± 1.50 b | |
| 0.25 | 0.25 | 100 | 19.72 ± 1.4 d | 8.68 ± 1.32 a |
| 0.5 | 0.5 | 100 | 26.17 ± 1.5 a | 8.67 ± 1.27 a |
| 0.75 | 0.75 | 100 | 22.57 ± 1.5 c | 6.84 ± 1.31 cd |
| 1.0 | 1.0 | 100 | 23.64 ± 2.4 b | 7.18 ± 1.23 bc |
| CV% | 6.5 | 5.6 | ||
| Potting Mixture | No. of Plantlets Transferred | Survival Rate (%) | New Leaf Formation (%) | Shoot Length (cm) (mean ± S.E.) |
|---|---|---|---|---|
| Burned rice husk | 36 | 16.13 | 43.70 | 10.20 ± 3.35 e |
| Soil | 36 | 43.54 | 53.60 | 15.10 ± 3.81 d |
| Coconut fiber | 36 | 91.64 | 72.40 | 25.00 ± 3.84 b |
| Sand | 36 | 58.70 | 61.50 | 13.80 ± 4.24 d |
| Soil:sand:compost (1:1:1) | 36 | 100.00 | 88.60 | 29.40 ± 3.59 a |
| Soil:paddy husk (1:1) | 36 | 80.40 | 66.70 | 25.70 ± 3.69 b |
| Soil:paddy husk (1:2) | 36 | 30.27 | 59.30 | 19.50 ± 3.46 c |
| CV% | 7.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Yen, D.; Li, J. Effect of Growth Regulators on In Vitro Micropropagation of Stahlianthus thorelii Gagnep. Agriculture 2022, 12, 1766. https://doi.org/10.3390/agriculture12111766
Van Yen D, Li J. Effect of Growth Regulators on In Vitro Micropropagation of Stahlianthus thorelii Gagnep. Agriculture. 2022; 12(11):1766. https://doi.org/10.3390/agriculture12111766
Chicago/Turabian StyleVan Yen, Duong, and Jing Li. 2022. "Effect of Growth Regulators on In Vitro Micropropagation of Stahlianthus thorelii Gagnep" Agriculture 12, no. 11: 1766. https://doi.org/10.3390/agriculture12111766
APA StyleVan Yen, D., & Li, J. (2022). Effect of Growth Regulators on In Vitro Micropropagation of Stahlianthus thorelii Gagnep. Agriculture, 12(11), 1766. https://doi.org/10.3390/agriculture12111766






