Impacts of Biochar-Based Controlled-Release Nitrogen Fertilizers on Soil Prokaryotic and Fungal Communities
Abstract
1. Introduction
2. Materials and Methods
2.1. Biochar-Based Controlled-Release Fertilizers Preparation
2.2. Evaluation of BCRNFs with Corn
2.3. DNA Extraction and Sequencing
2.4. Sequence Data Analysis
3. Results
3.1. Taxonomic Diversity
3.2. Prokaryotic Diversity across the Treatments
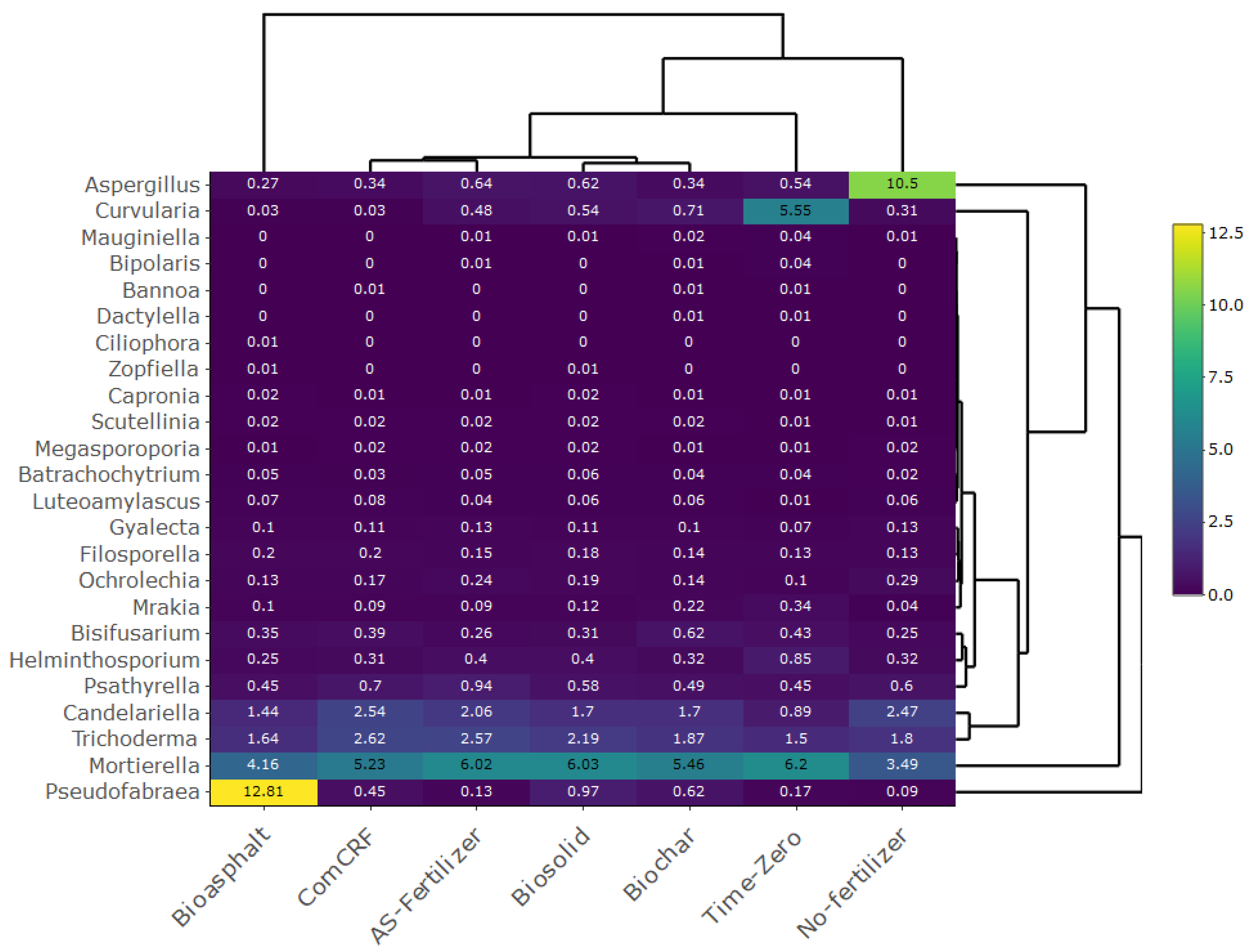
3.3. Fungal Diversities across the Treatments
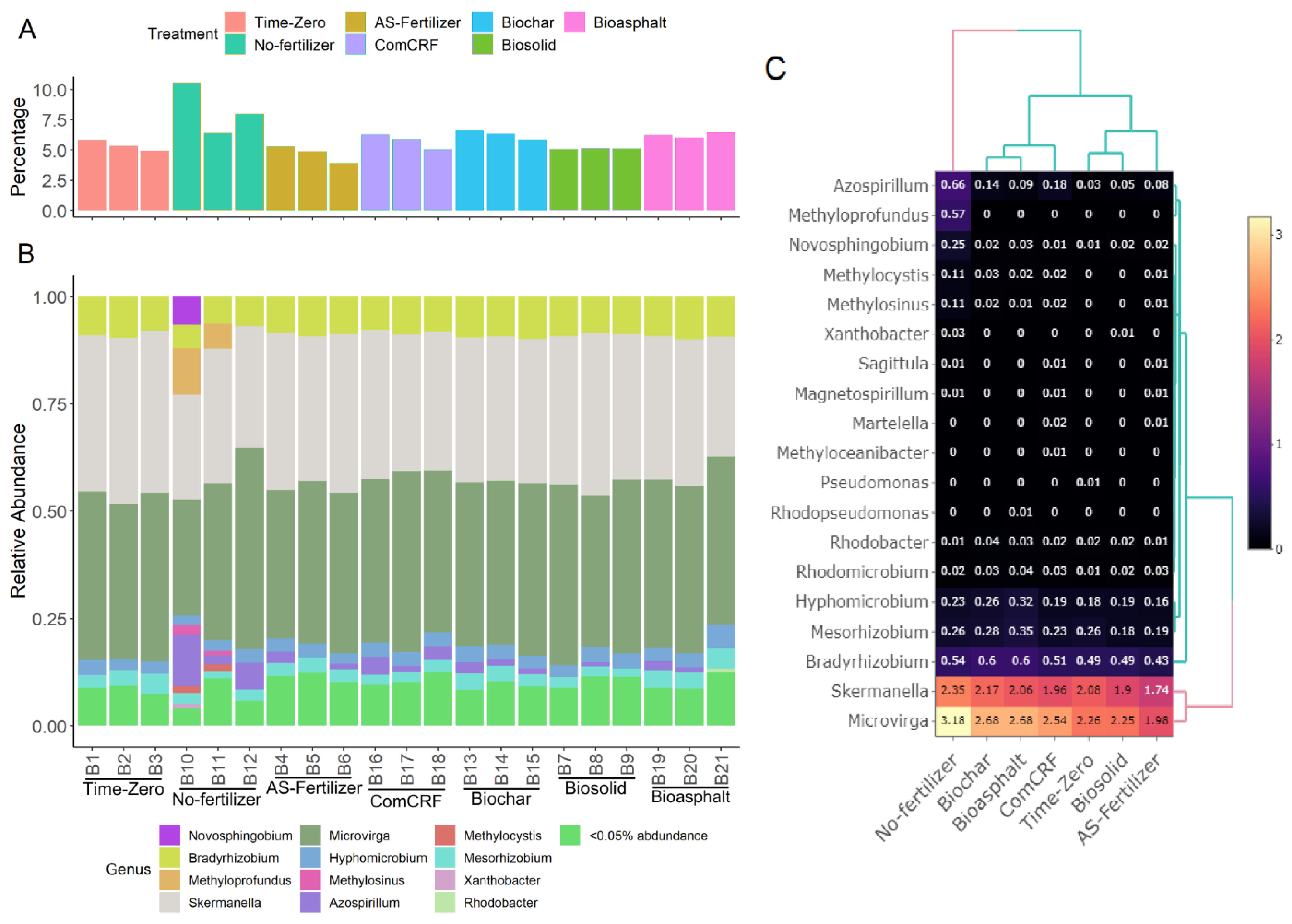
3.4. The N-Fixing Bacterial Diversity
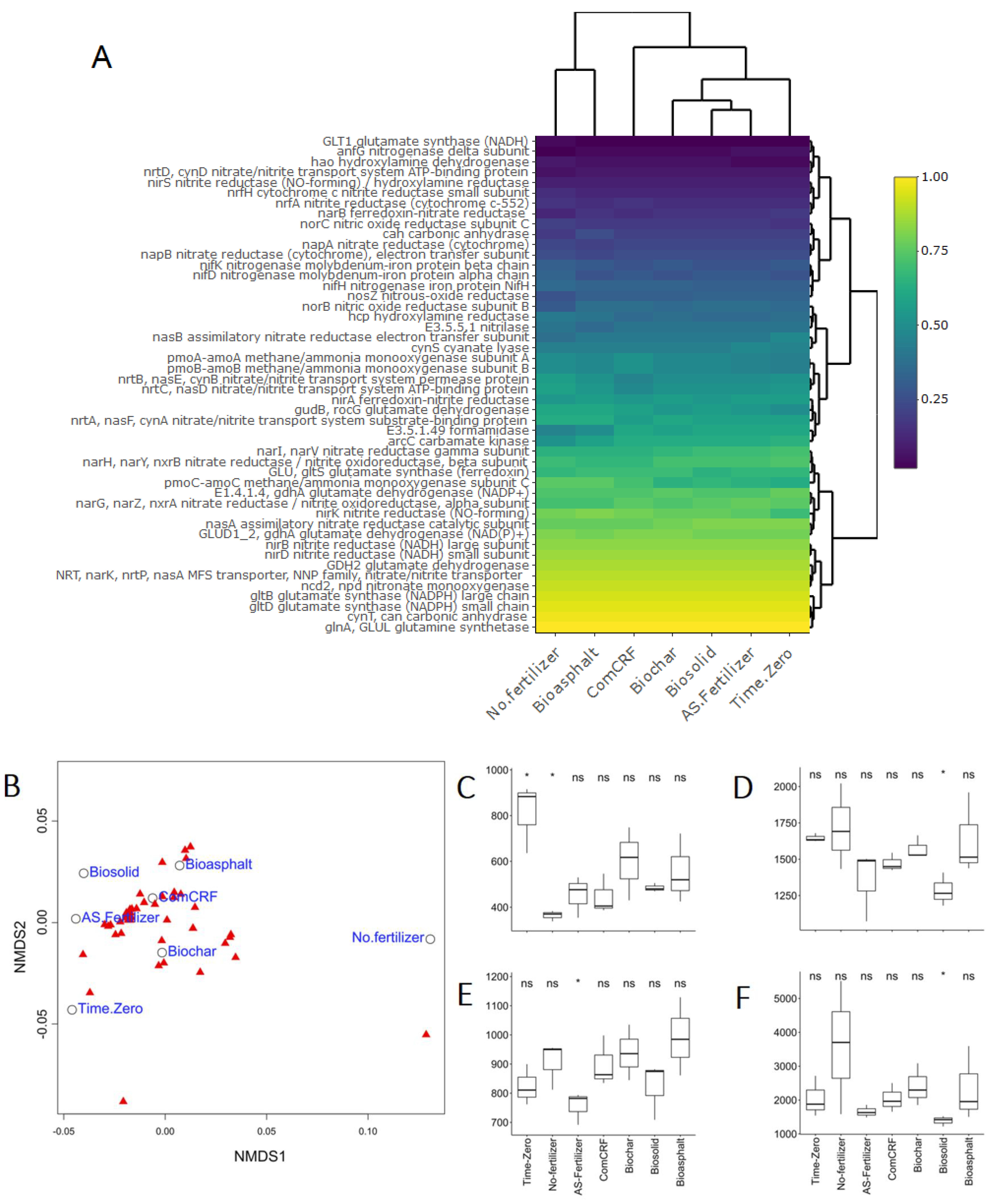
3.5. The Functional Potential of Soil Bacteria in Each Treatment
4. Discussion
4.1. BCRNFs Do Not Cause Major Shifts in the Soil Prokaryotic Communities
4.2. Fungal Diversity Remained Unchanged across the BCRNFs
4.3. N-Fixing Diversity and N-Cycle Functional Potential Were Different in Soil without Fertilizer
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, Y.; Wang, T.; Wang, C.; Anane, P.-S.; Liu, S.; Paz-Ferreiro, J. Nitrogen fertilizer is a key factor affecting the soil chemical and microbial communities in a Mollisol. Can. J. Microbiol. 2019, 65, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, W.; Wang, X.; Wang, F.; Dong, W.; Hu, C.; Liu, B.; Sun, R. Nitrogen leaching greatly impacts bacterial community and denitrifiers abundance in subsoil under long-term fertilization. Agric. Ecosyst. Environ. 2020, 294, 106885. [Google Scholar] [CrossRef]
- Luo, J.; Tillman, R.; Ball, P. Nitrogen loss through denitrification in a soil under pasture in New Zealand. Soil Biol. Biochem. 2000, 32, 497–509. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, H.; Xiao, H.; Shi, W.; Müller, K.; Van Zwieten, L.; Wang, H. Wheat straw biochar application increases ammonia volatilization from an urban compacted soil giving a short-term reduction in fertilizer nitrogen use efficiency. J. Soils Sediments 2019, 19, 1624–1631. [Google Scholar] [CrossRef]
- Yadav, S.N.; Peterson, W.; Easter, K.W. Do farmers overuse nitrogen fertilizer to the detriment of the environment? Environ. Resour. Econ. 1997, 9, 323–340. [Google Scholar] [CrossRef]
- Shaviv, A.; Mikkelsen, R. Controlled-release fertilizers to increase efficiency of nutrient use and minimize environmental degradation-A review. Fertil. Res. 1993, 35, 1–12. [Google Scholar] [CrossRef]
- Liao, J.; Liu, X.; Hu, A.; Song, H.; Chen, X.; Zhang, Z. Effects of biochar-based controlled release nitrogen fertilizer on nitrogen-use efficiency of oilseed rape (Brassica napus L.). Sci. Rep. 2020, 10, 11063. [Google Scholar] [CrossRef]
- Mihok, F.; Macko, J.; Oriňak, A.; Oriňaková, R.; Kovaľ, K.; Sisáková, K.; Petruš, O.; Kostecká, Z. Controlled nitrogen release fertilizer based on zeolite clinoptilolite: Study of preparation process and release properties using molecular dynamics. Curr. Res. Green Sustain. Chem. 2020, 3, 100030. [Google Scholar] [CrossRef]
- Dubey, A.; Mailapalli, D.R. Zeolite coated urea fertilizer using different binders: Fabrication, material properties and nitrogen release studies. Environ. Technol. Innov. 2019, 16, 100452. [Google Scholar] [CrossRef]
- Beig, B.; Niazi, M.B.K.; Jahan, Z.; Kakar, S.J.; Shah, G.A.; Shahid, M.; Zia, M.; Haq, M.U.; Rashid, M.I. Biodegradable Polymer Coated Granular Urea Slows Down N Release Kinetics and Improves Spinach Productivity. Polymers 2020, 12, 2623. [Google Scholar] [CrossRef]
- Chen, L.; Xie, Z.; Zhuang, X.; Chen, X.; Jing, X. Controlled release of urea encapsulated by starch-g-poly(l-lactide). Carbohydr. Polym. 2008, 72, 342–348. [Google Scholar] [CrossRef]
- Li, Y.; Jia, C.; Zhang, X.; Jiang, Y.; Zhang, M.; Lu, P.; Chen, H. Synthesis and performance of bio-based epoxy coated urea as controlled release fertilizer. Prog. Org. Coat. 2018, 119, 50–56. [Google Scholar] [CrossRef]
- Rubel, R.I.; Wei, L. Biochar-Based Controlled Release Nitrogen Fertilizer Coated with Polylactic Acid. J. Polym. Environ. 2022, 30, 4406–4417. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K. Biochemical cycling of nitrogen and phosphorus in biochar-amended soils. Soil Biol. Biochem. 2016, 103, 1–15. [Google Scholar] [CrossRef]
- Melo, L.C.A.; Lehmann, J.; Carneiro, J.S.d.S.; Camps-Arbestain, M. Biochar-based fertilizer effects on crop productivity: A meta-analysis. Plant and Soil 2022, 472, 45–58. [Google Scholar] [CrossRef]
- Cao, X.; Ma, L.; Gao, B.; Harris, W. Dairy-manure derived biochar effectively sorbs lead and atrazine. Environ Sci Technol 2009, 43, 3285–3291. [Google Scholar] [CrossRef]
- Cen, Z.; Wei, L.; Muthukumarappan, K.; Sobhan, A.; McDaniel, R. Assessment of a Biochar-Based Controlled Release Nitrogen Fertilizer Coated with Polylactic Acid. J. Soil Sci. Plant Nutr. 2021, 21, 2007–2019. [Google Scholar] [CrossRef]
- Rubel, R.I.; Wei, L. Improve biochar-based controlled release fertilizer’s performance by coating multiple layers of polylactic acid. In Proceedings of the 2021 ASABE Annual International Virtual Meeting, St. Joseph, MI, USA, 12–16 July 2021; p. 1. [Google Scholar]
- Dutta, S.; Pal, S.; Panwar, P.; Sharma, R.K.; Bhutia, P.L. Biopolymeric Nanocarriers for Nutrient Delivery and Crop Biofortification. ACS Omega 2022, 7, 25909–25920. [Google Scholar] [CrossRef]
- Rubel, R.I. Developed Bio-Based Controlled-Release Nitrogen Fertilizer to Improve Corn Yield via Greenhouse Trials. Master’s Thesis, South Dakota State University, Brookings, SD, USA, 2022. [Google Scholar]
- Yan, T.; Xue, J.; Zhou, Z.; Wu, Y. Biochar-based fertilizer amendments improve the soil microbial community structure in a karst mountainous area. Sci. Total Environ. 2021, 794, 148757. [Google Scholar] [CrossRef]
- Gao, M.; Yang, J.; Liu, C.; Gu, B.; Han, M.; Li, J.; Li, N.; Liu, N.; An, N.; Dai, J. Effects of long-term biochar and biochar-based fertilizer application on brown earth soil bacterial communities. Agric. Ecosyst. Environ. 2021, 309, 107285. [Google Scholar] [CrossRef]
- Anderson, C.R.; Condron, L.M.; Clough, T.J.; Fiers, M.; Stewart, A.; Hill, R.A.; Sherlock, R.R. Biochar induced soil microbial community change: Implications for biogeochemical cycling of carbon, nitrogen and phosphorus. Pedobiologia 2011, 54, 309–320. [Google Scholar] [CrossRef]
- O’neill, B.; Grossman, J.; Tsai, M.; Gomes, J.; Lehmann, J.; Peterson, J.; Neves, E.; Thies, J.E. Bacterial community composition in Brazilian Anthrosols and adjacent soils characterized using culturing and molecular identification. Microb. Ecol. 2009, 58, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Lévesque, V.; Rochette, P.; Hogue, R.; Jeanne, T.; Ziadi, N.; Chantigny, M.H.; Dorais, M.; Antoun, H. Greenhouse gas emissions and soil bacterial community as affected by biochar amendments after periodic mineral fertilizer applications. Biol. Fertil. Soils 2020, 56, 907–925. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.; Malla, M.A.; Khan, F.; Chowdhary, K.; Yadav, S.; Kumar, A.; Sharma, S.; Khare, P.K.; Khan, M.L. Soil microbiome: A key player for conservation of soil health under changing climate. Biodivers. Conserv. 2019, 28, 2405–2429. [Google Scholar] [CrossRef]
- Jing, J.; Cong, W.-F.; Bezemer, T.M. Legacies at work: Plant–soil–microbiome interactions underpinning agricultural sustainability. Trends Plant Sci. 2022, 27, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Harter, J.; Krause, H.M.; Schuettler, S.; Ruser, R.; Fromme, M.; Scholten, T.; Kappler, A.; Behrens, S. Linking N2O emissions from biochar-amended soil to the structure and function of the N-cycling microbial community. ISME J. 2014, 8, 660–674. [Google Scholar] [CrossRef]
- Zhou, Z.; Gao, T.; Van Zwieten, L.; Zhu, Q.; Yan, T.; Xue, J.; Wu, Y. Soil Microbial Community Structure Shifts Induced by Biochar and Biochar-Based Fertilizer Amendment to Karst Calcareous Soil. Soil Sci. Soc. Am. J. 2019, 83, 398–408. [Google Scholar] [CrossRef]
- Hermans, S.M.; Buckley, H.L.; Case, B.S.; Curran-Cournane, F.; Taylor, M.; Lear, G. Using soil bacterial communities to predict physico-chemical variables and soil quality. Microbiome 2020, 8, 79. [Google Scholar] [CrossRef]
- Moore, R.E.; Young, M.K.; Lee, T.D. Qscore: An algorithm for evaluating SEQUEST database search results. J. Am. Soc. Mass Spectrom. 2002, 13, 378–386. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Robeson, M.S., II; O’Rourke, D.R.; Kaehler, B.D.; Ziemski, M.; Dillon, M.R.; Foster, J.T.; Bokulich, N.A. RESCRIPt: Reproducible sequence taxonomy reference database management. PLoS Comput. Biol. 2021, 17, e1009581. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- McKinney, W. Data structures for statistical computing in python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010; pp. 51–56. [Google Scholar]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahe, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, R.H.; Larsson, K.H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res 2019, 47, D259–D264. [Google Scholar] [CrossRef]
- Abarenkov, K.; Zirk, A.; Piirmann, T.; Pöhönen, R.; Ivanov, F.; Nilsson, R.; Kõljalg, U. UNITE QIIME release for Fungi 2. Version 10.05. 2021. UNITE Community 2021, 7, 1264763. [Google Scholar]
- McDonald, D.; Clemente, J.C.; Kuczynski, J.; Rideout, J.R.; Stombaugh, J.; Wendel, D.; Wilke, A.; Huse, S.; Hufnagle, J.; Meyer, F.; et al. The Biological Observation Matrix (BIOM) format or: How I learned to stop worrying and love the ome-ome. GigaScience 2012, 1. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2–Approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Xu, Z.Z.; Peddada, S.; Amir, A.; Bittinger, K.; Gonzalez, A.; Lozupone, C.; Zaneveld, J.R.; Vázquez-Baeza, Y.; Birmingham, A. Normalization and microbial differential abundance strategies depend upon data characteristics. Microbiome 2017, 5, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Team, R.C. R: A language and environment for statistical computing. R Found. Stat. Comput. 2021. [Google Scholar]
- Team, R. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020. [Google Scholar]
- Wickham, H.; François, R.; Henry, L.; Müller, K. dplyr: A Grammar of Data Manipulation; R Package Version 1.0.8; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Wickham, H.; Henry, L. Tidyr: Tidy Messy Data; R Package Version 1.1.2; R Foundation for Statistical Computing: Vienna, Austria, 2020; Volume 1, p. 397. [Google Scholar]
- Wickham, H. Reshaping Data with the reshape Package. J. Stat. Softw. 2007, 21, 1–20. [Google Scholar] [CrossRef]
- Koirala, A.; Brözel, V.S. Phylogeny of Nitrogenase Structural and Assembly Components Reveals New Insights into the Origin and Distribution of Nitrogen Fixation across Bacteria and Archaea. Microorganisms 2021, 9, 1662. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. Elegant Graphics for Data Analysis. In ggplot2; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Kassambara, A. ggpubr: ‘ggplot2’ Based Publication Ready Plots; R Package Version 0.4.0; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Erich, N. RColorBrewer: ColorBrewer Palettes; R Package Version 1.1-2; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Garnier, S.; Ross, N.; Rudis, R.; Camargo, P.A.; Sciaini, M.; Scherer, C. Viridis—Colorblind-Friendly Color Maps for R; R Package Version 0.6; R Foundation for Statistical Computing: Vienna, Austria, 2021; Volume 2. [Google Scholar] [CrossRef]
- Sievert, C. Interactive Web-Based Data Visualization with R, Plotly, and Shiny; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Galili, T.; O’Callaghan, A.; Sidi, J.; Sievert, C. heatmaply: An R package for creating interactive cluster heatmaps for online publishing. Bioinformatics 2018, 34, 1600–1602. [Google Scholar] [CrossRef]
- Kõljalg, U.; Nilsson, H.R.; Schigel, D.; Tedersoo, L.; Larsson, K.-H.; May, T.W.; Taylor, A.F.S.; Jeppesen, T.S.; Frøslev, T.G.; Lindahl, B.D.; et al. The Taxon Hypothesis Paradigm—On the Unambiguous Detection and Communication of Taxa. Microorganisms 2020, 8, 1910. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Oliverio, A.M.; Brewer, T.E.; Benavent-González, A.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K.; Fierer, N. A global atlas of the dominant bacteria found in soil. Science 2018, 359, 320–325. [Google Scholar] [CrossRef]
- Bai, L.; Zhang, X.; Li, B.; Sun, F.; Zhao, X.; Wang, Y.; Lu, Z.; Zhang, D.; Fang, J. Fungal communities are more sensitive to nitrogen fertilization than bacteria in different spatial structures of silage maize under short-term nitrogen fertilization. Appl. Soil Ecol. 2022, 170, 104275. [Google Scholar] [CrossRef]
- Semenov, M.V.; Krasnov, G.S.; Semenov, V.M.; van Bruggen, A.H.C. Long-term fertilization rather than plant species shapes rhizosphere and bulk soil prokaryotic communities in agroecosystems. Appl. Soil Ecol. 2020, 154, 103641. [Google Scholar] [CrossRef]
- Ramirez, K.S.; Craine, J.M.; Fierer, N. Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Glob. Change Biol. 2012, 18, 1918–1927. [Google Scholar] [CrossRef]
- Brennan, E.B.; Acosta-Martinez, V. Cover cropping frequency is the main driver of soil microbial changes during six years of organic vegetable production. Soil Biol. Biochem. 2017, 109, 188–204. [Google Scholar] [CrossRef]
- Du, T.-Y.; He, H.-Y.; Zhang, Q.; Lu, L.; Mao, W.-J.; Zhai, M.-Z. Positive effects of organic fertilizers and biofertilizers on soil microbial community composition and walnut yield. Appl. Soil Ecol. 2022, 175, 104457. [Google Scholar] [CrossRef]
- Wang, L.; Huang, D. Soil ammonia-oxidizing archaea in a paddy field with different irrigation and fertilization managements. Sci. Rep. 2021, 11, 14563. [Google Scholar] [CrossRef]
- Muneer, M.A.; Hou, W.; Li, J.; Huang, X.; Ur Rehman Kayani, M.; Cai, Y.; Yang, W.; Wu, L.; Ji, B.; Zheng, C. Soil pH: A key edaphic factor regulating distribution and functions of bacterial community along vertical soil profiles in red soil of pomelo orchard. BMC Microbiol. 2022, 22, 38. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, A.; Yu, Y.; Bi, C.; Zhou, C. Integrated transcriptomic and secretomic approaches reveal critical pathogenicity factors in Pseudofabraea citricarpa inciting citrus target spot. Microb. Biotechnol. 2019, 12, 1260–1273. [Google Scholar] [CrossRef]
- Gomes, N.C.; Fagbola, O.; Costa, R.; Rumjanek, N.G.; Buchner, A.; Mendona-Hagler, L.; Smalla, K. Dynamics of fungal communities in bulk and maize rhizosphere soil in the tropics. Appl. Environ. Microbiol. 2003, 69, 3758–3766. [Google Scholar] [CrossRef]
- Brimecombe, M.J.; De Leij, F.A.; Lynch, J.M. The effect of root exudates on rhizosphere microbial populations. In The Rhizosphere, Biochemistry and Organic Substances at the Soil-Plant Interface; CRC Press: Boca Raton, FL, USA, 2000; pp. 95–140. [Google Scholar]
- Wu, X.; Wang, R.; Hu, H.; Xiu, W.M.; Li, G.; Zhao, J.N.; Yang, D.L.; Wang, L.L.; Wang, X.Y. Response of Bacterial and Fungal Communities to Chemical Fertilizer Reduction Combined with Organic Fertilizer and Straw in Fluvo-aquic Soil. Huan Jing Ke Xue 2020, 41, 4669–4681. [Google Scholar] [CrossRef]
- Egidi, E.; Delgado-Baquerizo, M.; Plett, J.M.; Wang, J.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K. A few Ascomycota taxa dominate soil fungal communities worldwide. Nat. Commun. 2019, 10, 2369. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Zhuang, X.; Wu, J.; Cui, M.; Lv, D.; Liu, C.; Zhuang, G. Ascomycota members dominate fungal communities during straw residue decomposition in arable soil. PLoS ONE 2013, 8, e66146. [Google Scholar] [CrossRef] [PubMed]
- Challacombe, J.F.; Hesse, C.N.; Bramer, L.M.; McCue, L.A.; Lipton, M.; Purvine, S.; Nicora, C.; Gallegos-Graves, V.; Porras-Alfaro, A.; Kuske, C.R. Genomes and secretomes of Ascomycota fungi reveal diverse functions in plant biomass decomposition and pathogenesis. BMC Genomics 2019, 20, 976. [Google Scholar] [CrossRef] [PubMed]
- Ardley, J.K.; Parker, M.A.; De Meyer, S.E.; Trengove, R.D.; O’Hara, G.W.; Reeve, W.G.; Yates, R.J.; Dilworth, M.J.; Willems, A.; Howieson, J.G. Microvirga lupini sp. nov., Microvirga lotononidis sp. nov. and Microvirga zambiensis sp. nov. are alphaproteobacterial root-nodule bacteria that specifically nodulate and fix nitrogen with geographically and taxonomically separate legume hosts. Int. J. Syst. Evol. Microbiol. 2012, 62, 2579–2588. [Google Scholar] [CrossRef]
- Normand, P.; Orso, S.; Cournoyer, B.; Jeannin, P.; Chapelon, C.; Dawson, J.; Evtushenko, L.; Misra, A.K. Molecular phylogeny of the genus Frankia and related genera and emendation of the family Frankiaceae. Int. J. Syst. Bacteriol. 1996, 46, 1–9. [Google Scholar] [CrossRef]
- Fuentes-Ramirez, L.E.; Bustillos-Cristales, R.; Tapia-Hernandez, A.; Jimenez-Salgado, T.; Wang, E.T.; Martinez-Romero, E.; Caballero-Mellado, J. Novel nitrogen-fixing acetic acid bacteria, Gluconacetobacter johannae sp. nov. and Gluconacetobacter azotocaptans sp. nov., associated with coffee plants. Int. J. Syst. Evol. Microbiol. 2001, 51, 1305–1314. [Google Scholar] [CrossRef]
- Poulsen, P.H.B.; Al-Soud, W.A.; Bergmark, L.; Magid, J.; Hansen, L.H.; Sørensen, S.J. Effects of fertilization with urban and agricultural organic wastes in a field trial–Prokaryotic diversity investigated by pyrosequencing. Soil Biol. Biochem. 2013, 57, 784–793. [Google Scholar] [CrossRef]
- Geisseler, D.; Doane, T.A.; Horwath, W.R. Determining potential glutamine synthetase and glutamate dehydrogenase activity in soil. Soil Biol. Biochem. 2009, 41, 1741–1749. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, B.K.; Rubel, R.I.; Gupta, S.; Wu, Y.; Wei, L.; Brözel, V.S. Impacts of Biochar-Based Controlled-Release Nitrogen Fertilizers on Soil Prokaryotic and Fungal Communities. Agriculture 2022, 12, 1706. https://doi.org/10.3390/agriculture12101706
Das BK, Rubel RI, Gupta S, Wu Y, Wei L, Brözel VS. Impacts of Biochar-Based Controlled-Release Nitrogen Fertilizers on Soil Prokaryotic and Fungal Communities. Agriculture. 2022; 12(10):1706. https://doi.org/10.3390/agriculture12101706
Chicago/Turabian StyleDas, Bikram K., Robiul Islam Rubel, Surbhi Gupta, Yajun Wu, Lin Wei, and Volker S. Brözel. 2022. "Impacts of Biochar-Based Controlled-Release Nitrogen Fertilizers on Soil Prokaryotic and Fungal Communities" Agriculture 12, no. 10: 1706. https://doi.org/10.3390/agriculture12101706
APA StyleDas, B. K., Rubel, R. I., Gupta, S., Wu, Y., Wei, L., & Brözel, V. S. (2022). Impacts of Biochar-Based Controlled-Release Nitrogen Fertilizers on Soil Prokaryotic and Fungal Communities. Agriculture, 12(10), 1706. https://doi.org/10.3390/agriculture12101706







