Traditional and Conditional QTL Analysis of Kernel Size- and Shape-Related Traits in Wheat (Triticum aestivum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Field Trials
2.2. Phenotypic Evaluation and Statistical Analysis
2.3. QTL Analysis
2.4. Development of Kompetitive Allele-Specific PCR (KASP) Markers
3. Results
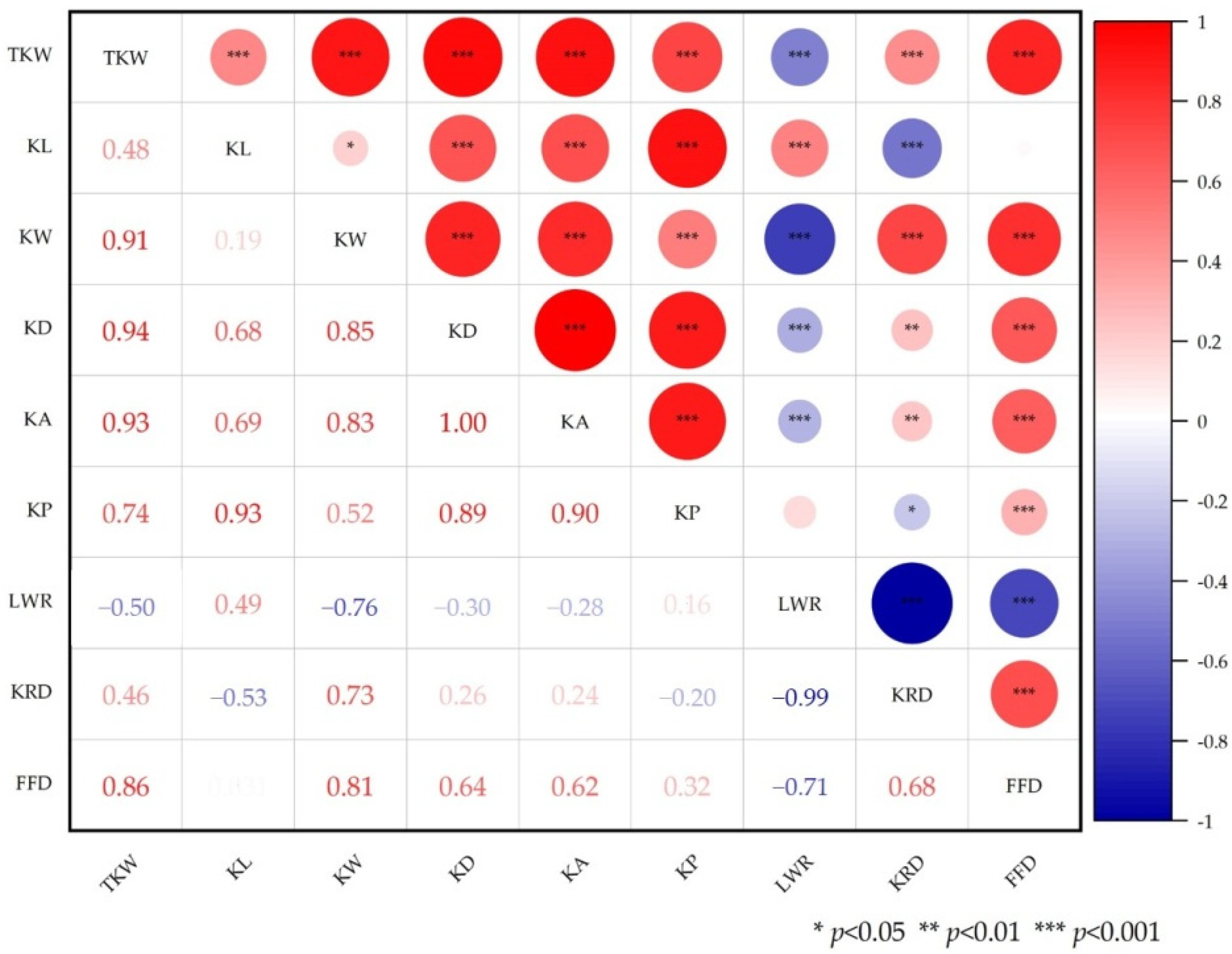
3.1. Phenotypic Evaluation and Correlation Analysis
3.2. Traditional QTL Analysis
3.2.1. The Traditional QTL for Kernel Components (KCs)
3.2.2. The Traditional QTL for Kernel Size Related Traits (KSzieRTs)
3.2.3. The Traditional QTL for Kernel Shape-Related Traits (KShapeRTs)
3.3. Conditional QTL Analysis
3.3.1. The Conditional QTL Mapping for KSizeRTs Independent of a Given KC
3.3.2. The Conditional QTL Mapping for KShapeRTs Independent of a Given KC
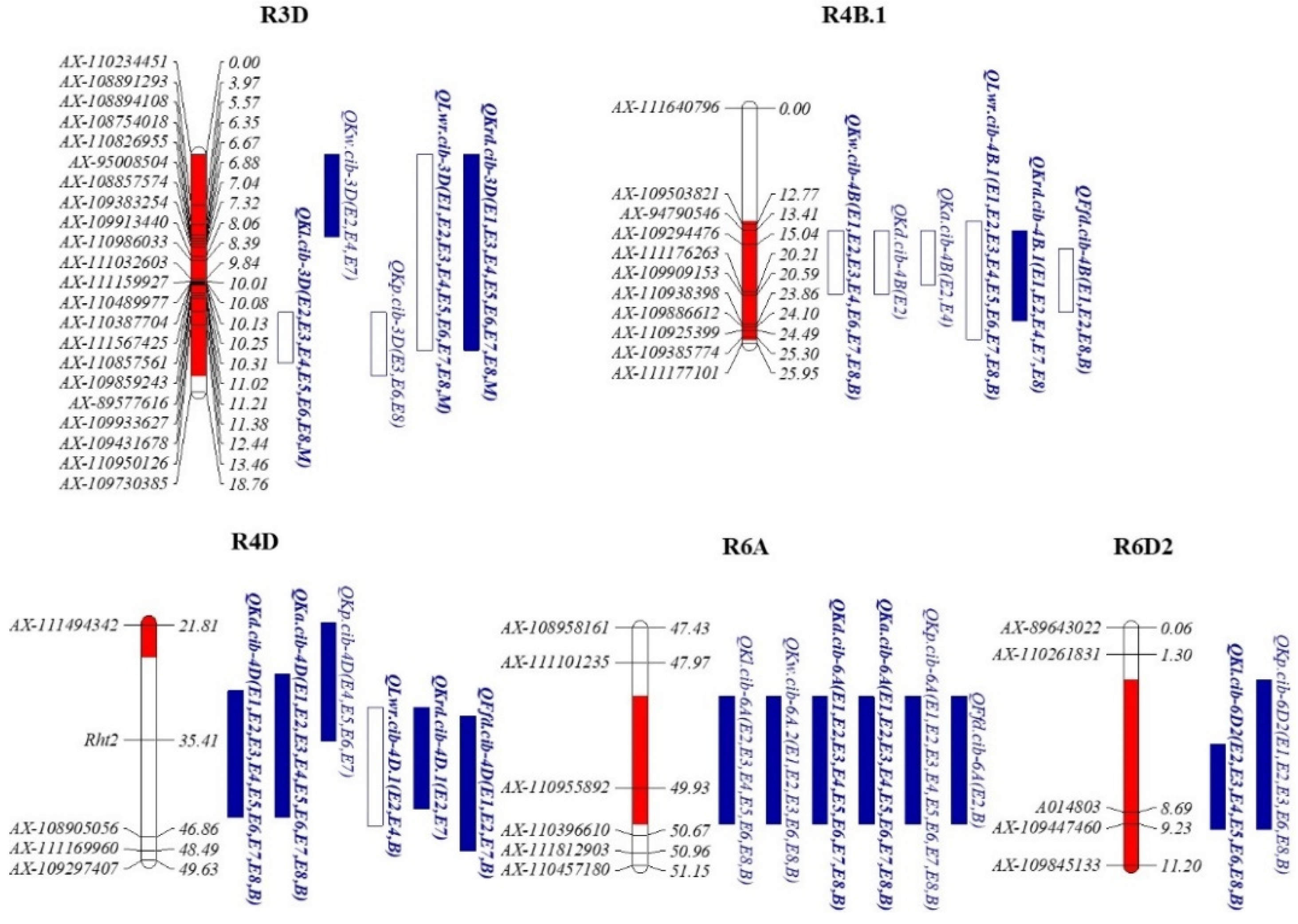
3.4. QTL Hot Regions for KRTs
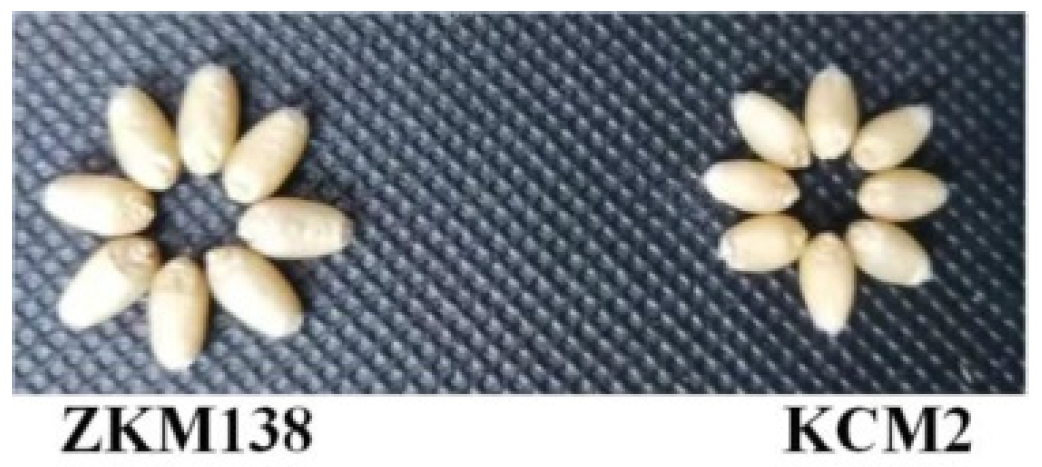
3.5. Validation of the Target Loci by Markers
3.6. The Transmissibility and Distribution of R3D, R4B.1 and R6D2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef] [PubMed]
- Dholakia, B.; Ammiraju, J.; Singh, H.; Lagu, M.; Röder, M.; Rao, V.; Dhaliwal, H.; Ranjekar, P.; Gupta, V.; Weber, W. Molecular marker analysis of kernel size and shape in bread wheat. Plant Breed. 2003, 122, 392–395. [Google Scholar] [CrossRef]
- Wang, L.; Ge, H.; Hao, C.; Dong, Y.; Zhang, X. Identifying loci influencing 1,000-kernel weight in wheat by microsatellite screening for evidence of selection during breeding. PLoS ONE 2012, 7, e29432. [Google Scholar] [CrossRef]
- Ramya, P.; Chaubal, A.; Kulkarni, K.; Gupta, L.; Kadoo, N.; Dhaliwal, H.S.; Chhuneja, P.; Lagu, M.; Gupt, V. QTL mapping of 1000-kernel weight, kernel length, and kernel width in bread wheat (Triticum aestivum L.). J. Appl. Genet. 2010, 51, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Gegas, V.C.; Nazari, A.; Griffiths, S.; Simmonds, J.; Fish, L.; Orford, S.; Sayers, L.; Doonan, J.H.; Snape, J.W. A Genetic framework for grain size and shape variation in wheat. Plant Cell 2010, 22, 1046–1056. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.A.; Ding, A.; Li, J.U.N.; Zhao, C.; Li, X.; Feng, D.; Wang, X.; Wang, L.I.N.; Gao, J.; Wang, H. Wheat kernel dimensions: How do they contribute to kernel weight at an individual QTL level? J. Genet. 2011, 90, 409–425. [Google Scholar] [CrossRef]
- Mohler, V.; Albrecht, T.; Castell, A.; Diethelm, M.; Schweizer, G.; Hartl, L. Considering causal genes in the genetic dissection of kernel traits in common wheat. J. Appl. Genet. 2016, 57, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, H.; Mai, C.; Yu, G.; Li, H.; Meng, L.; Jian, D.; Yang, L.; Zhou, Y.; Zhang, H. Variation in allelic frequencies at loci associated with kernel weight and their effects on kernel weight-related traits in winter wheat. Crop J. 2019, 7, 30–37. [Google Scholar] [CrossRef]
- Xin, F.; Zhu, T.; Wei, S.; Han, Y.; Zhao, Y.; Zhang, D.; Ma, L.; Ding, Q. QTL mapping of kernel traits and validation of a major QTL for kernel length-width ratio using SNP and bulked segregant analysis in wheat. Sci. Rep. 2020, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Li, C.; Liu, H.; Liu, J.; Luo, W.; Xu, Q.; Tang, H.; Mu, Y.; Deng, M.; Pu, Z. Quick mapping and characterization of a co-located kernel length and thousand-kernel weight-related QTL in wheat. Theor. Appl. Genet. 2022, 135, 2849–2860. [Google Scholar] [CrossRef] [PubMed]
- Chastain, T.; Ward, K.; Wysocki, D. Stand establishment response of soft white winter wheat to seedbed residue and seed size. Crop Sci. 1995, 35, 213–218. [Google Scholar] [CrossRef]
- Botwright, T.; Condon, A.; Rebetzke, G.; Richards, R. Field evaluation of early vigour for genetic improvement of grain yield in wheat. Aust. J. Agric. Res. 2002, 53, 1137–1145. [Google Scholar] [CrossRef]
- Ma, L.; Li, T.; Hao, C.; Wang, Y.; Chen, X.; Zhang, X. TaGS5-3A, a grain size gene selected during wheat improvement for larger kernel and yield. Plant Biotechnol. J. 2016, 14, 1269–1280. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Z.; Feng, B.; Zhou, Q.; Ji, G.; Guo, S.; Liao, S.; Lin, D.; Fan, X.; Wang, T. Quantitative trait loci identification and breeding value estimation of grain weight-related traits based on a new wheat 50K single nucleotide polymorphism array-derived genetic map. Front. Plant Sci. 2022, 13, 967432. [Google Scholar] [CrossRef]
- Su, Z.; Hao, C.; Wang, L.; Dong, Y.; Zhang, X. Identification and development of a functional marker of TaGW2 associated with grain weight in bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 2011, 122, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Wang, F.; Liu, T.; Dong, Z.; Li, A.; Jing, R.; Mao, L.; Li, Y.; Liu, X.; Zhang, K. Natural variation of TaGASR7-A1 affects grain length in common wheat under multiple cultivation conditions. Mol. Breed. 2014, 34, 937–947. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Xia, X.; He, Z. TaGS-D1, an ortholog of rice OsGS3, is associated with grain weight and grain length in common wheat. Mol. Breed. 2014, 34, 1097–1107. [Google Scholar] [CrossRef]
- Breseghello, F.; Sorrells, M.E. QTL analysis of kernel size and shape in two hexaploid wheat mapping populations. Field Crops Res. 2007, 101, 172–179. [Google Scholar] [CrossRef]
- Evers, A.D.; Cox, R.I.; Shaheedullah, M.Z.; Withey, R.P. Predicting milling extraction rate by image analysis of wheat grains. Asp. Appl. Biol. 1990, 25, 417–426. [Google Scholar]
- Kumar, A.; Mantovani, E.; Seetan, R.; Soltani, A.; Echeverry-Solarte, M.; Jain, S.; Simsek, S.; Doehlert, D.; Alamri, M.; Elias, E. Dissection of genetic factors underlying wheat kernel shape and size in an elite × nonadapted cross using a high density SNP linkage map. Plant Genome 2016, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Troccoli, A.; Di Fonzo, N. Relationship between kernel size features and test weight in Triticum durum. Cereal Chem. 1999, 76, 45–49. [Google Scholar] [CrossRef]
- Schuler, S.F.; Bacon, R.K.; Finney, P.L.; Gbur, E.E. Relationship of test weight and kernel properties to milling and baking quality in soft red winter wheat. Crop Sci. 1995, 35, 949–953. [Google Scholar] [CrossRef]
- Cabral, A.L.; Jordan, M.C.; Larson, G.; Somers, D.J.; Humphreys, D.G.; McCartney, C.A. Relationship between QTL for grain shape, grain weight, test weight, milling yield, and plant height in the spring wheat cross RL4452/‘AC Domain’. PLoS ONE 2018, 13, e0190681. [Google Scholar] [CrossRef]
- Campbell, K.G.; Bergman, C.J.; Gualberto, D.G.; Anderson, J.A.; Giroux, M.J.; Hareland, G.; Fulcher, R.G.; Sorrells, M.E.; Finney, P.L. Quantitative trait loci associated with kernel traits in a soft × hard wheat cross. Crop Sci. 1999, 39, 1184–1195. [Google Scholar] [CrossRef]
- Wang, S.; Wong, D.; Forrest, K.; Allen, A.; Chao, S.; Huang, B.E.; Maccaferri, M.; Salvi, S.; Milner, S.G.; Cattivelli, L. Characterization of polyploid wheat genomic diversity using a high-density 90 000 single nucleotide polymorphism array. Plant Biotechnol. J. 2014, 12, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Zhang, N.; Fan, X.L.; Zhang, W.; Zhao, C.H.; Yang, L.J.; Pan, R.Q.; Chen, M.; Han, J.; Zhao, X.Q. Utilization of a Wheat660K SNP array-derived high-density genetic map for high-resolution mapping of a major QTL for kernel number. Sci. Rep. 2017, 7, 3788. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Luo, W.; Qin, N.; Ding, P.; Zhang, H.; Yang, C.; Mu, Y.; Tang, H.; Liu, Y.; Li, W. A 55 K SNP array-based genetic map and its utilization in QTL mapping for productive tiller number in common wheat. Theor. Appl. Genet. 2018, 131, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Dong, Z.; Zhao, L.; Ren, Y.; Zhang, N.; Chen, F. The Wheat 660K SNP array demonstrates great potential for marker-assisted selection in polyploid wheat. Plant Biotechnol. J. 2020, 18, 1354–1360. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Liu, X.; Feng, B.; Zhou, Q.; Deng, G.; Long, H.; Cao, J.; Guo, S.; Ji, G.; Xu, Z.; et al. Construction of a novel Wheat 55 K SNP array-derived genetic map and its utilization in QTL mapping for grain yield and quality related traits. Front. Genet. 2022, 13, 978880. [Google Scholar] [CrossRef]
- Sun, X.; Wu, K.; Zhao, Y.; Kong, F.; Han, G.; Jiang, H.; Huang, X.; Li, R.; Wang, H.; Li, S. QTL analysis of kernel shape and weight using recombinant inbred lines in wheat. Euphytica 2009, 165, 615–624. [Google Scholar] [CrossRef]
- Zhu, J. Analysis of conditional genetic effects and variance components in developmental genetics. Genetics 1995, 141, 1633–1639. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Wang, J.; Liu, Q.; Zhang, M.; Zou, K.; Fu, X. Genetic relationships among panicle characteristics of rice (Oryza sativa L.) using unconditional and conditional QTL analyses. J. Plant Biol. 2009, 52, 259–267. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, Z.; Wang, Y.; Li, J.; Tian, J. Unconditional and conditional QTL analysis of kernel weight related traits in wheat (Triticum aestivum L.) in multiple genetic backgrounds. Genetica 2014, 142, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, Y.; Liu, T.; Wang, F.; Liu, K.; Chen, J.; Tian, J. Genetic analysis of kernel weight and kernel size in wheat (Triticum aestivum L.) using unconditional and conditional QTL mapping. Mol. Breed. 2015, 35, 194. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.; Li, R.; Deng, Z.; Zhang, K.; Liu, B.; Tian, J. Conditional QTL mapping of three yield components in common wheat (Triticum aestivum L.). Crop J. 2016, 4, 220–228. [Google Scholar] [CrossRef]
- Li, C.; Tang, H.; Luo, W.; Zhang, X.; Mu, Y.; Deng, M.; Liu, Y.; Jiang, Q.; Chen, G.; Wang, J. A novel, validated, and plant height-independent QTL for spike extension length is associated with yield-related traits in wheat. Theor. Appl. Genet. 2020, 133, 3381–3393. [Google Scholar] [CrossRef] [PubMed]
- Giura, A.; Saulescu, N. Chromosomal location of genes controlling grain size in a large grained selection of wheat (Triticum aestivum L.). Euphytica 1996, 89, 77–80. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Li, H.; Zhang, L.; Wang, J. QTL IciMapping: Integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. Crop J. 2015, 3, 269–283. [Google Scholar] [CrossRef]
- Li, T.; Deng, G.; Tang, Y.; Su, Y.; Wang, J.; Cheng, J.; Yang, Z.; Qiu, X.; Pu, X.; Zhang, H. Identification and validation of a novel locus controlling spikelet number in bread wheat (Triticum aestivum L.). Front. Plant Sci. 2021, 12, 611106. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.X.; Zhu, J. Multivariable conditional analysis for complex trait and its components. Acta Genet. Sin. 2005, 32, 289–296. [Google Scholar] [PubMed]
- Fuller, D.Q. Contrasting patterns in crop domestication and domestication rates: Recent archaeobotanical insights from the Old World. Ann. Bot. 2007, 100, 903–924. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.A.; Jones, M.K.; Powell, W.; Allaby, R.G. The complex origins of domesticated crops in the Fertile Crescent. Trends Ecol. Evol. 2009, 24, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Keyes, G.; Paolillo, D.; Sorrells, M. The effects of dwarfing genes Rht1 and Rht2 on cellular dimensions and rate of leaf elongation in wheat. Ann. Bot. 1989, 64, 683–690. [Google Scholar] [CrossRef]
- Manske, G.; Ortiz-Monasterio, J.; Van Ginkel, R.; Rajaram, S.; Vlek, P. Phosphorus use efficiency in tall, semi-dwarf and dwarf near-isogenic lines of spring wheat. Euphytica 2002, 125, 113–119. [Google Scholar] [CrossRef]
- Velu, G.; Singh, R.P.; Huerta, J.; Guzmán, C. Genetic impact of Rht dwarfing genes on grain micronutrients concentration in wheat. Field Crops Res. 2017, 214, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Rebetzke, G.; Richards, R. Gibberellic acid-sensitive dwarfing genes reduce plant height to increase kernel number and grain yield of wheat. Aust. J. Agric. Res. 2000, 51, 235–246. [Google Scholar] [CrossRef]
| Traits | QTLs | Environments | Linkages | Marker Intervals | LOD | PVE(%) | Add | Confidence Intervals |
|---|---|---|---|---|---|---|---|---|
| KL (mm) | QKl.cib-3B | E2,E3,E4,E5,E6,E8,B | 3B | AX-108855934—AX-109984220 | 4.74 | 6.45 | −0.07 | 79.50–81.00 |
| QKl.cib-3D | E2,E3,E4,E5,E6,E8,B | 3D | AX-110950126—AX-109730385 | 8.14 | 12.38 | −0.10 | 12.50–16.50 | |
| QKl.cib-6A | E2,E3,E4,E5,E6,E8,B | 6A | AX-110955892—AX-110396610 | 4.40 | 6.02 | 0.07 | 48.50–50.50 | |
| QKl.cib-6D2 | E2,E3,E4,E5,E6,E8,B | 6D2 | AX-110261831—KASP14803 | 6.86 | 10.21 | 0.09 | 5.50–9.50 | |
| KW (mm) | QKw.cib-3D | E2,E4,E7 | 3D | AX-110234451—AX-108754018 | 4.17 | 6.93 | 0.05 | 0–6.50 |
| QKw.cib-4B | E1,E2,E3,E4,E6,E7,E8,B | 4B | AX-109294476—AX-111176263 | 6.72 | 11.77 | −0.06 | 13.50–20.50 | |
| QKw.cib-4D | E1,E2,E3,E4,E5,E6,E7,E8,B | 4D | Rht2—AX-108905056 | 7.32 | 13.88 | 0.06 | 30.50–45.50 | |
| QKw.cib-6A.1 | E7 | 6A | AX-110423063—AX-110607111 | 3.91 | 6.02 | 0.04 | 26.50–32.50 | |
| QKw.cib-6A.2 | E1,E2,E3,E6,E8,B | 6A | AX-110955892—AX-110396610 | 6.06 | 9.86 | 0.05 | 48.50–50.50 | |
| KD (mm) | QKd.cib-1A | E7 | 1A | AX-110963581—AX-108804089 | 3.81 | 5.92 | −0.04 | 0–1.50 |
| QKd.cib-4A | E8 | 4A | AX-109926421—AX-111508583 | 5.12 | 8.55 | 0.05 | 75.50–77.50 | |
| QKd.cib-4B | E2 | 4B | AX-109294476—AX-111176263 | 3.86 | 5.94 | −0.04 | 13.50–20.50 | |
| QKd.cib-4D | E1,E2,E3,E4,E5,E6,E7,E8,B | 4D | Rht2—AX-108905056 | 5.27 | 11.46 | 0.06 | 29.50–44.50 | |
| QKd.cib-6A | E1,E2,E3,E4,E5,E6,E7,E8,B | 6A | AX-110955892—AX-110396610 | 6.75 | 12.39 | 0.06 | 48.50–50.50 | |
| KA (mm2) | QKa.cib-1A | E4 | 1A | AX-110963581—AX-108804089 | 4.73 | 6.78 | −0.34 | 0–2.50 |
| QKa.cib-4A | E8 | 4A | AX-109926421—AX-111508583 | 4.87 | 8.21 | 0.34 | 75.50–77.50 | |
| QKa.cib-4B | E2,E4 | 4B | AX-94790546—AX-111176263 | 4.01 | 5.84 | −0.32 | 13.50–19.50 | |
| QKa.cib-4D | E1,E2,E3,E4,E5,E6,E7,E8,B | 4D | Rht2—AX-108905056 | 5.29 | 11.62 | 0.43 | 27.50–44.50 | |
| QKa.cib-6A | E1,E2,E3,E4,E5,E6,E7,E8,B | 6A | AX-110955892—AX-110396610 | 6.28 | 11.82 | 0.41 | 48.50–50.50 | |
| KP (mm) | QKp.cib-3D | E3,E6,E8 | 3D | AX-110950126—AX-109730385 | 3.92 | 6.58 | −0.16 | 12.50–17.50 |
| QKp.cib-4D | E4,E5,E6,E7 | 4D | AX-111494342—Rht2 | 3.93 | 8.74 | 0.18 | 21.50–35.50 | |
| QKp.cib-6A | E1,E2,E3,E4,E5,E6,E7,E8,B | 6A | AX-110955892—AX-110396610 | 5.59 | 9.54 | 0.18 | 48.50–50.50 | |
| QKp.cib-6D2 | E1,E2,E3,E6,E8,B | 6D2 | AX-110261831—KASP14803 | 4.45 | 7.58 | 0.16 | 2.50–9.50 | |
| LWR | QLwr.cib-2A | E1,E7 | 2A | AX-111042596—AX-110433540 | 3.87 | 7.31 | −0.03 | 1.50–3.50 |
| QLwr.cib-3D | E1,E2,E3,E4,E5,E6,E7,E8,B | 3D | AX-110234451—AX-110950126 | 6.70 | 12.13 | −0.04 | 0–15.50 | |
| QLwr.cib-4B.1 | E1,E2,E3,E4,E5,E6,E7,E8,B | 4B | AX-111640796—AX-111176263 | 5.85 | 11.34 | 0.04 | 12.50–25.50 | |
| QLwr.cib-4B.2 | E2 | 4B | AX-108828132—AX-110103911 | 4.06 | 6.51 | 0.03 | 28.50–29.50 | |
| QLwr.cib-4D | E2,E4,B | 4D | Rht2—AX-108905056 | 5.40 | 10.29 | −0.04 | 31.50–45.50 | |
| QLwr.cib-5D | E4,E5,B | 5D | AX-89591395—AX-111019979 | 4.91 | 6.95 | −0.03 | 96.50–97.50 | |
| QLwr.cib-6A | E5 | 6A | AX-110561762—KASP13603 | 14.20 | 14.63 | 0.05 | 1.50–2.50 | |
| QLwr.cib-7A | E2 | 7A | AX-110529210—AX-110925530 | 4.12 | 7.00 | 0.03 | 94.50–99.50 | |
| KRD | QKrd.cib-2A | E4 | 2A | AX-111042596—AX-110433540 | 3.96 | 7.58 | 0.01 | 0–3.50 |
| QKrd.cib-3D | E1,E3,E4,E5,E6,E7,E8,B | 3D | AX-110234451—AX-108891293 | 7.82 | 14.77 | 0.01 | 0–15.50 | |
| QKrd.cib-4B.1 | E1,E2,E4,E7,E8 | 4B | AX-109909153—AX-110938398 | 6.62 | 12.03 | −0.01 | 13.50–23.50 | |
| QKrd.cib-4B.2 | E2,E3 | 4B | AX-94425728—AX-110103911 | 4.34 | 6.71 | −0.01 | 26.50–29.50 | |
| QKrd.cib-4D.1 | E2,E7 | 4D | Rht2—AX-108905056 | 6.63 | 11.28 | 0.01 | 31.50–43.50 | |
| QKrd.cib-4D.2 | E5 | 4D | AX-89617545—AX-108742747 | 3.91 | 5.60 | 0.01 | 70.50–77.50 | |
| QKrd.cib-5A | E6,E8,B | 5A | AX-110587222—AX-110644627 | 3.69 | 7.58 | −0.01 | 0–10.50 | |
| QKrd.cib-5D.1 | E5 | 5D | AX-111512534—AX-89633041 | 4.04 | 7.53 | −0.01 | 58.50–60.50 | |
| QKrd.cib-5D.2 | E3,E5,E6 | 5D | AX-89591395—AX-111019979 | 3.95 | 8.27 | 0.01 | 96.50–98.50 | |
| QKrd.cib-7A | E2 | 7A | AX-110529210—AX-110925530 | 4.13 | 7.14 | −0.01 | 94.50–99.50 | |
| FFD | QFfd.cib-2A | E8 | 2A | AX-110562886—AX-110919081 | 6.58 | 9.04 | 0.05 | 8.50–9.50 |
| QFfd.cib-2D | E8 | 2D | AX-109998182—AX-109330666 | 3.90 | 4.53 | −0.04 | 68.50–70.50 | |
| QFfd.cib-3B | E4 | 3B | AX-109911340—AX-111536910 | 3.79 | 8.95 | 0.03 | 75.50–77.50 | |
| QFfd.cib-4B | E1,E2,E8,B | 4B | AX-109294476—AX-111176263 | 6.57 | 10.57 | −0.05 | 15.50–22.50 | |
| QFfd.cib-4D | E1,E2,E7,B | 4D | Rht2—AX-108905056 | 5.09 | 11.28 | 0.05 | 32.50–48.50 | |
| QFfd.cib-6A | E2,B | 6A | AX-110955892—AX-110396610 | 4.30 | 6.76 | 0.04 | 48.50–50.50 | |
| QFfd.cib-7B | E8 | 7B | AX-94611818—AX-109976283 | 5.72 | 9.27 | 0.05 | 77.50–83.50 |
| QTLs | Change Percentage of Additive Effect (%) when Conditioned on KL | Change Percentage of Additive Effect (%) when Conditioned on KW | Change Percentage of Additive Effect (%) when Conditioned on KD |
|---|---|---|---|
| QKa.cib-1A | / | / | / |
| QKa.cib-4A | / | / | / |
| QKa.cib-4B | −12.54 | / | / |
| QKa.cib-4D | −22.17 | / | / |
| QKa.cib-6A | / | −57.60 | / |
| QKp.cib-3D | −131.36 | 24.2 | −45.58 |
| QKp.cib-4D | −53.4 | / | / |
| QKp.cib-6A | / | −28.08 | / |
| QKp.cib-6D2 | / | 0 | / |
| QLwr.cib-2A | / | −18.71 | 21.94 |
| QLwr.cib-3D | −19.7 | −32.74 | / |
| QLwr.cib-4B.1 | 0 | −36.34 | 0 |
| QLwr.cib-4B.2 | / | / | / |
| QLwr.cib-4D | 0 | / | / |
| QLwr.cib-5D | −12.94 | / | / |
| QLwr.cib-6A | −150.73 | −63 | / |
| QLwr.cib-7A | / | / | / |
| QKrd.cib-2A | / | 14.29 | 22.22 |
| QKrd.cib-3D | 0 | −35.44 | 0 |
| QKrd.cib-4B.1 | 0 | 73.49 | 0 |
| QKrd.cib-4B.2 | / | / | / |
| QKrd.cib-4D.1 | 0.27 | −168.22 | −14.02 |
| QKrd.cib-4D.2 | / | / | / |
| QKrd.cib-5A | / | −22.97 | 0 |
| QKrd.cib-5D.1 | / | / | / |
| QKrd.cib-5D.2 | / | / | / |
| QKrd.cib-7A | / | / | −8 |
| QFfd.cib-2A | / | −39.31 | −38.73 |
| QFfd.cib-2D | / | / | −80.72 |
| QFfd.cib-3B | 2.9 | / | / |
| QFfd.cib-4B | −4.47 | −46.6 | 48.09 |
| QFfd.cib-4D | −2.09 | / | −34.41 |
| QFfd.cib-6A | 1.54 | / | / |
| QFfd.cib-7B | / | / | / |
| Genomic Regions | Chromosomes | Markers Intervals | Intervals (cM) | Physical Location (bp) | Involved QTLs |
|---|---|---|---|---|---|
| R1A | 1A | AX-110963581—AX-108804089 | 0–2.50 | 0–14,167,187 | QKd.cib-1A (1, −), QKa.cib-1A (1, −) |
| R2D | 2D | AX-111042596—AX-110433540 | 0–3.50 | 317,84,518–358,91,228 | QLwr.cib-2A (2, −), QKrd.cib-2A (1, +) |
| R3B | 3B | AX-109911340—AX-109984220 | 77.50–81.50 | 818,890,758–820,411,093 | QKl.cib-3B (7, −), QFfd.cib-3B |
| R3D | 3D | AX-110234451—AX-109730385 | 0–17.50 | 0–452,409,067 | QKl.cib-3D (7, −), QKw.cib-3D (3, +), QKp.cib-3D (3, −), QLwr.cib-3D (9, −), QKrd.cib-3D (8, +) |
| R4A | 4A | AX-109926421—AX-111508583 | 75.50–77.50 | 684,899,178–685,743,278 | QKd.cib-4A (1, +),QKa.cib-4A (1, +) |
| R4B.1 | 4B | AX-111640796—AX-111176263 | 12.50–25.50 | 12,536,661–41,550,962 | QKw.cib-4B (8, −), QKd.cib-4B (1, −), QKa.cib-4B (2, −), QLwr.cib-4B.1 (9, +), QKrd.cib-4B.1 (5, −), QFfd.cib-4B (4, −) |
| R4B.2 | 4B | AX-94425728—AX-110103911 | 26.50–29.50 | 103,527,224–361,732,573 | QKrd.cib-4B.2 (2, −), QLwr.cib-4B.2 (1, +), |
| R4D | 4D | Rht2—AX-108905056 | 27.50–48.50 | 18,781,000–54,131,805 | QKw.cib-4D (9, +), QKd.cib-4D (9, +), QKa.cib-4D (9, +), QKp.cib-4D (4, +), QLwr.cib-4D.1 (3, −), QKrd.cib-4D.1 (2, +), QFfd.cib-4D (4, +) |
| R5D | 5D | AX-89591395—AX-111019979 | 96.50–98.50 | 290,069,021–304,497,167 | QLwr.cib-5D (3, −), QKrd.cib-5D.2 (3, +) |
| R6A | 6A | AX-110955892—AX-110396610 | 48.50–50.50 | 268,089,973–322,766,933 | QKl.cib-6A (7, +), QKw.cib-6A.2(6, +), QKd.cib-6A (9, +), QKa.cib-6A (9, +), QKp.cib-6A (9, +), QFfd.cib-6A (2, +), |
| R6D2 | 6D2 | AX-110261831—KASP14803 | 2.50–9.50 | 75,878,004–89,262,056 | QKl.cib-6D2 (9, +), QKp.cib-6D2 (6, +) |
| Traits | Genotypes | R3D | R4B.1 | R4D | R6A | R6D2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Numbers | Average | Significance | Numbers | Average | Significance | Numbers | Average | Significance | Numbers | Average | Significance | Numbers | Average | Significance | ||
| TKW (g) | A | 61 | 48.18 | 73 | 47.10 | 97 | 48.42 | *** | 89 | 48.40 | ** | 74 | 48.30 | * | ||
| B | 71 | 47.15 | 68 | 48.14 | 50 | 45.72 | 58 | 46.29 | 63 | 46.57 | ||||||
| KL (mm) | A | 61 | 6.89 | ** | 73 | 6.91 | 97 | 6.97 | * | 89 | 7.18 | * | 74 | 7.00 | ** | |
| B | 71 | 7.02 | 68 | 6.90 | 50 | 6.90 | 58 | 6.91 | 63 | 6.88 | ||||||
| KW (mm) | A | 61 | 3.44 | ** | 73 | 3.36 | ** | 97 | 3.43 | ** | 89 | 3.42 | ** | 74 | 3.41 | |
| B | 71 | 3.37 | 68 | 3.44 | 50 | 3.34 | 58 | 3.36 | 63 | 3.38 | ||||||
| KD (mm) | A | 61 | 4.89 | 73 | 4.87 | 97 | 4.91 | *** | 89 | 4.91 | ** | 74 | 4.90 | * | ||
| B | 71 | 4.88 | 68 | 4.89 | 50 | 4.82 | 58 | 4.84 | 63 | 4.84 | ||||||
| KA (mm2) | A | 61 | 19.07 | 73 | 18.97 | 97 | 19.20 | *** | 89 | 19.21 | ** | 74 | 19.18 | * | ||
| B | 71 | 19.01 | 68 | 19.08 | 50 | 18.56 | 58 | 18.69 | 63 | 18.74 | ||||||
| KP (mm) | A | 61 | 17.64 | 73 | 17.77 | 97 | 17.78 | ** | 89 | 17.79 | * | 74 | 17.81 | ** | ||
| B | 71 | 17.80 | 68 | 17.66 | 50 | 17.53 | 58 | 17.58 | 63 | 17.55 | ||||||
| LWR | A | 61 | 2.03 | *** | 73 | 2.12 | *** | 97 | 2.07 | 89 | 2.07 | 74 | 2.09 | |||
| B | 71 | 2.12 | 68 | 2.04 | 50 | 2.10 | 58 | 2.09 | 63 | 2.07 | ||||||
| KRD | A | 61 | 0.50 | *** | 73 | 0.48 | *** | 97 | 0.50 | 89 | 0.50 | 74 | 0.49 | |||
| B | 71 | 0.48 | 68 | 0.50 | 50 | 0.49 | 58 | 0.49 | 63 | 0.50 | ||||||
| FFD | A | 61 | 2.03 | ** | 73 | 1.99 | ** | 97 | 2.03 | ** | 89 | 2.03 | * | 74 | 2.02 | |
| B | 71 | 1.99 | 68 | 2.03 | 50 | 1.98 | 58 | 1.99 | 63 | 2.00 | ||||||
| Genotypes of ZKM138-Derivatives | KASP3D32 | KASP4B61 | KASP14803 | Genotypes of Global Varieties | KASP3D32 | KASP4B61 | KASP14803 | ||
|---|---|---|---|---|---|---|---|---|---|
| Numbers of genotype | A | 85 | 79 | 83 | Numbers of genotype | A | 201 | 135 | 248 |
| B | 0 | 6 | 3 | B | 104 | 93 | 72 | ||
| Percentage of genotypes (%) | A | 100.00 | 92.94 | 96.51 | Percentage of genotypes (%) | A | 65.90 | 59.21 | 77.50 |
| B | 0 | 6.06 | 3.01 | B | 34.10 | 40.79 | 22.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, X.; Liu, X.; Guo, S.; Feng, B.; Zhou, Q.; Deng, G.; Long, H.; Xu, Z.; Wang, T. Traditional and Conditional QTL Analysis of Kernel Size- and Shape-Related Traits in Wheat (Triticum aestivum L.). Agriculture 2022, 12, 1718. https://doi.org/10.3390/agriculture12101718
Fan X, Liu X, Guo S, Feng B, Zhou Q, Deng G, Long H, Xu Z, Wang T. Traditional and Conditional QTL Analysis of Kernel Size- and Shape-Related Traits in Wheat (Triticum aestivum L.). Agriculture. 2022; 12(10):1718. https://doi.org/10.3390/agriculture12101718
Chicago/Turabian StyleFan, Xiaoli, Xiaofeng Liu, Shaodan Guo, Bo Feng, Qiang Zhou, Guangbing Deng, Hai Long, Zhibin Xu, and Tao Wang. 2022. "Traditional and Conditional QTL Analysis of Kernel Size- and Shape-Related Traits in Wheat (Triticum aestivum L.)" Agriculture 12, no. 10: 1718. https://doi.org/10.3390/agriculture12101718
APA StyleFan, X., Liu, X., Guo, S., Feng, B., Zhou, Q., Deng, G., Long, H., Xu, Z., & Wang, T. (2022). Traditional and Conditional QTL Analysis of Kernel Size- and Shape-Related Traits in Wheat (Triticum aestivum L.). Agriculture, 12(10), 1718. https://doi.org/10.3390/agriculture12101718





