The Role of Methionine Supplementation on Oxidative Stress and Antioxidant Status of Poultry-A Review
Abstract
1. Introduction
1.1. Methionine Supplementation of Poultry
1.2. The Antioxidant System of Poultry
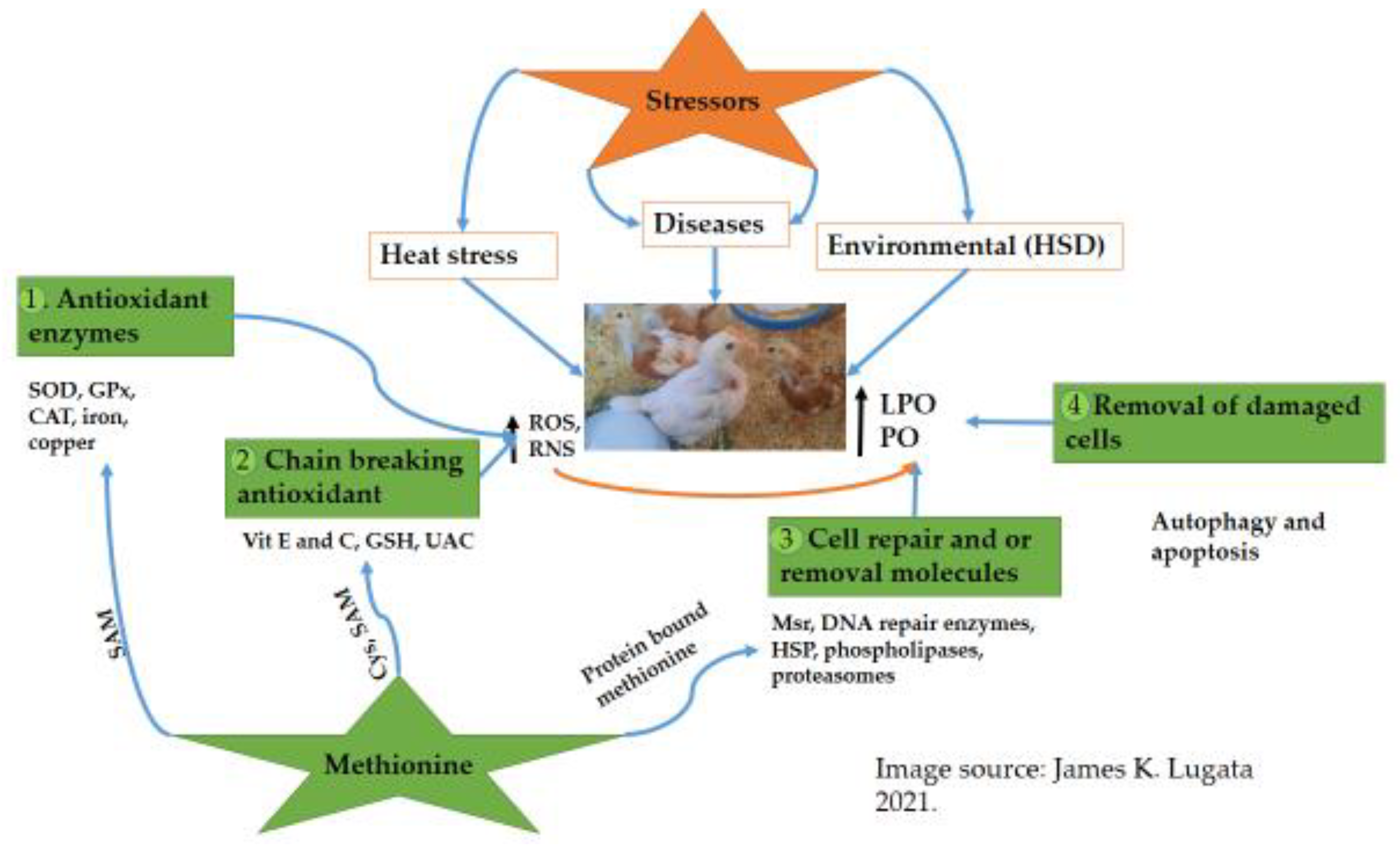
1.3. How Antioxidants Network Work under Oxidative Stress
2. Role of Methionine on Oxidative Stress/Status of Poultry under Heat Stress
3. Effect of Met on Poultry Oxidative Stress Underlying Health Conditions
4. Influence of Met Sources and Levels on the Antioxidants Defense System in Poultry (MDA SOD, CAT, GPx, and GSH)
4.1. Effects of Met Sources on Oxidative Stress/Antioxidants of poultry
4.2. Effect of Dietary Met on Antioxidants/Oxidative Stress of Poultry at Different Supplementation Levels
4.3. Superoxide Dismutase (SOD)
4.4. Catalase CAT
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Havenstein, G.B.; Ferket, P.R.; Qureshi, M.A. Growth, Livability, and Feed Conversion of 1957 versus 2001 Broilers When Fed Representative 1957 and 2001 Broiler Diets. Poult. Sci. 2003, 82, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Djumadil, N.; Syafie, Y. Analyses of Factors Affecting the Production of Broiler Chickens in Ternate. In Proceedings of the 5th International Conference on Food, Agriculture and Natural Resources (FANRes 2019), Ternate, Indonesia, 17–18 September 2019; Atlantis Press: Dordrecht, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Baracho, M.S.; Nääs, I.A.; Lima, N.D.S.; Cordeiro, A.F.S.; Moura, D.J. Factors Affecting Broiler Production: A Meta-Analysis. Braz. J. Poult. Sci. 2019, 21, 001–010. [Google Scholar] [CrossRef]
- Zhao, P.Y.; Kim, I.H. Effect of Diets with Different Energy and Lysophospholipids Levels on Performance, Nutrient Metabolism, and Body Composition in Broilers. Poult. Sci. 2017, 96, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Das, T.K.; Mondal, M.K.; Biswas, P.; Bairagi, B.; Samanta, C.C. Influence of Level of Dietary Inorganic and Organic Copper and Energy Level on the Performance and Nutrient Utilization of Broiler Chickens. Asian-Australas. J. Anim. Sci. 2009, 23, 82–89. [Google Scholar] [CrossRef]
- Maharjan, P.; Mullenix, G.; Hilton, K.; Caldas, J.; Beitia, A.; Weil, J.; Suesuttajit, N.; Kalinowski, A.; Yacoubi, N.; Naranjo, V.; et al. Effect of Digestible Amino Acids to Energy Ratios on Performance and Yield of Two Broiler Lines Housed in Different Grow-out Environmental Temperatures. Poult. Sci. 2020, 99, 6884–6898. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Magnuson, A.D.; Sun, T.; Tolba, S.A.; Starkey, C.; Whelan, R.; Lei, X.G. Supplemental Methionine Exerted Chemical Form-Dependent Effects on Antioxidant Status, Inflammation-Related Gene Expression, and Fatty Acid Profiles of Broiler Chicks Raised at High Ambient Temperature. J. Anim. Sci. 2019, 97, 4883–4894. [Google Scholar] [CrossRef] [PubMed]
- Magnuson, A.D.; Liu, G.; Sun, T.; Tolba, S.A.; Xi, L.; Whelan, R.; Lei, X.G. Supplemental Methionine and Stocking Density Affect Antioxidant Status, Fatty Acid Profiles, and Growth Performance of Broiler Chickens. J. Anim. Sci. 2020, 98, skaa092. [Google Scholar] [CrossRef] [PubMed]
- Lara, L.J.; Rostagno, M.H. Impact of Heat Stress on Poultry Production. Animals 2013, 3, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Selye, H. Forty Years of Stress Research: Principal Remaining Problems and Misconceptions. Can. Med. Assoc. J. 1976, 115, 53–56. [Google Scholar] [PubMed]
- Yunianto, V.D.; Hayashi, K.; Kaneda, S.; Ohtsuka, A.; Tomita, Y. Effect of Environmental Temperature on Muscle Protein Turnover and Heat Production in Tube-Fed Broiler Chickens. Br. J. Nutr. 1997, 77, 897–909. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Fry, J.L.; Stadelman, W.J. The Effect of Dietary Methionine on the Methionine and Cystine Content of Poultry Meat. Poult. Sci. 1960, 39, 614–617. [Google Scholar] [CrossRef]
- Graber, G.; Scott, H.M.; Baker, D.H. Sulfur Amino Acid Nutrition of the Growing Chick: Effect of Age on the Dietary Methionine Requirement. Poult. Sci. 1971, 50, 854–858. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y. Amino Acid Nutrition and Chicken Gut Health. Worlds Poult. Sci. J. 2020, 76, 563–576. [Google Scholar] [CrossRef]
- Stipanuk, M.H. Metabolism of Sulfur-Containing Amino Acids: How the Body Copes with Excess Methionine, Cysteine, and Sulfide. J. Nutr. 2020, 150, 2494S–2505S. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Saremi, B.; Gilbert, E.R.; Wong, E.A. Physiological and Biochemical Aspects of Methionine Isomers and a Methionine Analogue in Broilers. Poult. Sci. 2017, 96, 425–439. [Google Scholar] [CrossRef]
- Ren, Z.; Bütz, D.E.; Whelan, R.; Naranjo, V.; Arendt, M.K.; Ramuta, M.D.; Yang, X.; Crenshaw, T.D.; Cook, M.E. Effects of Dietary Methionine plus Cysteine Levels on Growth Performance and Intestinal Antibody Production in Broilers during Eimeria Challenge. Poult. Sci. 2020, 99, 374–384. [Google Scholar] [CrossRef]
- Sigolo, S.; Deldar, E.; Seidavi, A.; Bouyeh, M.; Gallo, A.; Prandini, A. Effects of Dietary Surpluses of Methionine and Lysine on Growth Performance, Blood Serum Parameters, Immune Responses, and Carcass Traits of Broilers. J. Appl. Anim. Res. 2019, 47, 146–153. [Google Scholar] [CrossRef]
- Zhang, S.; Gilbert, E.R.; Saremi, B.; Wong, E.A. Supplemental Methionine Sources Have a Neutral Impact on Oxidative Status in Broiler Chickens. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1274–1283. [Google Scholar] [CrossRef]
- Akter, N.; Islam, M.; Zaman, S.; Jahan, I.; Hossain, M. The Impact of Different Levels of L-Methionine (L-Met) on Carcass Yield Traits, Serum Metabolites, Tibial Characters, and Profitability of Broilers Fed Conventional Diet. J. Adv. Vet. Anim. Res. 2020, 7, 253. [Google Scholar] [CrossRef]
- Esteve-Garcia, E.; Khan, D.R. Relative Bioavailability of DL and L-Methionine in Broilers. Open J. Anim. Sci. 2018, 8, 151–162. [Google Scholar] [CrossRef]
- Jankowski, J.; Ognik, K.; Kubińska, M.; Czech, A.; Juśkiewicz, J.; Zduńczyk, Z. The Effect of DL-, L-Isomers and DL-Hydroxy Analog Administered at 2 Levels as Dietary Sources of Methionine on the Metabolic and Antioxidant Parameters and Growth Performance of Turkeys. Poult. Sci. 2017, 96, 3229–3238. [Google Scholar] [CrossRef] [PubMed]
- Murawska, D.; Kubińska, M.; Gesek, M.; Zduńczyk, Z.; Brzostowska, U.; Jankowski, J. The Effect of Different Dietary Levels and Sources of Methionine on the Growth Performance of Turkeys, Carcass and Meat Quality. Ann. Anim. Sci. 2018, 18, 525–540. [Google Scholar] [CrossRef]
- Park, I.; Pasquetti, T.; Malheiros, R.D.; Ferket, P.R.; Kim, S.W. Effects of Supplemental L-Methionine on Growth Performance and Redox Status of Turkey Poults Compared with the Use of DL-Methionine. Poult. Sci. 2018, 97, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Abouelezz, K.F.M.; Cheng, Z.; Gad-Elkareem, A.E.G.; Fan, Q.; Ding, F.; Gao, J.; Jiang, S.; Jiang, Z. Modelling Methionine Requirements of Fast- and Slow-Growing Chinese Yellow-Feathered Chickens during the Starter Phase. Animals 2020, 10, 443. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (U.S.). Nutrient Requirements of Poultry. In Nutrient Requirements of Domestic Animals, 9th ed.; National Academy Press: Washington, DC, USA, 1994; ISBN 978-0-309-04892-7. [Google Scholar]
- Rehman, A.U.; Arif, M.; Husnain, M.M.; Alagawany, M.; Abd El-Hack, M.E.; Taha, A.E.; Elnesr, S.S.; Abdel-Latif, M.A.; Othman, S.I.; Allam, A.A. Growth Performance of Broilers as Influenced by Different Levels and Sources of Methionine Plus Cysteine. Animals 2019, 9, 1056. [Google Scholar] [CrossRef]
- Tian, Q.Y.; Zeng, Z.K.; Zhang, Y.X.; Long, S.F.; Piao, X.S. Effect of L- or DL-Methionine Supplementation on Nitrogen Retention, Serum Amino Acid Concentrations and Blood Metabolites Profile in Starter Pigs. Asian-Australas. J. Anim. Sci. 2016, 29, 689–694. [Google Scholar] [CrossRef]
- Ullrich, C.; Langeheine, M.; Brehm, R.; Taube, V.; Rosillo Galera, M.; Rohn, K.; Popp, J.; Visscher, C. Influence of Different Methionine Sources on Performance and Slaughter Characteristics of Broilers. Animals 2019, 9, 984. [Google Scholar] [CrossRef]
- Agostini, P.S.; Dalibard, P.; Mercier, Y.; Van der Aar, P.; Van der Klis, J.D. Comparison of Methionine Sources around Requirement Levels Using a Methionine Efficacy Method in 0 to 28 Day Old Broilers. Poult. Sci. 2016, 95, 560–569. [Google Scholar] [CrossRef]
- Fouad, A.M.; Ruan, D.; Lin, Y.C.; Zheng, C.T.; Zhang, H.X.; Chen, W.; Wang, S.; Xia, W.G.; Li, Y. Effects of Dietary Methionine on Performance, Egg Quality and Glutathione Redox System in Egg-Laying Ducks. Br. Poult. Sci. 2016, 57, 818–823. [Google Scholar] [CrossRef]
- Zduńczyk, Z.; Jankowski, J.; Kubińska, M.; Ognik, K.; Czech, A.; Juśkiewicz, J. The Effect of Different Dietary Levels of Dl-Methionine and Dl-Methionine Hydroxy Analogue on the Antioxidant and Immune Status of Young Turkeys. Arch. Anim. Nutr. 2017, 71, 347–361. [Google Scholar] [CrossRef]
- Murillo, M.G.; Jensen, L.S.; Ruff, M.D.; Rahn, A.P. Effect of Dietary Methionine Status on Response of Chicks to Coccidial Infection. Poult. Sci. 1976, 55, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Meirelles, H.T.; Albuquerque, R.; Borgatti, L.M.O.; Souza, L.W.O.; Meister, N.C.; Lima, F.R. Performance of Broilers Fed with Different Levels of Methionine Hydroxy Analogue and DL-Methionine. Braz. J. Poult. Sci. 2003, 5, 69–74. [Google Scholar] [CrossRef]
- Swennen, Q.; Geraert, P.-A.; Mercier, Y.; Everaert, N.; Stinckens, A.; Willemsen, H.; Li, Y.; Decuypere, E.; Buyse, J. Effects of Dietary Protein Content and 2-Hydroxy-4-Methylthiobutanoic Acid or DL-Methionine Supplementation on Performance and Oxidative Status of Broiler Chickens. Br. J. Nutr. 2011, 106, 1845–1854. [Google Scholar] [CrossRef]
- Yodseranee, R.; Bunchasak, C. Effects of Dietary Methionine Source on Productive Performance, Blood Chemical, and Hematological Profiles in Broiler Chickens under Tropical Conditions. Trop. Anim. Health Prod. 2012, 44, 1957–1963. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Wang, Y.; Liu, W.; Ju, T.; Zhan, X. Effects of Different Methionine Sources on Production and Reproduction Performance, Egg Quality and Serum Biochemical Indices of Broiler Breeders. Asian-Australas. J. Anim. Sci. 2017, 30, 828–833. [Google Scholar] [CrossRef]
- Albrecht, A.; Herbert, U.; Miskel, D.; Heinemann, C.; Braun, C.; Dohlen, S.; Zeitz, J.O.; Eder, K.; Saremi, B.; Kreyenschmidt, J. Effect of Methionine Supplementation in Chicken Feed on the Quality and Shelf Life of Fresh Poultry Meat. Poult. Sci. 2017, 96, 2853–2861. [Google Scholar] [CrossRef]
- Jankowski, J.; Tykałowski, B.; Ognik, K.; Koncicki, A.; Kubińska, M.; Zduńczyk, Z. The Effect of Different Dietary Levels of DL-Methionine and DL-Hydroxy Analogue on the Antioxidant Status of Young Turkeys Infected with the Haemorrhagic Enteritis Virus. BMC Vet. Res. 2018, 14, 404. [Google Scholar] [CrossRef]
- Wan, J.; Ding, X.; Wang, J.; Bai, S.; Peng, H.; Luo, Y.; Su, Z.; Xuan, Y.; Zhang, K. Dietary Methionine Source and Level Affect Hepatic Sulfur Amino Acid Metabolism of Broiler Breeder Hens. Anim. Sci. J. 2017, 88, 2016–2024. [Google Scholar] [CrossRef]
- Sangali, C.P.; Bruno, L.D.G.; Nunes, R.V.; de Oliveira Neto, A.R.; Pozza, P.C.; de Oliveira, T.M.M.; Frank, R.; Schöne, R.A. Bioavailability of Different Methionine Sources for Growing Broilers. R. Bras. Zootec. 2014, 43, 140–145. [Google Scholar] [CrossRef]
- Millecam, J.; Khan, D.R.; Dedeurwaerder, A.; Saremi, B. Optimal Methionine plus Cystine Requirements in Diets Supplemented with L-Methionine in Starter, Grower, and Finisher Broilers. Poult. Sci. 2021, 100, 910–917. [Google Scholar] [CrossRef]
- Shen, Y.B.; Ferket, P.; Park, I.; Malheiros, R.D.; Kim, S.W. Effects of Feed Grade L-Methionine on Intestinal Redox Status, Intestinal Development, and Growth Performance of Young Chickens Compared with Conventional DL-Methionine. J. Anim. Sci. 2015, 93, 2977–2986. [Google Scholar] [CrossRef] [PubMed]
- Martínez, Y.; Li, X.; Liu, G.; Bin, P.; Yan, W.; Más, D.; Valdivié, M.; Hu, C.-A.A.; Ren, W.; Yin, Y. The Role of Methionine on Metabolism, Oxidative Stress, and Diseases. Amino Acids 2017, 49, 2091–2098. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I.; Kidd, M.T. Antioxidant Defence Systems and Oxidative Stress in Poultry Biology: An Update. Antioxidants 2019, 8, 235. [Google Scholar] [CrossRef] [PubMed]
- Horváth, M.; Babinszky, L. Impact of Selected Antioxidant Vitamins (Vitamin A, E and C) and Micro Minerals (Zn, Se) on the Antioxidant Status and Performance under High Environmental Temperature in Poultry. A Review. Acta Agric. Scand. Sect. A—Anim. Sci. 2018, 68, 152–160. [Google Scholar] [CrossRef]
- Mishra, B.; Jha, R. Oxidative Stress in the Poultry Gut: Potential Challenges and Interventions. Front. Vet. Sci. 2019, 6, 60. [Google Scholar] [CrossRef]
- Luo, S.; Levine, R.L. Methionine in Proteins Defends against Oxidative Stress. FASEB J. 2009, 23, 464–472. [Google Scholar] [CrossRef]
- Willemsen, H.; Swennen, Q.; Everaert, N.; Geraert, P.-A.; Mercier, Y.; Stinckens, A.; Decuypere, E.; Buyse, J. Effects of Dietary Supplementation of Methionine and Its Hydroxy Analog DL-2-Hydroxy-4-Methylthiobutanoic Acid on Growth Performance, Plasma Hormone Levels, and the Redox Status of Broiler Chickens Exposed to High Temperatures. Poult. Sci. 2011, 90, 2311–2320. [Google Scholar] [CrossRef]
- Song, B.; Fu, M.; He, F.; Zhao, H.; Wang, Y.; Nie, Q.; Wu, B. Methionine Deficiency Affects Liver and Kidney Health, Oxidative Stress, and Ileum Mucosal Immunity in Broilers. Front. Vet. Sci. 2021, 8, 722567. [Google Scholar] [CrossRef]
- Erfani, M.; Eila, N.; Zarei, A.; Noshary, A. The Effects of Vitamin C and Methionine Hydroxy Analog Supplementation on Performance, Blood Parameters, Liver Enzymes, Thyroid Hormones, Antioxidant Activity of Blood Plasma, Intestine Morphology, and HSP70 Gene Expression of Broilers under Heat Stress. Trop. Anim. Health Prod. 2021, 53, 296. [Google Scholar] [CrossRef]
- Santana, T.P.; Gasparino, E.; de Sousa, F.C.B.; Khatlab, A.S.; Zancanela, V.; Brito, C.O.; Barbosa, L.T.; Fernandes, R.P.M.; Del Vesco, A.P. Effects of Free and Dipeptide Forms of Methionine Supplementation on Oxidative Metabolism of Broilers under High Temperature. Animal 2021, 15, 100173. [Google Scholar] [CrossRef]
- Del Vesco, A.P.; Gasparino, E.; Grieser, D.d.O.; Zancanela, V.; Soares, M.A.M.; Neto, A.R.d.O. Effects of Methionine Supplementation on the Expression of Oxidative Stress-Related Genes in Acute Heat Stress-Exposed Broilers. Br. J. Nutr. 2015, 113, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Gasparino, E.; Del Vesco, A.P.; Khatlab, A.S.; Zancanela, V.; Grieser, D.O.; Silva, S.C.C. Effects of Methionine Hydroxy Analogue Supplementation on the Expression of Antioxidant-Related Genes of Acute Heat Stress-Exposed Broilers. Animal 2018, 12, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Zeitz, J.O.; Fleischmann, A.; Ehbrecht, T.; Most, E.; Friedrichs, S.; Whelan, R.; Gessner, D.K.; Failing, K.; Lütjohann, D.; Eder, K. Effects of Supplementation of DL-Methionine on Tissue and Plasma Antioxidant Status during Heat-Induced Oxidative Stress in Broilers. Poult. Sci. 2020, 99, 6837–6847. [Google Scholar] [CrossRef]
- Elwan, H.; Elnesr, S.; Xu, Q.; Xie, C.; Dong, X.; Zou, X. Effects of In Ovo Methionine-Cysteine Injection on Embryonic Development, Antioxidant Status, IGF-I and TLR4 Gene Expression, and Jejunum Histomorphometry in Newly Hatched Broiler Chicks Exposed to Heat Stress during Incubation. Animals 2019, 9, 25. [Google Scholar] [CrossRef]
- Elnesr, S.S.; Elwan, H.A.M.; Xu, Q.Q.; Xie, C.; Dong, X.Y.; Zou, X.T. Effects of in Ovo Injection of Sulfur-Containing Amino Acids on Heat Shock Protein 70, Corticosterone Hormone, Antioxidant Indices, and Lipid Profile of Newly Hatched Broiler Chicks Exposed to Heat Stress during Incubation. Poult. Sci. 2019, 98, 2290–2298. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Li, R.; Zhang, Y.F.; Qin, T.Y.; Li, Q.S.; Li, X.X.; Qi, Z.L. A Comparison of Two Sources of Methionine Supplemented at Different Levels on Heat Shock Protein 70 Expression and Oxidative Stress Product of Peking Ducks Subjected to Heat Stress. J. Anim. Physiol. Anim. Nutr. 2018, 102, e147–e154. [Google Scholar] [CrossRef]
- Bolek, J.K. Mitigation Strategies to Ameliorate Acute and Chronic Heat Stress Utilizing Supplemental Methionine or Embryonic Thermal Conditioning in Chickens. Master’s Thesis, Iowa State University, Digital Repository, Ames, IA, USA, 2013; pp. 29–42. [Google Scholar]
- Del Vesco, A.P.; Gasparino, E.; Grieser, D.O.; Zancanela, V.; Gasparin, F.R.S.; Constantin, J.; Oliveira Neto, A.R. Effects of Methionine Supplementation on the Redox State of Acute Heat Stress-Exposed Quails. J. Anim. Sci. 2014, 92, 806–815. [Google Scholar] [CrossRef]
- Kalvandi, O.; Sadeghi, A.; Karimi, A. Methionine Supplementation Improves Reproductive Performance, Antioxidant Status, Immunity and Maternal Antibody Transmission in Breeder Japanese Quail under Heat Stress Conditions. Arch. Anim. Breed. 2019, 62, 275–286. [Google Scholar] [CrossRef]
- Wang, J.; Yang, G.; Zhang, K.; Ding, X.; Bai, S.; Zeng, Q. Effects of Dietary Supplementation of DL-2-Hydroxy-4(Methylthio) Butanoic Acid on Antioxidant Capacity and Its Related Gene Expression in Lung and Liver of Broilers Exposed to Low Temperature. Poult. Sci. 2019, 98, 341–349. [Google Scholar] [CrossRef]
- Yang, G.L.; Zhang, K.Y.; Ding, X.M.; Zheng, P.; Luo, Y.H.; Bai, S.P.; Wang, J.P.; Xuan, Y.; Su, Z.W.; Zeng, Q.F. Effects of Dietary DL-2-Hydroxy-4(Methylthio)Butanoic Acid Supplementation on Growth Performance, Indices of Ascites Syndrome, and Antioxidant Capacity of Broilers Reared at Low Ambient Temperature. Int. J. Biometeorol. 2016, 60, 1193–1203. [Google Scholar] [CrossRef]
- Saleh, A.A.; Ragab, M.M.; Ahmed, E.A.M.; Abudabos, A.M.; Ebeid, T.A. Effect of Dietary Zinc-Methionine Supplementation on Growth Performance, Nutrient Utilization, Antioxidative Properties and Immune Response in Broiler Chickens under High Ambient Temperature. J. Appl. Anim. Res. 2018, 46, 820–827. [Google Scholar] [CrossRef]
- Maupin-Furlow, J.A. Methionine Sulfoxide Reductases of Archaea. Antioxidants 2018, 7, 124. [Google Scholar] [CrossRef] [PubMed]
- Savino, R.J.; Kempisty, B.; Mozdziak, P. The Potential of a Protein Model Synthesized Absent of Methionine. Molecules 2022, 27, 3679. [Google Scholar] [CrossRef] [PubMed]
- de Souza Castro, F.L.; Kim, W.K. Secondary Functions of Arginine and Sulfur Amino Acids in Poultry Health: Review. Animals 2020, 10, 2106. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.-Z.; Yang, S.; Wu, G. Free Radicals, Antioxidants, and Nutrition. Nutrition 2002, 18, 872–879. [Google Scholar] [CrossRef]
- Simitzis, P.E.; Kalogeraki, E.; Goliomytis, M.; Charismiadou, M.A.; Triantaphyllopoulos, K.; Ayoutanti, A.; Niforou, K.; Hager-Theodorides, A.L.; Deligeorgis, S.G. Impact of Stocking Density on Broiler Growth Performance, Meat Characteristics, Behavioural Components and Indicators of Physiological and Oxidative Stress. Br. Poult. Sci. 2012, 53, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.Q.; Dong, Y.Y.; Qin, X.; Yuan, J.M.; Han, M.M.; Zhang, K.K.; Shi, S.R.; Song, X.Y.; Zhang, J.Z.; Li, J.H. Dietary Supplementation of Methionine Mitigates Oxidative Stress in Broilers under High Stocking Density. Poult. Sci. 2021, 100, 101231. [Google Scholar] [CrossRef]
- Selvam, R.; Saravanakumar, M.; Suresh, S.; Sureshbabu, G.; Sasikumar, M.; Prashanth, D. Effect of Vitamin E Supplementation and High Stocking Density on the Performance and Stress Parameters of Broilers. Rev. Bras. Ciência Avícola 2017, 19, 587–594. [Google Scholar] [CrossRef]
- Ismail, F.S.A.; El-Gogary, M.R.; El-Nadi, M.I. Influence of Vitamin E Supplementation and Stocking Density on Performance, Thyroid Status, Some Blood Parameters, Immunity and Antioxidant Status in Broiler Chickens. Asian J. Anim. Vet. Adv. 2014, 9, 702–712. [Google Scholar] [CrossRef]
- Li, W.; Wei, F.; Xu, B.; Sun, Q.; Deng, W.; Ma, H.; Bai, J.; Li, S. Effect of Stocking Density and Alpha-Lipoic Acid on the Growth Performance, Physiological and Oxidative Stress and Immune Response of Broilers. Asian-Australas. J. Anim. Sci. 2019, 32, 1914–1922. [Google Scholar] [CrossRef]
- Castro, F.L.S.; Tompkins, Y.H.; Pazdro, R.; Kim, W.K. The Effects of Total Sulfur Amino Acids on the Intestinal Health Status of Broilers Challenged with Eimeria Spp. Poult. Sci. 2020, 99, 5027–5036. [Google Scholar] [CrossRef] [PubMed]
- Lai, A.; Dong, G.; Song, D.; Yang, T.; Zhang, X. Responses to Dietary Levels of Methionine in Broilers Medicated or Vaccinated against Coccidia under Eimeria Tenella-Challenged Condition. BMC Vet. Res. 2018, 14, 140. [Google Scholar] [CrossRef] [PubMed]
- Khatlab, A.d.S.; de Souza Khatlab, A.; Del Vesco, A.P.; de Oliveira Neto, A.R.; Fernandes, R.P.M.; Gasparino, E. Dietary Supplementation with Free Methionine or Methionine Dipeptide Mitigates Intestinal Oxidative Stress Induced by Eimeria spp. Challenge in Broiler Chickens. J. Anim. Sci. Biotechnol. 2019, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Reda, F.M.; Swelum, A.A.; Hussein, E.O.S.; Elnesr, S.S.; Alhimaidi, A.R.; Alagawany, M. Effects of Varying Dietary DL-Methionine Levels on Productive and Reproductive Performance, Egg Quality, and Blood Biochemical Parameters of Quail Breeders. Animals 2020, 10, 1839. [Google Scholar] [CrossRef]
- Ruan, D.; Fouad, A.M.; Fan, Q.; Xia, W.; Wang, S.; Chen, W.; Lin, C.; Wang, Y.; Yang, L.; Zheng, C. Effects of Dietary Methionine on Productivity, Reproductive Performance, Antioxidant Capacity, Ovalbumin and Antioxidant-Related Gene Expression in Laying Duck Breeders. Br. J. Nutr. 2018, 119, 121–130. [Google Scholar] [CrossRef]
- Zeitz, J.O.; Kaltenböck, S.; Most, E.; Eder, K. Effects of L-Methionine on Performance, Gut Morphology and Antioxidant Status in Gut and Liver of Piglets in Relation to DL-Methionine. J. Anim. Physiol. Anim. Nutr. 2019, 103, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, N.-Y.; Pan, Y.-X.; Zhu, L.-Y.; Batonon-Alavo, D.I.; Ma, L.-B.; Khalil, M.M.; Qi, D.-S.; Sun, L.-H. Efficacy of 2-Hydroxy-4-Methylthiobutanoic Acid Compared to DL-Methionine on Growth Performance, Carcass Traits, Feather Growth, and Redox Status of Cherry Valley Ducks. Poult. Sci. 2018, 97, 3166–3175. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Chen, X.; Zhang, H.; Zhou, Y.M. Effects of Dietary Concentrations of Methionine on Growth Performance and Oxidative Status of Broiler Chickens with Different Hatching Weight. Br. Poult. Sci. 2013, 54, 531–537. [Google Scholar] [CrossRef]
- Jankowski, J.; Kubinska, M.; Juskiewicz, J.; Czech, A.; Ognik, K.; Zdunczyk, Z. Effect of Different Dietary Methionine Levels on the Growth Performance and Tissue Redox Parameters of Turkeys. Poult. Sci. 2017, 96, 1235–1243. [Google Scholar] [CrossRef]
- Huber, P.C.; Almeida, W.P.; de Fátima, Â. Glutationa e enzimas relacionadas: Papel biológico e importância em processos patológicos. Quím. Nova 2008, 31, 1170–1179. [Google Scholar] [CrossRef]
- Németh, K.; Mézes, M.; Gaál, T.; Bartos, A.; Balogh, K.; Husvéth, F. Effect of Supplementation with Methionine and Different Fat Sources on the Glutathione Redox System of Growing Chickens. Acta Vet. Hung. 2004, 52, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.G.; Pan, X.J.; Zhang, W.Q.; Peng, Z.Q.; Zhao, R.Q.; Zhou, G.H. Methionine and Selenium Yeast Supplementation of the Maternal Diets Affects Antioxidant Activity of Breeding Eggs. Poult. Sci. 2010, 89, 931–937. [Google Scholar] [CrossRef]
- Wu, B.; Cui, H.; Peng, X.; Fang, J.; Cui, W.; Liu, X. Pathology of Spleen in Chickens Fed on a Diet Deficient in Methionine. Health 2012, 4, 32–38. [Google Scholar] [CrossRef][Green Version]
- Li, L.L.; Gong, Y.J.; Zhan, H.Q.; Zheng, Y.X.; Zou, X.T. Effects of Dietary Zn-Methionine Supplementation on the Laying Performance, Egg Quality, Antioxidant Capacity, and Serum Parameters of Laying Hens. Poult. Sci. 2019, 98, 923–931. [Google Scholar] [CrossRef]
- Chang, Y.; Tang, H.; Zhang, Z.; Yang, T.; Wu, B.; Zhao, H.; Liu, G.; Chen, X.; Tian, G.; Cai, J.; et al. Zinc Methionine Improves the Growth Performance of Meat Ducks by Enhancing the Antioxidant Capacity and Intestinal Barrier Function. Front. Vet. Sci. 2022, 9, 774160. [Google Scholar] [CrossRef]
- Jankowski, J.; Ognik, K.; Całyniuk, Z.; Stępniowska, A.; Konieczka, P.; Mikulski, D. The Effect of Different Dietary Ratios of Lysine, Arginine and Methionine on Protein Nitration and Oxidation Reactions in Turkey Tissues and DNA. Animal 2021, 15, 100183. [Google Scholar] [CrossRef]
- Kubińska, M.; Jankowski, J.; Juśkiewicz, J.; Ognik, K.; Czech, A.; Celej, J.; Zduńczyk, Z. Growth Rate and Metabolic Parameters in Young Turkeys Fed Diets with Different Inclusion Levels of Methionine. J. Animal. Feed Sci. 2016, 25, 152–159. [Google Scholar] [CrossRef]
- Wen, C.; Jiang, X.Y.; Ding, L.R.; Wang, T.; Zhou, Y.M. Effects of Dietary Methionine on Growth Performance, Meat Quality and Oxidative Status of Breast Muscle in Fast- and Slow-Growing Broilers. Poult. Sci. 2017, 96, 1707–1714. [Google Scholar] [CrossRef]
- Dang, D.X.; Zhou, H.; Lou, Y.; Li, D. Effects of in Ovo Feeding of Disaccharide and/or Methionine on Hatchability, Growth Performance, Blood Hematology, and Serum Antioxidant Parameters in Geese. J. Anim. Sci. 2022, 100, skac014. [Google Scholar] [CrossRef]
- Maddineni, S.; Nichenametla, S.; Sinha, R.; Wilson, R.P.; Richie, J.P., Jr. Methionine Restriction Affects Oxidative Stress and Glutathione-Related Redox Pathways in the Rat. Exp. Biol. Med. 2013, 238, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.A.; Zaghari, M.; Lotfollahian, H.; Shivazad, M.; Moravaj, H. Reevaluation of Methionine Requirement Based on Performance and Immune Responses in Broiler Breeder Hens. J. Poult. Sci. 2012, 49, 26–33. [Google Scholar] [CrossRef]
- Sheng, Y.; Abreu, I.A.; Cabelli, D.E.; Maroney, M.J.; Miller, A.-F.; Teixeira, M.; Valentine, J.S. Superoxide Dismutases and Superoxide Reductases. Chem. Rev. 2014, 114, 3854–3918. [Google Scholar] [CrossRef] [PubMed]
- Ogunbode, S.M.; Iyayi, E.A. Biochemical Effects of Low Crude Protein Diets Supplemented with Varying Methionine Concentrations. Jordan J. Biol. Sci. 2021, 14, 205–211. [Google Scholar] [CrossRef]
- Surai, P.F. Antioxidant Systems in Poultry Biology: Superoxide Dismutase. J. Animal Res. Nutr. 2015, 1, 8. [Google Scholar] [CrossRef]


| Met Sources/Poultry Species | Met Levels | Results | References | Remarks |
|---|---|---|---|---|
| DLM and HMTBA Cornish Cross cockerels 31 °C from 14 to 42 days | BD = 0.276% MS (DLM = 0.309 and 0.404% in grower; 0.277 and 0.360% in finisher correspond to 100% or 130% required Met, respectively) MS (HMTBA = 0.719 and 0.934% in grower; 0.644 and 0.837%) (88% DLM and 58% HMTBA used as sources of Met) | 130% ↑ hepatic GSH, GSSG in grower, and plasma FRAP finisher. 130% ↓ GPx, GST, and SOD in grower adipose tissues and ↓ plasma GSH, hepatic GSSG but ↑breast GSSG in the finisher broiler. In grower; DLM ↑ breast GSH and thigh GSH and GSSG than HMTBA 130% ↓ hepatic GPx and adipose tissue GPx and GST activities of the finisher broiler. In grower broiler: DLM; ↓ activities of breast GPx, thigh GR, adipose GST and all assayed antioxidant enzymes in the liver as compared with HMTBA | [7] | Chronic heat stress 30% extra Met improved the antioxidant status of broilers under HSNo effect of MS (either source or levels) on BW, average daily gain and feed conversion of birds throughout the study. DLM increased the FI in finisher birds as compared with HMTBA. |
| DLM DL-HMTBA HS 32 °C until 6 weeks of age Male Ross broiler | BD = 0.28% Met MS = 1.0 or 1.2 g/kg for each source (0.10% or 0.12% of either DLM or DLHMTBA added to basal diet) | In week 4: ⥊ FRAP on HS and MS ↓ MAD, but ↑UA in HS chicks In week 6: ⥊ plasma FRAP, SOD, UA by either HS or MS HS ↑ MDA in DLM but not DL-HMTBA ↑ hepatic ratios of reduced GSH to total GSH and reduced GSH to GSSG in HMTBA at HS | [50] | Chronic HS (4 weeks exposure): 0.1% and 0.12% of either DLM or HMTBA did not affect the antioxidants of broilers. HMTBA improved growth performance (FI and BW) at 5 and 6 weeks of age |
| DL-Met Male cobb broilers for 42 days | Met deficient (MD)-without Met supplementation (MS) Control (MD + 0.24% Met) in starter diet, (MD + 0.12% Met) in grower diet) | Met deficiency leads to oxidative stress in both the liver and kidney of growing chicks ↑ MDA ↓ SOD, CAT, GSH-PX, and GSH | [51] | Met-deficient (MD) causes injury to the kidney and liver. MD depressed body weight (BW) from 14 to 42 days |
| MHA Broilers | Basal diet + 0.46, 0.36 and 0.32% in starter, grower, and finisher, respectively | The antioxidants activity was improved by MHA supplementation | [52] | 0.2% of vitamin C supplemented together with MHA, no effect on BW |
| Free Met DL-Met, and Met dipeptide (DL-MM Male cobb broilers, from 21 to 42 days | MD-without MS DL-M (MD + 0.27%) DL-MM (MD + 0.28%) (MD = 0.543, DL-M = 0.810, DL-MM = 0.809 digestible Met + Cys) | Heat stress increased the expression of GSS and GPX. Met supplementation (MS) reduced the expression of GPX | [53] | Thermoneutral group 27–18˚C; Heat stress continuous exposure 30˚C, 60% RH from 21 to 42 days MS increased BW |
| DL-Met Male cobb 500 Heat stress (HS) 38 °C for 24 h. | Starter: MD-, DL1 = MD + 0.295%; DL2 = MD + 1% Grower: MD-; DL1 = MD + 0.275%; DL2 = MD + 1% | Broilers reared in HS and fed DL1 and DL2 increased expression levels of GSSG and GPx7 in both feeding phases | [54] | Acute heat stress MS increased BW, DL2 reduced FI |
| DL-2-hydroxy-4-methylthiobutanic acid (DL-HMTBA) HS 38 °C for 24 h at 41 day Male cobb 500 | Starter MD; DL-HMTBA: = 0.45% Grower DL-HMTBA = 0.42% (MD = 0.58, and 0.54; MS = 0.88 and 0.81 in starter and grower digestible Met + Cys, respectively) | ↑ TRxR1, SOD, and MsrA in starter under HS and fed DL-HMTBA diet. Interaction of the HS and MS observed In grower: HS ↑ SOD, TRxR1, and MsrA; MS also ↑ levels of expression than MD but not the interaction between temperature and diet in a grower phase. | [55] | Acute heat stress HS lowered the feed intake (FI), and BW gain MS did not influence FI and BW gain |
| DL-Met Male cobb 500 broilers HS 27.4 °C ± 0.3 °C, and mean relative humidity was 63.7 ± 0.6% from 21 days until day 35 | Control diet = 0.50, 0.42 and 0.39% in starter, grower, and finisher DLM 1 = 0.19, 0.16 and 0.15% added in starter, grower, and finisher diets, respectively. DLM2 = 0.37, 0.32, and 0.29% added in starter grower and finisher diets, respectively. | HS: ↓ plasma tocopherol, GSH: GSSG and ↑ GSSG, ↑ hepatic GSH, GSSG and ↓ Vit C than the thermoneutral group. High Met levels ↑ GSH and GSSG levels in the liver and thigh muscle of broilers subjected to HS High Met levels also ↑ tocopherols in the liver and plasma of heat-exposed ⥊ TBARS, GSH: GSSG | [56] | Chronic heat stress HS affected broilers’ performance negatively. MS did not improve the birds’ performance under HS |
| In ovo Met –cysteine (Cys) injection HS (39.6 °C for 6 h/d) from day 10 until day 18 | 5.90 mg L-Met plus 3.40 mg L-cysteine | ↑ TAC and GSH in serum and tissues of newly hatched chicks in Met + Cys injected group | [57] | Met-Cys injection elevated the antioxidant capacity of chicks exposed to HS during incubation. No effect on hatchability |
| In ovo Met-Cys injection Ross broilers HS (39.6 °C for six h daily) between 10 and 18 days of the incubation | 5.90 mg L-Met plus 3.40 mg L-cysteine | ↓ HSP70 and ↑GSH-Px expression in the liver, jejunum, cardiac muscles, and pectoral muscles tissues ↑ T-SOD in serum and tissues ↑ GSH-Px and GSH/GSSG ratio in serum and tissues ↓ MDA in serum and tissues ⥊CAT except in pectoral muscles | [58] | In ovo injection of Met + Cys improved the antioxidant status of the newly hatched chicks exposed to heat stress during incubation. |
| DLM and HMTBA Peking ducks HS (summer temperature range (mean of highest) 32.1 °C to 24.7 °C (mean of lowest) | Basal diet (BD) = 0.45 and 0.40% of Met in starter and grower period 0.05%, 0.2% and 0.35% of either DLM or HMTBA were added to the basal diet during starter and grower periods. | 0.35% ↑ HSP70 mRNA expression of the intestine and liver ↑ MDA by 0.35% of DLM on day 16. 0.35% DLM ↑ MDA on day 35. 0.35% HMTBA ↑ HSP70 expression compared with other treatment groups. | [59] | HMTBA improved the antioxidant status of the liver and intestine on day 35. Growth performance was not reported. |
| DLM and HMTBA HS 35 °C and TN 24 °C Male Ross 308. | MS; DLM = 0.31 and 0.51% = starter, 0.26 and 0.43% for grower) HMTBA = 0.34 and 0.57% for starter, 0.29 and 0.49% for grower diet. | TGSH and GSH were higher in the HS group. DLM had lower TBARS in the acute phase. ⥊ GPx. ↓ GSSG in super-adequate digestible sulfur amino acid (DSAA). GSH: GSSG ratio was higher than inadequate DSAA. | [60] | MS did not have significant influences on the antioxidants or the bird performance |
| DLM HS (38 °C for 24 h, starting on the sixth day TN (25 °C) Male meat quails. | MD, MS = 0.27% (MD = 0.57% MS = 0.84% Met + Cys digestible) | HS induced ↑ GPx, ↑UA, ↑ H2O2 production, ↓GSH, ↓ MAD levels on MS diet. Interaction between HS and diet on GPx and CAT | [61] | MS can mitigate ROS-induced damage by increasing the activities of antioxidants. MS did not affect feed consumption and weight gain. |
| DLM. Breeder Japanese quail | BD = 0.70% Met + Cys; BD + MS with DLM to provide proportions concentrations of 1.15, 1.30 and 1.45 times the quail requirements per NRC (1994) recommendations. | MS ↑ plasma and liver SOD, CAT, GPx, and ↓ MAD. 1.15 times the NRC ↑ plasma and liver SOD, CAT, GPx and ↓ MAD in HS quails. MS ↑ plasma TAC compared to HS and TN groups. | [62] | Met could improve the performance, immunity, and antioxidant status of quails by reducing the adverse effects of HS. MS improved the productive performance of birds. |
| DL-HMTBA Broilers. [Low (12 to 14 °C) vs. control temperature (thermoneutral, 24 to 26 °C)] from 8 to 28 days of age. | BD = 0.32% Met MS = 0.17% or 0.51% of DL-DL-HMTBA added to BD (HMTBA containing 88% of active substance) | 0.51% ↑ hepatic GSH and GSH-Px and lung SOD activity of broiler. Low temperature reduces the expression of GSH synthetase and increases GSH reductase gene expression. Higher DL-HMTBA induced ↑ GST in the lung, GSH synthetase in the liver and lung, and ↓ GSH reductase in the lung at low temperatures. | [63] | At low temperatures, 0.51% MS ↑ GSH synthesis gene expression hence increases the antioxidant capacity in the liver and lung No effect of MS on the growth performance |
| DL-HMTBA Broilers. 12–14 and 24–26 °C for Low ambient temperature (LAT) and normal ambient temperature.NAT | BD = 0.32% Met as-fed basis MS (0.17, 0.34, 0.51, and 0.68% DL-HMTBA added to BD) | LAT ↑ serum MAD and PC on day 21; ↓ serum GSH, GSH-Px and TAC on days 21 and 28. MS ↑ serum GSH content, GSH-Px activity, TAC and SOD at day 28, and SOD and GSH-PX activities and ↓ PC at day 21 under LAT conditions. | [64] | MS improved the serum antioxidant activities in a dose-dependent manner under LAT conditions. LAT ↓ BWG and ADFI at days 7–14 and 15–21 of the experiment. |
| Zinc- Met Male cobb 500 The average daily temperature ranged from 33 °C to 36 °C | BD = 0.25, 0.23 and 0.15% Met for starter, grower, and finisher diets, respectively. MS = 0, 0.025, 0.05, and 0.10% ZnMet (98% ZnMet purity) | The higher level of Zn-M ↑ plasma GPx activity and ↓ muscles MDA in broilers reared in higher ambient temperature | [65] | Chronic stress ZnM supplementation increased the BWG and BW and improved FCR under HS |
| Stocking Densities/Poultry Species | Met Source/Met Levels | Antioxidants Studied | Results | References | Remarks |
|---|---|---|---|---|---|
| Male Cornish Cross cockerels SD: (9 and 12 birds/m2) | MS (DLM: (grower: 0.29 or 0.377% and finisher: 0.260 or 0.338%) representing 100% and 130% of the recommendations. | GSH, GSSG, MDA, FRAP, GPx, SOD, glutathione S-transferase (GST), and glutathione reductase (GR). | HSD ↑ GSH in the liver but ↓ GSH in other tissues. In finisher: HSD ↓ GSSG in plasma, liver, breast, and GSH in the thigh. 130% ↓ GSSG in the plasma but ↑ GSH and GSSG 130% MS ↓ hepatic SOD, MDA in the breast and thigh muscles ⥊ FRAP, PC, and corticosterone HSD ↓ thigh GST in both phases. | [8] | 30% extra MS partially attenuated the adverse effects caused by stocking density. |
| MHA and DLM Female Hybrid Converter turkey Stress inducer: hemorrhagic enteritis virus (HEV) | BD = 0.40 and 0.35% for weeks 1–4 and weeks 5–8) MS = 0.15 and 0.38% in weeks 1–4 of age, and 0.10 and 0.30% in weeks 5–8 of age, respectively) | MDA, Lipid peroxides (LOOH), GSH + GSSG, FRAP, vitamin C, SOD and CAT. | In blood redox, High-level MS ↑ of GSH + GSSG, LOOH, and ↓ MDA MHA ↓ Vitamin C, GPx, FRAP, LOOH and ↑ SOD, and CAT than DLM. In the intestinal wall: Higher level ↑ CAT, LOOH, MDA, and lower SOD.\ DLM ↑ CAT and ↓ MDA than MHA In liver: ↑ Met; ↑ CAT, SOD LOOH, and ↓ MDA. DLM ↑vitamin C and ↓ SOD | [40] | DLM had more effects on the redox status in the small intestine, blood, and liver of turkeys than MHA |
| Arbor Acres Broiler: (14 and 20 broilers per m2) in the finisher phase (from 21 to 42 days) | Basal diet (BD) = 0.29% Met MS (DLM = 0.05, 0.1, 0.15, or 0.2% added to BD (99% DLM purity used) | Plasma T-SOD, Total antioxidant capacity (TAC), GPx, PCO, MDA, GSH/GSSG | HSD ↓ TAC, GPx, ↑ PCO, and MDA of plasma. ⥊ T-SOD and GSH/GSSG in HSD as compared with LSD. 0.2% MS ↑ GPx, GSH/GSSG ratio in the plasma. 0.15% MS ↑ T-SOD and ↓ GPx in the liver under HSD ⥊ SOD and GPx mRNA expression. 0.15–0.2% ↓ MDA and ↑ T-SOD in the jejunum. | [71] | MS mitigates the oxidative stress caused by HSD (0.1% to 0.15% should be supplemented. |
| DLM Male partridge shank broilers medicated or vaccinated against coccidia under EImeria tenella-challenged situation | BD = 0.30% Met MS (DLM = 0.15 0.27 and 0.39% from 22 to 42 days of life. | GSH-GPx, TAC, MDA | The serum antioxidative of chicken were not consistent ⥊ serum MDA by anticoccidial programs Interaction of anticoccidial program and Met level on TAC and GPx. High Met levels ↑ MDA in medicated and vaccinated chickens High Met levels ↑ GSH-GPx and TAC in medicated chickens only | [76] | Increased MS levels could not alleviate the oxidative stress effect in the mixed infection model. |
| Met Sources/Poultry Species | Met Levels | Main Results | References | Remarks |
|---|---|---|---|---|
| DLM and LM and HMTBA/male cobb 500 broiler | MS = 0.22% DLM, 0.22% LM or 0.31% HMTBA | In breast muscles: ↑ TGSH and rGSH in LM and HMTBA were observed. ⥊ by met source for MAD, FRAP, and the ratio of rGSH: GSSG and GSSG: TGSH. | [20] | Met + Cys deficiency did not compromise the antioxidant capacity of chickens |
| LM, DLM, and MHA Hybrid Converter turkey | BD = 0.40, 0.34, 0.29 and 0.26% Met for 1–4, 5–8, 9–12 and 13–16 weeks respectively; MS = NRC 1994 –Low and 40% extra NRC recommendation-High | Higher Met content ↑ SOD; CAT activity, Vit C concentration, and ↑ plasma FRAP, TGSH, and ↓ MDA ⥊ SOD, Vit C, MDA, or LOOH in the small intestinal or liver of turkey-fed diets with different Met levels. MHA ↑ TGSH and ↓ SOD, and MDA in the plasma compared with DLM. LM ↑ TGSH compared with DLM In the small intestine, SOD; DLM > LM > MHA ⥊ MDA and ↑ LOOH in LM compared with other Met sources. In liver: ⥊ Vit C, LOOH, and SOD but interaction for SOD, CAT, and LOOH. | [23] | A higher Met level improved the indicators of redox status MHA lowered plasma, and intestinal SOD more than DLM or M. LMH and LM decreased plasma and hepatic MDA and increased plasma glutathione levels. |
| DLM, LM, and MHA Hybrid converter turkey | Low = 100% and High = 150% of NRC (1994) recommendations for each feeding phase | Met source and Met level; ⥊ vit C, LOOH, and MDA Higher Met Level ↓ CAT, ↑ SOD DLM: ↑ SOD CAT: LM > DLM > MHA | [24] | MHA reduced the CAT activity in the breast meat compared with LM and DLM sources. |
| DLM and L-Met Hatched turkey. | basal diet (BD), the BD + 0.17 or 0.33% DL-Met or L-Met (60, 75, and 90% for SAA of NRC) | L-Met ↑ GSH and ↓ MAD in the liver than DLM during 28 days. MS regardless of the source ↓ MAD in the duodenal mucosa. ⥊ PC, TAC on day 7 but day 28 MS regardless of the source ↓ PC and ↑ TAC in the duodenum than BD | [25] | L-Met was more effective in reducing oxidative stress and improving glutathione in the liver than DL-Met. |
| MHA and DLM Female Hybrid converter turkey | 0.15% and 0.37% in weeks 1–4 of age, and 0.0% and 0.1% in weeks 5–8 of age added to BD (0.40 and 0.35% Met at 1–4 and 5–8 weeks of age, respectively) | Higher Met level ↑ SOD, GSH + GSSG, and FRAP values in the blood. ⥊ MAD, GPx, Vitamin C, and CAT. MHA ↓ SOD, CAT, and GSH + GSSG in the blood and ↑ MDA | [33] | DLM improved the activities of SOD, CAT, and GSH + GSSG levels |
| DLM and HMTBA Male ROss 308 | 0.25% of either DLM or HMTBA was added to either 18.3 or 23.2% of CP diets. | DLM ↑ plasma TBARS and FRAP compared with HMTBA at 4 weeks of age. At 6 weeks of age: interaction of protein and met source for FRAP and TBARS MHA ↑ SOD and reduced GSH but ⥊ GSH synthetase, glutathione reductase, or Msr-A gene expression in the liver at 6 weeks old chicken. | [36] | MHA presented a more pronounced antioxidants effect than DLM |
| DLM and LM 1-d-old Ross 308 | basal diet (BD), the BD + 0.095% LM or DLM, the BD + 0.190% LM or DLM, and the BD + 0.285% LM or DLM (representing 60, 70, 80, and 90% of the Met + Cys requirement) | 0.285% of LM ↑ GSH and TAC but ↓ PC in the duodenum compared to DLM at the same level. | [44] | L-Met served better in reducing protein oxidation and increasing antioxidant status in the duodenum/gut of chicks than DLM. |
| DLM and MHA Cherry Valley ducks | 0.04, 0.12, 0.16, and 0.20% of Met equivalents for the grower phase | Booth dietary Met source and level affected the TAC < GPx, GSSG, and MDA in pectoralis major muscles on day 42. MHA ↑ TAC and GPx in pectoralis major muscles and GSH in the breast muscles compared with DLM. ↑ Met level; ↑ GSH, GSSG, and MDA regardless of the Met source. | [81] | MHA improved the antioxidant status of cherry valley pectoralis major muscles compared with DLM (increased antioxidants capacity markers (TAC, GPX, and GSH) |
| Sources | Dietary Met Levels | Main Observation and Conclusion (Oxidative Stress Responses) |
|---|---|---|
| [8] Broilers | 100 and 130% of the recommendations and stocking density | The 130% of DL Met decreased the content of GSSG in the plasma but improved/enhanced both GSH and GSSG in the thigh of the growers compared to 100%. The 130% DL-Met decreased the MDA concentration in the breast and thigh of the finisher. |
| [32] Egg-laying ducks | 0.0, 0.5, 1.0, 1.5, 2.0, or 2.5 g Met/kg | Met supplementation had no effects on the plasma concentrations of GSH, GSSG, GPx, and MDA |
| [54] Broilers | Starter: MD-, DL1 = MD + 0.295%; DL2 = MD + 1% Grower: MD-; DL1 = MD + 0.275%; DL2 = MD + 1% | MD-without Met supplementation; The results indicated that heat stress (38 °C for 24 h) resulted in a higher expression of oxidative stress-related genes (CBS, GSS, and GPx7) levels in broilers supplemented with DL1 and DL2 diets. |
| [62] Breeder Japanese quail | 1.00-, 1.15-, 1.30-, and 1.45-times NRC 1994 quails recommended in heat stress (34 °C) | The dietary supplementation of Met increased the serum and liver activity of antioxidant enzymes (SOD, CAT, and HGPx) and reduced MDA concentration in heat-stressed quails. |
| [78] Quail breeders | (0, 0.5, 1.5, 2.5, and 3.5 g/kg) quail breeders | DL-Met supplementation (0.5 to 1.5 g/kg) increased the SOD, TAC, and CAT, quadratically reduced GSH and quadratically decreased the MDA levels compared to the control diet. |
| [79] Laying duck breeders | 2.00, 2.75, 3.50, 4.25, 5.00, and 5.75 g/kg | The dietary Met levels improved the hepatic levels of GSH and activities of GPx and TAC in laying duck breeders at 43 weeks and reduced MDA levels in a quadratic manner. |
| [82] Broilers | 4.9 and 5.9 g/kg (0.49% and 0.59%) | Broilers supplemented with 5.9 g/kg had enhanced serum SOD activity and lowered hepatic MDA at 7 days of age. Moreover, the hepatic GSH: GSSG ratio was small/reduced by high levels of Met on day 21. |
| [83] Turkeys | T1 2.86/2.44, T2 3.24/2.83, T3 4.01/3.44, T4 4.67/4.15, T5 5.55/4.67, T6 6.10/5.46 (9–12 weeks/13–16 weeks) | Increasing the amount of Met in the diet did not affect CAT and SOD activities or GSH + GSSG concentrations. Plasma FRAP values increased with increasing met levels. Low dietary Met levels had little effect; however, a significant rise in the Met content of turkey feeds accelerated the oxidation processes in the liver, as indicated by increased MDA levels. |
| [86] Langshan layer hens (dual purpose -indigenous breed of China)-52 wk old | 3.2, 4.0, and 5.4 g/kg (0.32%, 0.4%, and 0.54% Met) Se: 0, 0.3, and 0.6 mg/kg (0, 0.3 and 0.6% Se). | The high level of Met supplementation in maternal diets (5.4 g/kg) decreased the concentration of GSH, GPX, and MDA in the yolks of eggs. However, the same level seems to decrease the activity/level of GPX and carbonyl group but not the GSH in the albumen of eggs. Met supplementation at 4.0 and 5.4 reduced the carbonyl concentration compared with 3.2 g of Met/kg. |
| [87] Broilers | 0.26 and 0.50% starter 0.28 and 0.40% grower | Met deficiency diet (0.26 and 0.28%) resulted in a significantly lower serum level and splenic SOD and GPx activities. Still, it markedly increased the MDA contents of the same tissues in broilers at 28 and 42 days. |
| [88] Laying hens | 20, 40, 60, 80, and 100 mg of Zn/kg as Zn-Met (basal diet 80 mg of Zn/Kg as Zn-Sulphate) | The serum MDA level decreased linearly with increasing Zn-Met level. The liver CAT and serum GSH-Px activities had quadratic effects. The CAT activity in the liver increased with increasing Zn-Met levels. The 60 mg/kg Zn-Met increased the TAC and GSH-Px activity in the serum and liver of the laying hen compared to the control. |
| [89] Meat ducks | 0, 30, 60, 90, 120, or 150 mg/kg Zn-Met for 35 d | Dietary Zn-Met supplementation improved the intestinal antioxidant status in meat ducks by increasing the activity of SOD, CAT, and GSH and decreasing the level of MDA in the jejunum. |
| [90] Turkey | Dietary ratios of arginine and Met were relative to lysine. Arg90Met30, Arg90Met45, Arg100Met30, Arg100Met45, Arg110Met30, and Arg110Met45 | An increase in Met level from 30 to 45 of Lys content improved the antioxidants capacity of turkey regardless of the Arg levels by decreasing the plasma protein carbonyl concentration as well as CAT and SOD activity in the breast muscles and liver, respectively. |
| [91] Turkeys | 0.6, 1.5, 2.0, 2.7, 3.4 g/kg for 1–4 weeks 0.4, 0.9, 1.3, 2.8, 3.7 g/kg from 5–8 weeks | The redox status in the blood of turkeys is observed to deteriorate in unsupplemented diets. However, the highest and lowest dietary Met levels showed similar effects on antioxidant parameters. Therefore, their results were inconclusive and ambiguous. |
| [92] Arbor Acres and partridge shank (AA and PS) | MS (Low = LW, adequate AM and High HM) LW 0.0/0.0%, AM 0.15/0.13%, HM 0.30/0.26% (1–21/22–42 d, respectively) | The HM diets indicated strain-dependent changes in oxidative muscle status, AA broilers had high TAC, while PS broilers had increased MDA and GPx activity, but the SOD was high in both strains. |
| [93] Geese | In ovo injection of disaccharide (DS) and or Met DS injection (25 g/L maltose + 25 g/L sucrose + 7.5 g/L NaCl), Met injection (5 g/L Met + 7.5 g/L NaCl), or DS plus Met injection (25 g/L maltose + 25 g/L sucrose + 5 g/L Met + 7.5 g/L NaCl) | In ovo injection of Met enhanced serum uric acid, GSH, and glutathione peroxidase concentrations as well as lower GSSG/GSH ratio, serum glutathione disulfide (GSSG), and malondialdehyde (MDA) concentration than the noninjected group on the day of hatch. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kachungwa Lugata, J.; Ortega, A.D.S.V.; Szabó, C. The Role of Methionine Supplementation on Oxidative Stress and Antioxidant Status of Poultry-A Review. Agriculture 2022, 12, 1701. https://doi.org/10.3390/agriculture12101701
Kachungwa Lugata J, Ortega ADSV, Szabó C. The Role of Methionine Supplementation on Oxidative Stress and Antioxidant Status of Poultry-A Review. Agriculture. 2022; 12(10):1701. https://doi.org/10.3390/agriculture12101701
Chicago/Turabian StyleKachungwa Lugata, James, Arth David Sol Valmoria Ortega, and Csaba Szabó. 2022. "The Role of Methionine Supplementation on Oxidative Stress and Antioxidant Status of Poultry-A Review" Agriculture 12, no. 10: 1701. https://doi.org/10.3390/agriculture12101701
APA StyleKachungwa Lugata, J., Ortega, A. D. S. V., & Szabó, C. (2022). The Role of Methionine Supplementation on Oxidative Stress and Antioxidant Status of Poultry-A Review. Agriculture, 12(10), 1701. https://doi.org/10.3390/agriculture12101701






