Bio-Stimulants Extend Shelf Life and Maintain Quality of Okra Pods
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Preparation of Bio-Stimulants Solutions
2.3. Measurements
2.3.1. Weight Loss %
2.3.2. Appearance
2.3.3. Firmness kg/cm2
2.3.4. Decay %
2.3.5. Total Soluble Solids (TSS) and pH
2.3.6. Proximate Composition
2.3.7. Ascorbic Acid (V.C)
2.3.8. Total Phenolic Compounds (TPC)
2.3.9. Antioxidant Activity
2.3.10. Carotenoids and Chlorophyll Pigments
2.4. Statistical Analysis
3. Results
3.1. Effects of Bio-Stimulants, Storage Period and Storage Temperature on Weight Loss, Appearance, Firmness, Decay, TSS and pH Traits of Okra Pods
3.1.1. Weight Loss %
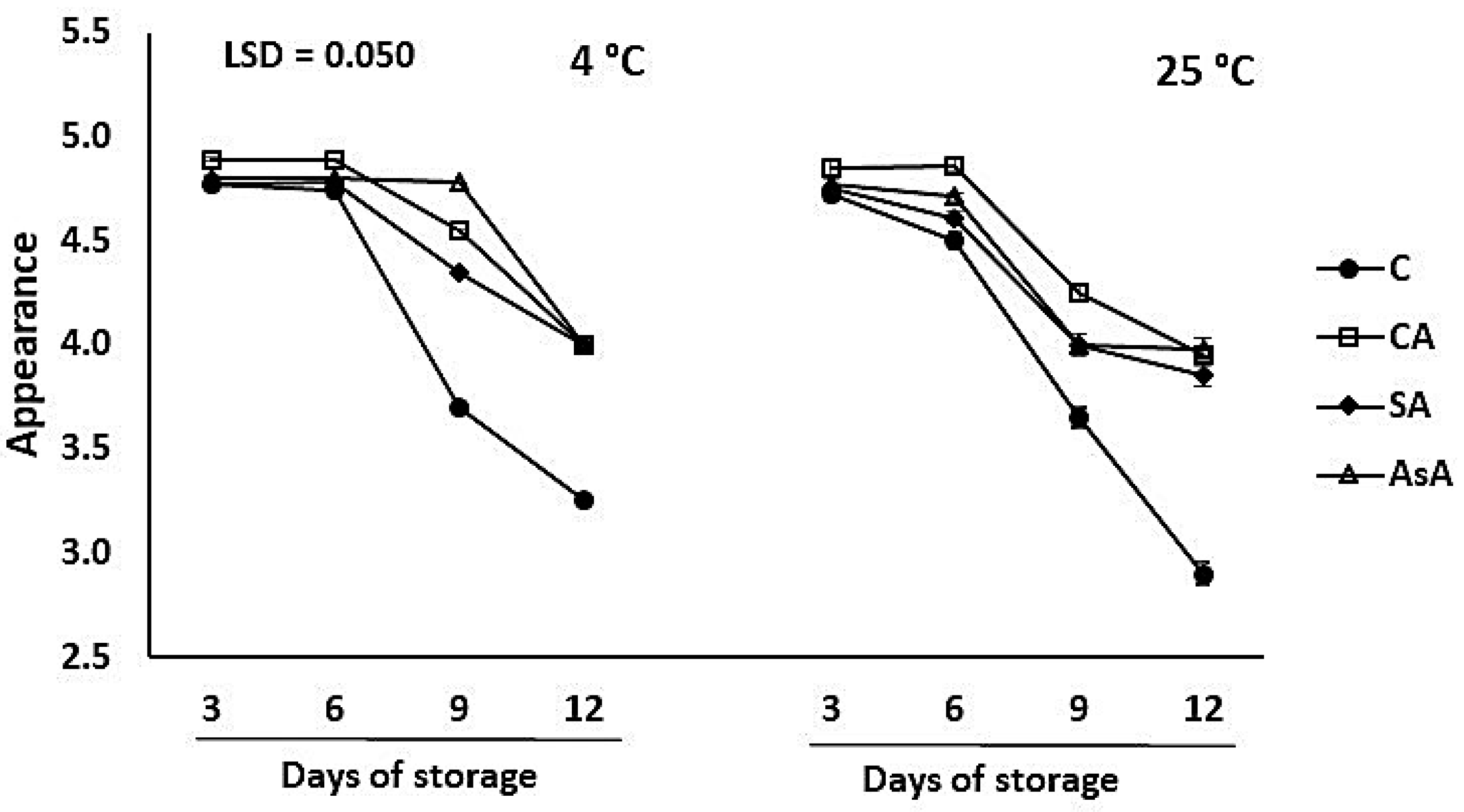
3.1.2. Appearance
3.1.3. Firmness kg/cm2
3.1.4. Decay %
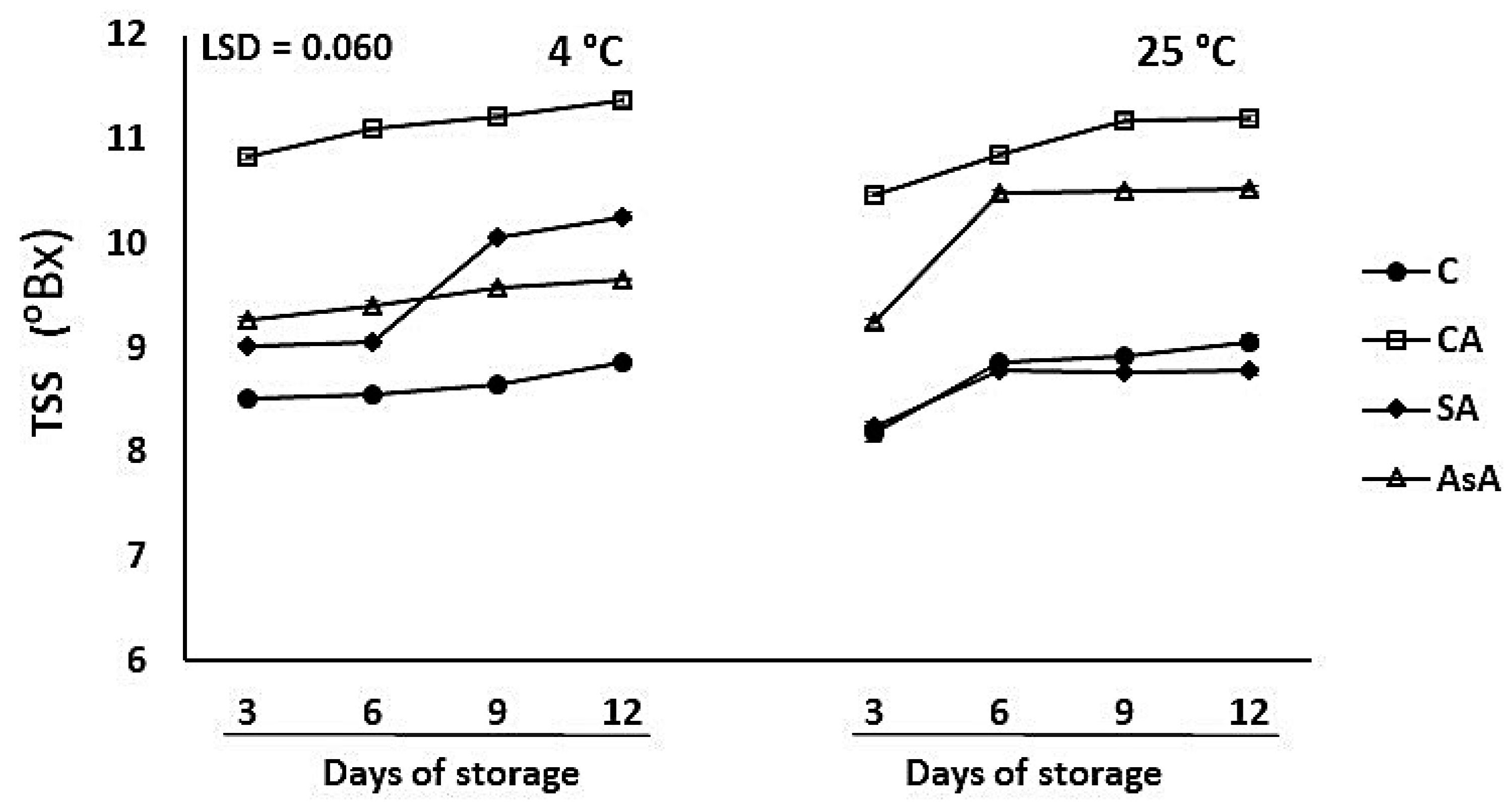
3.1.5. Total Soluble Solids (TSS)
3.1.6. pH
3.2. Effects of Bio-Stimulants and Storage Temperature on Nutritional Value and Biochemical Traits of Okra Pods Stored for 12 Days
3.2.1. Moisture and Ash %
3.2.2. Fat and Fiber Content for Dry Weight %
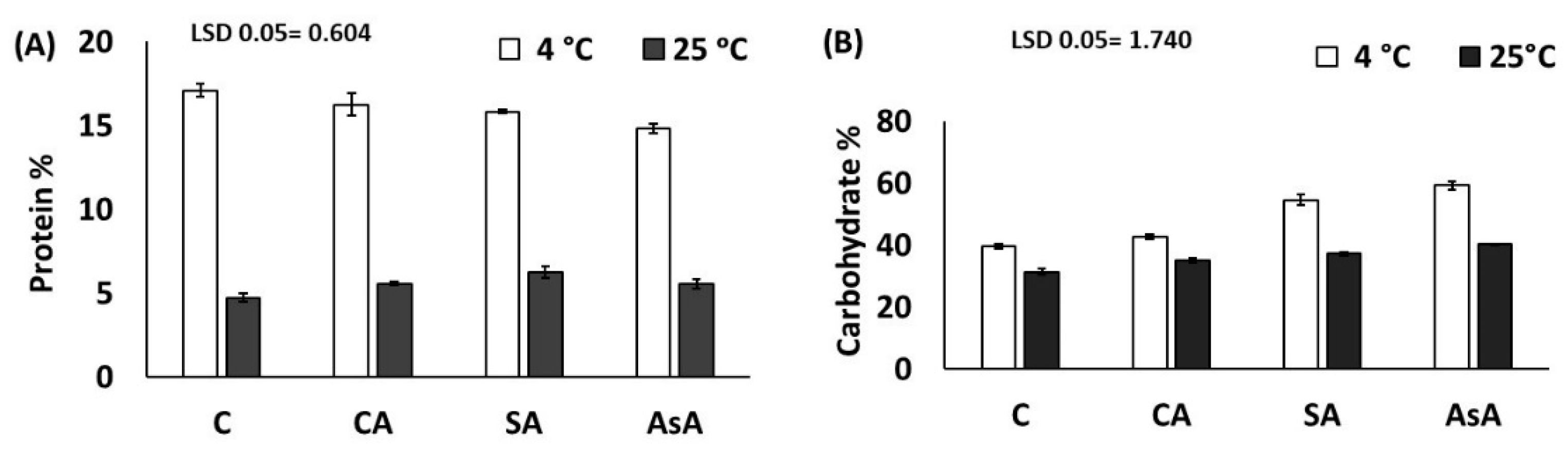
3.2.3. Crude Protein and Carbohydrates % (DW)
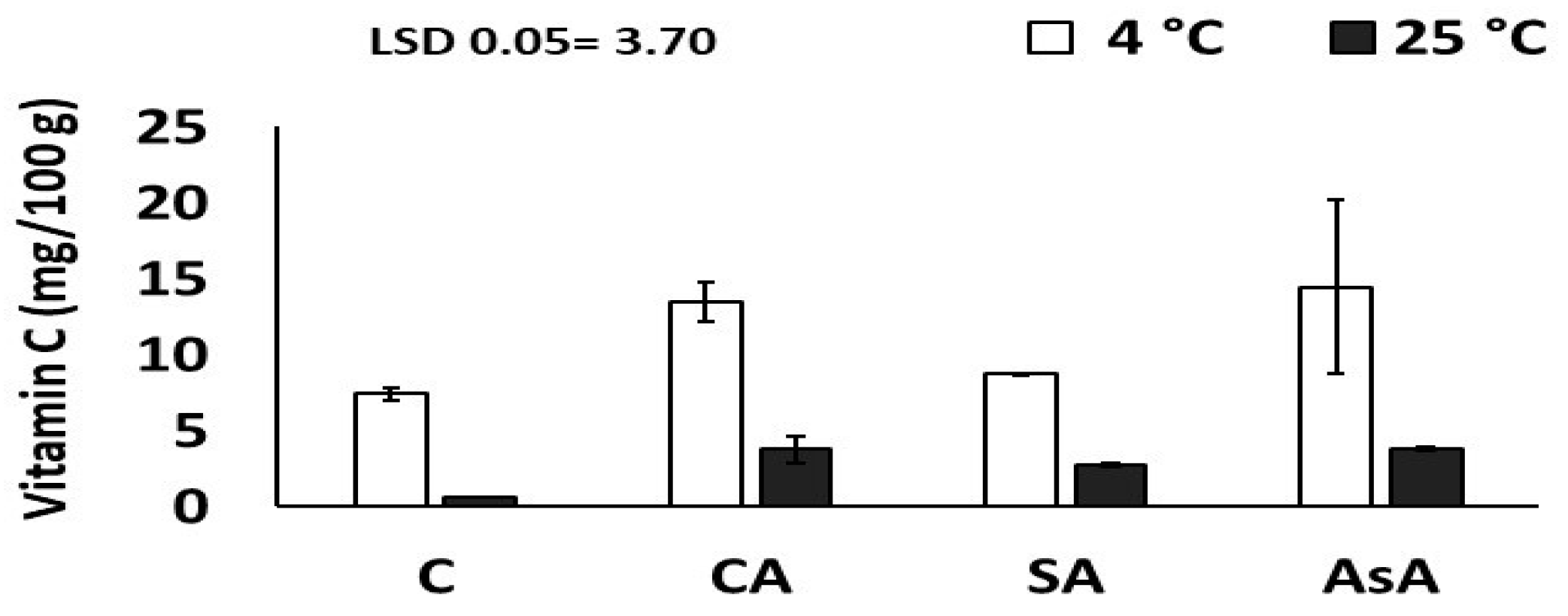
3.2.4. Ascorbic Acid (Vitamin C) (mg/100 g for Fresh Weight FW)
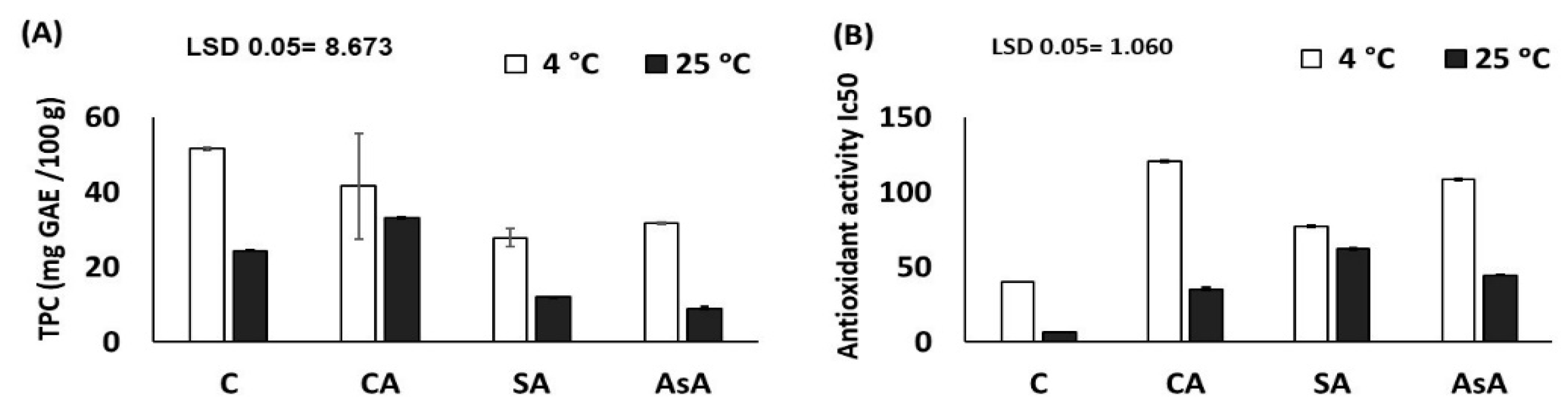
3.2.5. Total Phenolic Compounds TPC (mg GAE/100 g DW) and Antioxidants Activity (IC50/100 g DW)
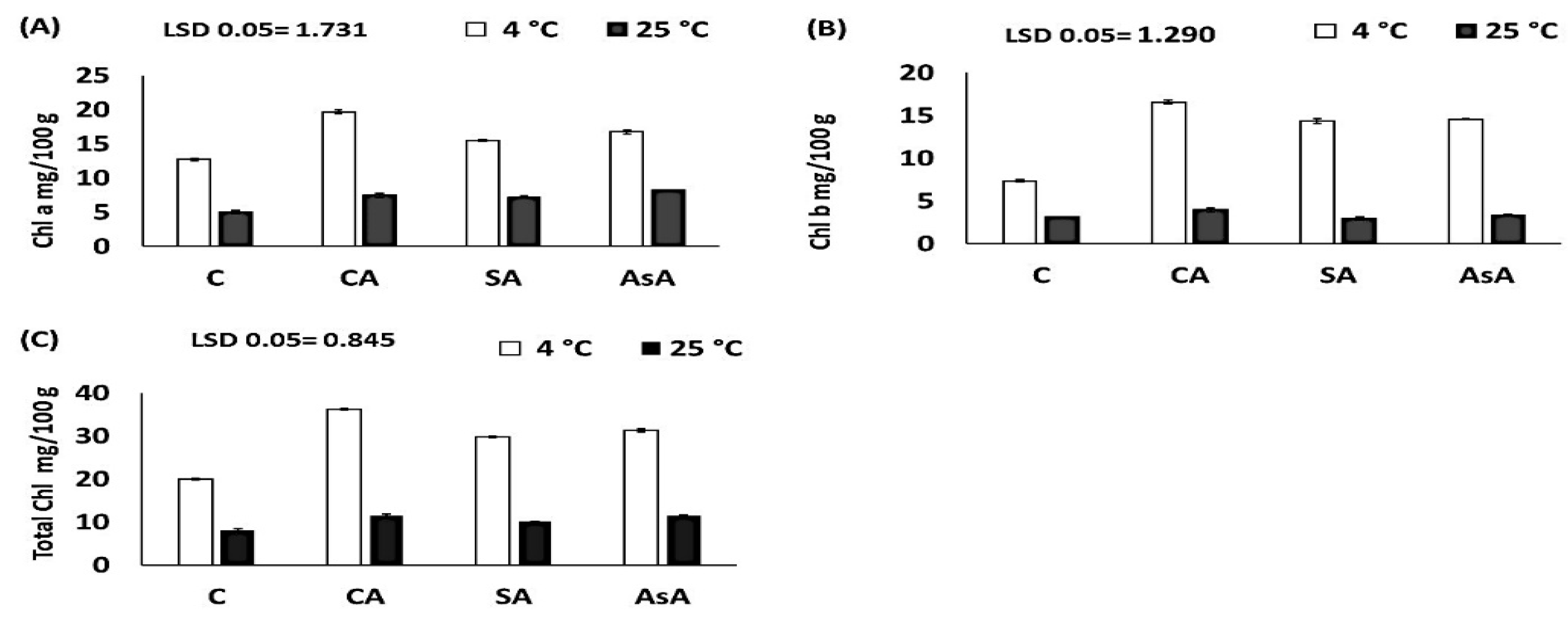
3.2.6. Carotenoids and Chlorophyll Pigments (mg/100 g DW)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dos Santos, R.F.; Gomes-Junior, F.G.; Marcos-Filho, J. Morphological and physiological changes during maturation of okra seeds evaluated through image analysis. Sci. Agric. 2020, 77, 1–9. [Google Scholar] [CrossRef]
- National Research Council. Lost Crops of Africa: Volume II: Vegetables. Crops II; National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Moyin-Jesu, E.I. Use of plant residues for improving soil fertility, pod nutrients, root growth and pod weight of okra (Abelmoschus esculentum L). Bioresour. Technol. 2007, 98, 2057–2064. [Google Scholar] [CrossRef]
- Charrier, A. Genetic Resources of the Genus Abelmoschus Med. (okra); IBPGR: Rome, Italy, 1984. [Google Scholar]
- Kumar, S.; Dagnoko, S.; Haougui, A.; Ratnadass, A.; Pasternak, D.; Kouame, C. Okra (Abelmoschus spp.) in west and central Africa: Potential and progress on its improvement. Afric. J. Agric. Res. 2010, 5, 3590–3598. [Google Scholar]
- Dagawi, A. Technology of Cultivation and Production of Vegetables; The Madbouly Library: Cairo, Egypt, 1996. [Google Scholar]
- Singh, B.P.; Bhatnagar, D.K.; Gupta, O.P. Effect of growth retardants and wax emulsion on the shelf life of okra (Abelmoschus esculentus L.). Haryana J. Hort. Sci. 1978, 7, 91–94. [Google Scholar]
- Huang, H.; Jiang, Y. Effect of plant growth regulators on banana fruit and broccoli during storage. Sci. Hortic. 2012, 145, 62–67. [Google Scholar] [CrossRef]
- Anonymous. Annual Report of Punjab Horticultural Postharvest Technology Center; Punjab Agricultural University: Ludhiana, India, 2004. [Google Scholar]
- Gilmer, P.H.; Carlos, A.L.; Ailton, R. A novel postharvest rot of okra caused by Rhizoctonia solani in Brazil. Fitopatol. Bras. 2007, 32, 237–240. [Google Scholar]
- Abdul Hadi, A.M.; Matlob, A.N.; Yousef, Y.H. Handling and Storage of Fruits and Vegetables; University of Baghdad: Baghdad, Iraq, 1989. [Google Scholar]
- Salunkhe, D.K.; Desai, B.B. Post-Harvest Biotechnology of Fruits. Vol. 1; CRC Press Inc.: Boca Raton, FL, USA, 1984. [Google Scholar]
- Adetuyi, F.O.; Osagie, A.U.; Adekunle, A.T. Antioxidant Degradation in Six Indigenous Okra Abelmoschus esculentus (L.) Moench Varieties During Storage in Nigeria. J. Food Tech. 2008, 6, 227–230. [Google Scholar]
- Joyce, W.N.; Joseph, N.A.; Lim, G. Interactive effect of packing and storage temperature on the shelf life of okra. ARPN J. Agric. Biol. Sci. 2009, 4, 176–184. [Google Scholar]
- Awad, A.; Parmar, A.; Ali, M.; El-Mogy, M.; Abdelgawad, K. Extending the Shelf-Life of Fresh-Cut Green Bean Pods by Ethanol, Ascorbic Acid, and Essential Oils. Foods 2021, 10, 1103. [Google Scholar] [CrossRef] [PubMed]
- El-Mogy, M.M.; Garchery, C.; Stevens, R. Irrigation with salt water affects growth, yield, fruit quality, storability and marker-gene expression in cherry tomato. Acta Agric. Scand. Sect. B —Soil Plant Sci. 2018, 68, 727–737. [Google Scholar] [CrossRef]
- Abdelgawad, K.F.; El-Mogy, M.M.; Mohamed, M.I.A.; Garchery, C.; Stevens, R.G. Increasing Ascorbic Acid Content and Salinity Tolerance of Cherry Tomato Plants by Suppressed Expression of the Ascorbate Oxidase Gene. Agronomy 2019, 9, 51. [Google Scholar] [CrossRef]
- Liu, K.; Yuan, C.; Chen, Y.; Li, H.; Liu, J. Combined effects of ascorbic acid and chitosan on the quality maintenance and shelf life of plums. Sci. Hortic. 2014, 176, 45–53. [Google Scholar] [CrossRef]
- Sikora, M.; Świeca, M. Effect of ascorbic acid postharvest treatment on enzymatic browning, phenolics and antioxidant capacity of stored mung bean sprouts. Food Chem. 2018, 239, 1160–1166. [Google Scholar] [CrossRef]
- El-Mogy, M.M.; Parmar, A.; Ali, M.R.; Abdel-Aziz, M.E.; Abdeldaym, E.A. Improving postharvest storage of fresh artichoke bottoms by an edible coating of Cordia myxa gum. Postharvest Biol. Technol. 2020, 163, 111143. [Google Scholar] [CrossRef]
- Asghari, M.; Aghdam, M.S. Impact of salicylic acid on post-harvest physiology of horticultural crops. Trends Food Sci. Technol. 2010, 21, 502–509. [Google Scholar] [CrossRef]
- Babalar, M.; Asghari, M.; Talaei, A.; Khosroshahi, A. Effect of pre- and postharvest salicylic acid treatment on ethylene production, fungal decay and overall quality of Selva strawberry fruit. Food Chem. 2007, 105, 449–453. [Google Scholar] [CrossRef]
- El-Mogya, M.M.; Alib, M.R.; Darwisha, O.S.; Rogersc, H.J. Impact of salicylic acid, abscisic acid, and methyl jasmonate on postharvest quality and bioactive compounds of cultivated strawberry fruit. J. Berry Rese 2019, 9, 333–348. [Google Scholar] [CrossRef]
- Asghari, M.R.; Hajitagilo, R.; Jalilimarandi, R. Postharvest application of salicylic acid before coating with chitosan affects the pattern of quality changes in table grape during cold storage. In Proceedings of the 6th International Postharvest Symposium, Antalya, Turkey, 8–12 April 2009. [Google Scholar]
- Asghari, M.R.; Hajitagilo, R.; Shirzad, H. Postharvest treatment of salicylic acid effectively controls pear fruit diseases and disorders during cold storage. In Proceedings of the COST Action International Congress on Novel Approaches for the Control of Postharvest Diseases and Disorders, Bologna, Italy, 3–5 May 2007. [Google Scholar]
- Youssef, S.M.; López-Orenes, A.; Ferrer, M.A.; Calderón, A.A. Salicylic-Acid-Regulated Antioxidant Capacity Contributes to Growth Improvement of Okra (Abelmoschus esculentus cv. Red Balady). Agronomy 2022, 12, 168. [Google Scholar] [CrossRef]
- Wills, R.; Lee, T.; Graham, D.; McGlasson, W.; Hall, E. Postharvest. An Introduction to the Physiology and Handling of Fruit and Vegetables; CAB International: Willingford, UK, 1981. [Google Scholar]
- Taain, D.A.; Jasim, A.M.; Al-Hij, J.H.H. A study of storage behavior of okra fruits (Abelmoschus esculentus L. Moenth cv. Khnesri). Int. J. Farming Allied Sci. 2014, 3, 760–766. [Google Scholar]
- Saleh, M.; El-Gizawy, A.; El-Bassiouny, R.; Ali, H. Effects of anti-coloring agents on blackening inhibition and maintaining physical and chemical quality of fresh-cut okra during storage. Ann. Agric. Sci. 2013, 58, 239–245. [Google Scholar] [CrossRef]
- Niketa, P.; Patel, N.L.; Sangani, S.L. Effect of Post-Harvest Treatments, Packaging Materials and Storage Conditions on Shelf Life and Quality of Okra. Int. J. Agric. Sci. 2016, 8, 2601–2605. [Google Scholar]
- Latimer, G.W., Jr. (Ed.) Official Methods of Analysis of AOAC International, 20th ed.; AOAC International: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Manzi, P.; Marconi, S.; Aguzzi, A.; Pizzoferrato, L. Commercial mushrooms: Nutritional quality and effect of cooking. Food Chem. 2004, 84, 201–206. [Google Scholar] [CrossRef]
- Kumar, V.G.; Kumar, A.; Raghu, K.; Patel, G.R.; Manjappa, S. Determination of Vitamin C in Some Fruits and Vegetables in Davanagere City, (Karanataka)–India. Int. J. Pharm. Biol. Sci. 2013, 4, 2489–2491. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Barros, L.; Pereira, E.; Calhelha, R.C.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Bioactivity and chemical characterization in hydrophilic and lipophilic compounds of Chenopodium ambrosioides L. J. Funct. Foods 2013, 5, 1732–1740. [Google Scholar] [CrossRef]
- Nagata, M.; Yamashita, I. Simple Method for Simultaneous Determination of Chlorophyll and Carotenoids in Tomato Fruit. Nippon. Shokuhin Kogyo Gakkaishi 1992, 39, 925–928. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS/STAT 9.4 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2013. [Google Scholar]
- Fayed, T. Effect of some antioxidants on growth, yield and bunch characteristics of Thompson seedless grapevine. Am. Eurasian J. Agric. Environ. Sci. 2010, 8, 322–328. [Google Scholar]
- Cheng, Z.; Gong, X.; Jing, W.; Peng, Z.; Li, J. Quality Change of Postharvest Okra at Different Storage Temperatures. J. Food Eng. 2018, 7, 43–46. [Google Scholar]
- Santi, R.; Bhowmilk, S.R.; Jung, C.P. Shelf life of mature green tomatoes stored in controlled atmosphere and high humidity. J. Food Sci. 1992, 57, 948–951. [Google Scholar]
- Babarinde, G.O.; Fabunmi, O.A. Effects of packaging materials and storage temperature on quality of fresh okra (Abelmoschus esculentus) fruit. Agric. Trop. Subtrop. 2009, 42, 151–156. [Google Scholar]
- Ouda, A.S.; Bahnasawy, A.H.; Khater, E.K. Effect of Storage System and Packages Type on The Self life and Quality of okra. Ann. Agric. Sci. Moshtohor 2021, 59, 883–892. [Google Scholar]
- Eman, A.A.; Kamel, H.M.; Zaki, Z.A.; Rehab, M.A. Effect of honey and citric acid treatments on postharvest quality of fruits and fresh cut of guava. World J. Agric. Sci. 2015, 5, 255–267. [Google Scholar]
- Ali, S.; Khan, A.S.; Malik, A.U.; Anwar, R.; Anjum, M.A.; Nawaz, A.; Shafique, M.; Naz, S. Combined application of ascorbic and oxalic acids delays postharvest browning of litchi fruits under controlled atmosphere conditions. Food Chem. 2021, 350, 129277. [Google Scholar] [CrossRef] [PubMed]
- Sargent, S.A.; Fox, A.J.; Coelho, E.C.; Locascio, S. Comparison of Cooling and Packaging Methods to Extend the Post-harvest Life of Okra. Proc. Fla. Sstate Hortic. Soc. 1996, 109, 285–290. [Google Scholar]
- Xylia, P.; Clark, A.; Chrysargyris, A.; Romanazzi, G.; Tzortzakis, N. Quality and safety attributes on shredded carrots by using Origanum majorana and ascorbic acid. Postharvest Biol. Technol. 2019, 155, 120–129. [Google Scholar] [CrossRef]
- Abdel-Hamid, A.N. Changes in Post-harvest Quality of Menthe and Sage fresh Cut Herbs Treated with Citric acid, Salicylic Acid and Chitosan under Cold storage. Middle East J. 2020, 9, 134–148. [Google Scholar]
- Shehata, S.A.; Abdeldaym, E.A.; Ali, M.R.; Mohamed, R.M.; Bob, R.I.; AbdelGawad, K.F. Effect of Some Citrus Essential Oils on Post-Harvest Shelf Life and Physicochemical Quality of Strawberries during Cold Storage. Agronomy 2020, 10, 1466. [Google Scholar] [CrossRef]
- Shehata, S.A.; Abdelrahman, S.Z.; Megahed, M.M.A.; Abdeldaym, E.A.; El-Mogy, M.M.; Abdelgawad, K.F. Extending Shelf Life and Maintaining Quality of Tomato Fruit by Calcium Chloride, Hydrogen Peroxide, Chitosan, and Ozonated Water. Horticulturae 2021, 7, 309. [Google Scholar] [CrossRef]
- Barzegar, T.; Fateh, M.; Razavi, F. Enhancement of postharvest sensory quality and antioxidant capacity of sweet pepper fruits by foliar applying calcium lactate and ascorbic acid. Sci. Hortic. 2018, 241, 293–303. [Google Scholar] [CrossRef]
- Mohammadi, S.; Pourakbar, L.; Moghaddam, S.S.; Popović-Djordjević, J. The effect of EDTA and citric acid on biochemical processes and changes in phenolic compounds profile of okra (Abelmoschus esculentus L.) under mercury stress. Ecotoxicol. Environ. Saf. 2021, 208, 111607. [Google Scholar] [CrossRef] [PubMed]
- Mostofi, Y.; Toivonen, P.M.; Lessani, H.; Babalar, M.; Lu, C. Effects of 1-methylcyclopropene on ripening of greenhouse tomatoes at three storage temperatures. Postharvest Biol. Technol. 2003, 27, 285–292. [Google Scholar] [CrossRef]
- Yang, C.; Chen, T.; Shen, B.; Sun, S.; Song, H.; Chen, D.; Xi, W. Citric acid treatment reduces decay and maintains the postharvest quality of peach (Prunus persica L.) fruit. Food Sci. Nutr. 2019, 7, 3635–3643. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Liu, J.; Li, H.; Yuan, C.; Zhong, J.; Chen, Y. Influence of postharvest citric acid and chitosan coating treatment on ripening attributes and expression of cell wall related genes in cherimoya (Annona cherimola Mill.) fruit. Sci. Hortic. 2016, 198, 1–11. [Google Scholar] [CrossRef]
- Perkins-Veazie, P.; Collins, J.K. Cultivar, Packaging, and Storage Temperature Differences in Postharvest Shelf Life of Okra. Hort. Technol. 1992, 2, 3. [Google Scholar] [CrossRef]
- Waskar, D.P.; Yadav, B.B.; Garande, V.K. Influence of various packaging materials on storage behaviour of bottle gourd under different storage conditions. Indian J. Agric. Res. 1999, 33, 287–292. [Google Scholar]
- Pal, R.K.; Roy, S.K. Zero-energy cool chamber for maintaining post-harvest quality of carrot (Daucus carota). Indian J. Agric. Sci. 1988, 58, 665–667. [Google Scholar]
- Sandooja, J.K.; Sharma, R.K.; Pandit, M.L.; Batra, B.R. Storage studies to tomato in zeroenergy cool chamber in relation to storage of maturity and packaging material used. Haryana Agric. Univ. J. Res. 1987, 17, 216–217. [Google Scholar]
- Shehata, S.A.; El-Mogy, M.M.; Hanaa, F.Y.M. Postharvest quality and nutrient contents of long sweet pepper enhanced by supplementary potassium foliar application. Int. J. Veg. Sci. 2019, 25, 196–209. [Google Scholar] [CrossRef]
- Shehata, S.A.; AbdelGawad, K.F.; Elmogy, M. Quality and Shelf-life of Onion Bulbs Influenced by Bio-stimulants. Int. J. Veg. Sci. 2017, 23, 362–371. [Google Scholar] [CrossRef]
- Menzel, C.M. Effect of Temperature on Soluble Solids Content in Strawberry in Queensland, Australia. Horticulturae 2022, 8, 367. [Google Scholar] [CrossRef]
- Yin, C.; Huang, C.; Wang, J.; Liu, Y.; Lu, P.; Huang, L. Effect of Chitosan- and Alginate-Based Coatings Enriched with Cinnamon Essential Oil Microcapsules to Improve the Postharvest Quality of Mangoes. Materials 2019, 12, 2039. [Google Scholar] [CrossRef] [PubMed]
- Razavi1, F.; Hajilou, J.; Dehgan, G.; Hassani, R.N.B.; Turchi, M. Enhancement of postharvest quality of peach fruit by salicylic acid treatment. Int. J. Biosci. 2014, 4, 177–184. [Google Scholar]
- Adetuyi, F.; Osagie, A. Nutrient, antinutrient, mineral and zinc bioavailability of okra Abelmoschus esculentus (L) Moench Variety. Am. J. Food Nutr. 2011, 1, 49–54. [Google Scholar] [CrossRef]
- Nwachukwu, E.C.; Nulit, R.; Go, R. Nutritional and biochemical properties of Malaysian okra variety. Adv. Med. Plant Res. 2014, 2, 16–19. [Google Scholar]
- Nwofia, G.; Victoria, N.N.; Blessing, K.N. Nutritional Variation in Fruits and Seeds of Pumpkins (Cucurbita Spp) Accessions from Nigeria. Pak. J. Nutr. 2012, 11, 946–956. [Google Scholar] [CrossRef]
- Aruah, B.C.; Uguru, M.I.; Oyiga, B.C. Genetic variability and inter-relationship among some Nigerian pumpkin accessions (Cucurbita spp). Int. J. Plant Breed. 2012, 6, 34–41. [Google Scholar]
- Gemede, H.F.; Haki, G.D.; Beyene, F.; Woldegiorgis, A.Z.; Rakshit, S.K. Proximate, Mineral and Antinutrient Compositions of Indigenous Okra (Abelmoschus esculentus) Pod Accessions: Implications for Mineral Bioavailability. J. Nutr. Food Sci. 2016, 4, 223–233. [Google Scholar] [CrossRef]
- Combo, A.M.-M.; Dakia, P.A.; Niaba, K.P.V.; Traorã©, N.; Beugrã©, G.A.M. Assessment of Chemical Composition and Nutritional Value of Some Varieties of Okra Available in the Market of Daloa (Côte d’Ivoire). Asian J. Agric. Food Sci. 2020, 8, 18–27. [Google Scholar] [CrossRef]
- Romdhane, M.H.; Chahdoura, H.; Barros, L.; Dias, M.I.; Corrêa, R.C.G.; Morales, P.; Ciudad-Mulero, M.; Flamini, G.; Majdoub, H.; Ferreira, I.C. Chemical composition, nutritional value, and biological evaluation of Tunisian okra pods (Abelmoschus esculentus L. Moench). Molecules 2020, 25, 4739. [Google Scholar] [CrossRef]
- Gunawardhana, M.; De Silva, C. Impact of Temperature and Water Stress on Growth Yield and Related Biochemical Parameters of Okra. Trop. Agric. Res. 2011, 23, 77. [Google Scholar] [CrossRef]
- Cachero, T.; Belonias, B.S. Morpho-anatomical and physiological changes of okra (Abelmoschus esculentus L. Moench var. ‘Smooth Green’) fruit and seed during development. J. Sci. Humanit. 2017, 11, 40–63. [Google Scholar] [CrossRef]
- Shi, Q.; Bao, Z.; Zhu, Z.; Ying, Q.; Qian, Q. Effects of Different Treatments of Salicylic Acid on Heat Tolerance, Chlorophyll Fluorescence, and Antioxidant Enzyme Activity in Seedlings of Cucumis sativa L. Plant Growth Regul. 2006, 48, 127–135. [Google Scholar] [CrossRef]
- Lee, S.K.; Kader, A.A. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar] [CrossRef]
- Kader, A.; Morris, L. Prompt handling reduces processing-tomato losses. Calif. Agric. 1978, 32, 21–22. [Google Scholar]
- Allahveran, A.; Farokhzad, A.; Asghari, M.; Sarkhosh, A. Foliar application of ascorbic and citric acids enhanced ‘Red Spur’ apple fruit quality, bioactive compounds and antioxidant activity. Physiol. Mol. Biol. Plants 2018, 24, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Sreeramulu, D.; Reddy, C.V.K.; Chauhan, A.; Balakrishna, N.; Raghunath, M. Natural Antioxidant Activity of Commonly Consumed Plant Foods in India: Effect of Domestic Processing. Oxidative Med. Cell. Longev. 2013, 2013, 369479. [Google Scholar] [CrossRef]
- Balasubramanian, T.; Sadasivam, S. Changes in starch, oil, protein and amino acids in developing seeds of okra (Abelmoschus esculentus L. Moench). Plant Foods Hum. Nutr. 1987, 37, 41–46. [Google Scholar] [CrossRef]
- Martínez Vidal, J.L.; González-Rodríguez, M.J.; Arrebola, F.J.; Garrido Frenich, A.; Sánchez López, F.J.; Díez, N.M. Selective extraction and determination of multiclass pesticide residues in post-harvest French beans by low-pressure gas chromatography/tandem mass spectrometry. J. AOAC Int. 2003, 86, 856–867. [Google Scholar] [CrossRef]







| Trait | MS | F (3,92) | p Value |
|---|---|---|---|
| Weight Loss % | 2.47 | 0.93 | 0.43 |
| Appearance % | 1.22 | 5.13 | 0.003 ** |
| Firmness (Kg/cm) | 0.10 | 0.18 | 0.91 |
| Decay % | 31.61 | 5.46 | 0.002 ** |
| TSS % | 24.98 | 112.76 | <0.001 *** |
| pH | 0.17 | 10.67 | <0.001 ** |
| Traits | Mean ± SD | Compared Group | p Value |
|---|---|---|---|
| Weight loss % | C 3.2 ± 2.0 CA 2.5 ± 1.6 SA 2.7 ± 1.5 AsA 2.6 ± 1.5 | AsA vs. C CA vs. C SA vs. C | NS |
| Appearance | C 4.0 ± 0.71 CA 4.5 ± 0.39 SA 4.4 ± 0.38 AsA 4.5 ± 0.39 | AsA vs. C CA vs. C SA vs. C | 0.01 ** |
| 0.00 ** | |||
| 0.03 * | |||
| Firmness | C 2.7 ± 0.71 CA 2.8 ± 0.73 SA 2.7 ± 0.74 AsA 2.8 ± 0.70 | AsA vs. C CA vs. C SA vs. C | NS |
| Decay % | C 8.1 ± 2.7 CA 5.4 ± 2.3 SA 6.2 ± 2.2 AsA 6.1 ± 0.55 | AsA vs. C CA vs. C SA vs. C | 0.02 * |
| 0.00 *** | |||
| 0.02 * | |||
| TSS % | C 8.7 ± 0.27 CA 11.0 ± 0.28 SA 9.1 ± 0.66 AsA 9.8 ± 0.55 | AsA vs. C CA vs. C SA vs. C | <0.00 *** |
| <0.00 *** | |||
| 0.01 ** | |||
| pH | C 3.9 ± 0.09 CA 3.7 ± 0.16 SA 3.8 ± 0.12 AsA 3.8 ± 0.13 | AsA vs. C CA vs. C SA vs. C | 0.03 * |
| <0.001 *** | |||
| 0.09 |
| Trait | MS | F(3,20) | P value |
|---|---|---|---|
| Moisture % | 73.26 | 0.78 | 0.52 |
| Ash % | 0.62 | 1.60 | 0.22 |
| Fat % | 6.79 | 3.24 | 0.044 * |
| Fiber % | 12.73 | 16.58 | <0.001 *** |
| Protein% | 0.91 | 0.03 | 0.994 |
| Carbohydrate % | 254.80 | 4.24 | 0.017 * |
| Vit. C | 35.23 | 1.38 | 0.28 |
| TPC | 615.82 | 4.47 | 0.015 * |
| Antioxidant | 4029.0 | 4.24 | 0.018 * |
| β-Carotene | 165.78 | 0.76 | 0.53 |
| Lycopene | 30.93 | 4.79 | 0.01 * |
| Chlorophyll a | 24.24 | 0.93 | 0.44 |
| Chlorophyll b | 27.74 | 0.85 | 0.48 |
| Total Chlorophyll | 102.87 | 0.89 | 0.46 |
| Trait | Mean ± SD | Compared Groups | P Value |
|---|---|---|---|
| Moisture % | C 74.4 ± 12.7 CA 80.8 ± 8.8 SA 79.4 ± 8.7 AsA 82.5 ± 7.8 | AsA vs. C | NS |
| CA vs. C | |||
| SA vs. C | |||
| Ash % | C 8.2 ± 1.0 CA 8.7 ± 0.55 SA 8.8 ± 0.44 AsA 8.9 ± 0.30 | AsA vs. C | NS |
| CA vs. C | |||
| SA vs. C | |||
| Fat | C 3.1 ± 1.9 CA 4.6 ± 1.6 SA 2.9 ± 1.4 AsA 5.0 ± 0.30 | AsA vs. C | 0.09 * |
| CA vs. C | 0.22 | ||
| SA vs. C | 0.97 | ||
| Fiber | C 20.1 ± 0.52 CA 17.2 ± 1.4 SA 19.6 ± 0.87 AsA 20.4 ± 0.30 | AsA vs. C | 0.95 |
| CA vs. C | <0.00 *** | ||
| SA vs. C | 0.60 | ||
| Protein % | C 10.9 ± 6.8 CA 10.9 ± 5.9 SA 11.0 ± 5.2 AsA 10.2 ± 5.1 | AsA vs. C | NS |
| CA vs. C | |||
| SA vs. C | |||
| Carbohydrate | C 35.5 ± 4.6 CA 38.8 ± 4.2 SA 45.9 ± 9.6 AsA 49.8 ± 10.5 | AsA vs. C | 0.01 * |
| CA vs. C | 0.80 | ||
| SA vs. C | 0.08 | ||
| Vitamin C | C 4.0 ± 3.8 CA 8.6 ± 5.4 SA 5.7 ± 3.3 AsA 9.1 ± 6.9 | AsA vs. C | NS |
| CA vs. C | |||
| SA vs. C | |||
| TP | C 38.0 ± 14.8 CA 37.3 ± 10.1 SA 19.9 ± 8.7 AsA 20.4 ± 12.4 | AsA vs. C | 0.04 * |
| CA vs. C | 1.0 | ||
| SA vs. C | 0.04 * | ||
| Antioxidant | C 23.3 ± 18.3 CA 77.8 ± 46.7 SA 69.7 ± 8.3 AsA 76.5 ± 35.0 | AsA vs. C | 0.02 * |
| CA vs. C | 0.02 * | ||
| SA vs. C | 0.04 * | ||
| β-Carotene | C 9.9 ± 9.4 CA 22.7 ± 18.7 SA 16.1 ± 15.3 AsA 17.1 ± 14.1 | AsA vs. C | NS |
| CA vs. C | |||
| SA vs. C | |||
| Lycopene | C 0.80 ± 0.66 CA 2.0 ± 1.9 SA 3.4 ± 2.3 AsA 6.1 ± 4.1 | AsA vs. C | 0.01 ** |
| CA vs. C | 0.74 | ||
| SA vs. C | 0.23 | ||
| Chlorophyll a | C 8.9 ± 4.2 CA 13.6 ± 6.7 SA 11.3 ± 4.5 AsA 12.5 ± 4.7 | AsA vs. C | NS |
| CA vs. C | |||
| SA vs. C | |||
| Chlorophyll b | C 5.2 ± 2.3 CA 10 ± 0.39 SA 8.7 ± 6.2 AsA 8.9 ± 6.2 | AsA vs. C | NS |
| CA vs. C | |||
| SA vs. C | |||
| Total Chlorophyll | C 14.1 ± 0 CA 23.8 ± 13.6 SA 20.0 ± 10.8 AsA 21.4 ± 10.8 | AsA vs. C | NS |
| CA vs. C | |||
| SA vs. C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Shaieny, A.-H.A.H.; Abd-Elkarim, N.A.A.; Taha, E.M.; Gebril, S. Bio-Stimulants Extend Shelf Life and Maintain Quality of Okra Pods. Agriculture 2022, 12, 1699. https://doi.org/10.3390/agriculture12101699
El-Shaieny A-HAH, Abd-Elkarim NAA, Taha EM, Gebril S. Bio-Stimulants Extend Shelf Life and Maintain Quality of Okra Pods. Agriculture. 2022; 12(10):1699. https://doi.org/10.3390/agriculture12101699
Chicago/Turabian StyleEl-Shaieny, Abdel-Haleem A. H., Naglaa A. A. Abd-Elkarim, Eman M. Taha, and Sayed Gebril. 2022. "Bio-Stimulants Extend Shelf Life and Maintain Quality of Okra Pods" Agriculture 12, no. 10: 1699. https://doi.org/10.3390/agriculture12101699
APA StyleEl-Shaieny, A.-H. A. H., Abd-Elkarim, N. A. A., Taha, E. M., & Gebril, S. (2022). Bio-Stimulants Extend Shelf Life and Maintain Quality of Okra Pods. Agriculture, 12(10), 1699. https://doi.org/10.3390/agriculture12101699






