Abstract
In recent years, studies on the potential use of essential oils (EOs) as pesticides have enormously increased owing to their remarkable biological activities and health benefits. However, given the scant knowledge on the mode(s) of action behind insecticidal activity of individual essential oils, as well as their mixtures, much more work has yet to be undertaken. Furthermore, the variable and complex mixtures of essential oils suggest that their biological activities are likely due to several mechanisms acting on different physiological processes. Here, we firstly assessed the toxicity of Illicium verum, Myristica fragrans and Schinus molle EOs on Drosophila suzukii adults. Then, their acetylcholinesterase inhibitory activity as a potential mode of action was investigated. Subsequently, we explored potential structural alterations caused by exposure to low concentrations (LC20 and LC50) of I. verum oil on the fat body, midgut and muscular tissues of female flies. The results showed that the three EOs had good insecticidal activity against D. suzukii flies and although I. verum oil was more toxic than the two others, its acetylcholinesterase inhibition was the lowest. However, exposure to I. verum EO promoted severe concentration-dependent histological and structural alterations in the carbohydrate contents, muscle fiber, midgut epithelium as well as fat droplets area of exposed females. Collectively, our findings revealed that the insect’s internal organs are potential target sites of I. verum EO’s acute toxicity. Further studies are needed to confirm I. verum as a promising insecticidal compound against insect pests, to better elucidate its physiological and molecular action sites as well as assess its toxicity to non-target organisms.
1. Introduction
In the agricultural sector, studies on plant-derived compounds have focused on obtaining new insecticides against novel molecular targets [1]. Attention has focused on the search for alternative control methods that are environmentally friendly, sustainable and effective [2]. In this sense, products of plant origin have gained prominence in research, especially essential oils, due to the availability of raw materials and the good cost–benefit ratio [3].
Essential oils (EOs) are complex mixtures of volatile organic compounds produced as secondary metabolites in plants. In recent years, the use of EOs and their constituents as low-risk insecticides, insect repellents, and oviposition deterrents has increased considerably owing to their popularity among organic growers and environmentally conscious consumers. EOs extracted from plant seeds, stems, leaves, and flowers have been widely tested for their fungicidal, bactericidal, and insecticidal properties [4]. They have yielded promising results under laboratory conditions and are being considered an important alternative strategy for sustainable insect pest management [5,6,7]. These botanical insecticides have shown strong insecticidal, repellent and fumigant activity against many insect pest species [4,8,9,10,11,12,13].
The mechanisms of the toxic action of EOs have not been fully elucidated, to date. In many cases, visible symptoms of the insecticidal activity of EOs such as hyperactivity, convulsions, tremors and paralysis suggest a neurotoxic mode of action [14]. Such symptoms indicate that the potential target site of these EOs may be the insect’s neural signaling, acting on the enzyme acetylcholinesterase, GABA ionotropic receptors and, more evidently, octopamine metabotropic receptors [15]. Moreover, as EOs are generally mixtures of many compounds, their mechanism of action is usually not specific to one target but includes a variety of actions. Thus, the intrinsic properties of EOs can interfere with basic metabolic, biochemical, and physiological functions of insect pests and their ability to reduce and suppress the activity of detoxifying enzymes have been frequently reported [3]. Besides metabolic and physiological alteration, the action of EOs may produce histological modifications. Previous studies have reported several histopathological changes in insects exposed to EOs from different plants. Such changes can help to elucidate mechanisms involved in the insecticidal action of EOs [16,17,18,19,20].
Drosophila suzukii (Diptera: Drosophilidae) is a species of fly that has great potential to cause economic damage [21,22]. These insects can infest a wide variety of hosts, such as cultivated and wild red-fruit species [23,24]. It is a native species from East Asia and was first reported in Brazil in the summer of 2012/2013 in the southern region of the country [25]. Males differ from females by the presence of a dark spot near the edge of each wing, a characteristic that gives rise to the popular name of spotted-wing fly [26]. Females have serrated ovipositor apparatus that allow them to infest immature and undamaged fruits before harvesting, a unique feature among members of the Drosophila genus [24,27]. The use of synthetic insecticides is an alternative to minimize the economic damage generated by this species despite their disadvantages such as toxicity to non-target organisms and relatively high cost [28]. In this sense, studies on EOs have shown to be promising for the management of different pest arthropods [29,30]. This fact can be very important for the management of D. suzukii, considered the primary pest of small fruits in Brazil [25]. However, the effect of EOs from plants of families such as Schisandraceae, Myristicaceae and Anacardiaceae has not yet been evaluated in D. suzukii. Furthermore, the effects of low concentrations of EOs on the internal morphology of these flies have not yet been explored.
The star anise Illicium verum belongs to the Schisandraceae family, native to tropical and subtropical regions of Asia, and it is used as a spice [31]. Myristica fragrans belongs to the Myristicaceae family, its ovoid seed is used as a condiment and it is popularly known as nutmeg [32]. Schinus molle belongs to the Anacardiaceae family, native to subtropical regions of South America, and is commonly called pink pepper. These plants stand out for their broad range of biological activities such as antibacterial [33], antifungal [34] and antioxidant [35]. Additionally, the insecticidal activity of these species has already been reported for several pest insects [36,37,38]. However, the effects of their essential oil on the control of D. suzukii have not yet been explored, to the best of our knowledge.
The present study aimed firstly to investigate the lethal effects of the EOs from Illicium verum, Myristica fragrans and Schinus molle, as well as their inhibitory activity of acetylcholinesterase enzyme on D. suzukii adults. Secondly, the histopathological alterations caused by the exposure to low concentrations of I. verum oil in the thoracic muscles, midgut and fat body of adult flies were assessed as potential alternative targets for this oil toxic action.
2. Material and Methods
2.1. Plant Material and Insect Population
The plant material used in the present study consisted of leaves of S. molle collected from trees growing on the Campus of the Federal University of Lavras (UFLA) (21°14′ latitude S, 45°00′ longitude W) in Minas Gerais state (Brazil). A voucher was deposited in the Herbarium of the Federal University of Alfenas, receiving the code HUNIFAL298. Seeds of M. fragrans (CHAMEL, Campo Largo—PR; Brazil) and fruits of I. verum (CHAMEL, Campo Largo—PR; Brazil) were obtained from the local market (21°24′ latitude S, 44°99′ longitude W). The plant materials were cut into smaller parts for subsequent extraction of the EO.
The D. suzukii adult flies used in the bioassays were obtained from a stock colony kept under controlled conditions (i.e., temperature: 25 ± 2 °C; relative humidity: 60 ± 5%; photoperiod: 12 h L:12 h D) in the Molecular Entomology and Ecotoxicology Laboratory of Entomology Department (DEN) at UFLA. Rearing was carried out on an artificial diet and followed previously described methods [39,40,41].
2.2. Essential Oils Extraction and Chemical Characterization
Extraction of the EOs was performed by hydrodistillation in a modified Clevenger apparatus and was carried out at the Laboratory of Organic Chemistry and Essential Oils of the Chemistry Department (DQI) at UFLA. For this purpose, 630 g of I. verum, 143 g of M. fragrans and 901 g of S. molle were added to a 5 L round-bottom flask half-filled with water. After 2 h of gentle boiling, the hydrolate was collected and subjected to centrifugation at 965× g for 15 min. Subsequently, the EO was pipetted and placed in a suitable container in the absence of light and under refrigeration (<5 °C) [42] until used in the experiments. Extractions were performed in triplicate and extraction yield was calculated by the difference in moisture-free base (%w/w BLU) [43].
The identification of chemical constituents was performed by gas chromatography coupled to mass spectrometry (GC-MS), using a model QP 2010 Plus equipment (Shimadzu Corporation, Kyoto, Japan) operating with a capillary column of fused silica (30 m × 0.25 mm) with a DB-5 bound phase (film thickness, 0.25 μm). Helium was used as carrier gas at a flow of 1.0 mL min−1. Injector and detector temperatures were 220 and 240 °C, respectively. The sample injection volume was 0.5 μL, diluted in hexane (1%) (Sigma-Aldrich®, St. Louis, MO, USA) and injection volume partition ratio (split) of 1:100. Temperature ramp started at 60 °C, with an increase at a rate of 3 °C min−1 to 240 °C, followed by an increase at 10 °C min−1 until reaching 300 °C, with the final temperature maintained for 7 min. Column pressure was around 71.0 kPa. The mass spectrometer was operated at ionization potential of 70 eV and ion source temperature of 200 °C. The mass analysis was performed in full-scan mode, ranging from 45 to 500 Da, with a scan speed of 1000 Da s−1 and a scanning interval of 0.5 fragments s−1. Data were obtained and processed using the Lab Solutions LC/GC Workstation 2.72 software(Shimadzu, Kyoto, Japan). The retention index of the compounds was calculated in relation to a homologous series of n-alkanes (nC9–nC18), using the equation of Van den Dool and Kratz [44]. The identification of compounds was performed by comparing the calculated retention indices with those described in the literature [45]. Comparisons of the mass spectra obtained with those existing in the FFNSC 1.2, NIST107 and NIST21 libraries were also performed.
Quantitative analysis was performed by gas chromatography with flame ionization detector (GC-FID), using model GC-2010 equipment (Shimadzu Corporation, Kyoto, Japan), with experimental conditions identical to those used in the qualitative analysis, except the detector temperature, which was 300 °C. The relative percentages of each constituent were obtained by the area normalization method.
2.3. Toxicity Assessment of Essential Oils against Drosophila suzukii
Concentration–mortality bioassays were conducted to determine contact and ingestion lethal activity of the three EOs to D. suzukii adults. The exposure procedure was based on the IRAC (Insecticide Resistance Action Committee) protocol number 026 [46], recommended for bioassays with adults of Musca domestica L. (Diptera: Muscidae), with minor modifications. Briefly, dental cotton (2 cm) treated with 1.9 mL of the solution composed of dimethyl sulfoxide (DMSO) (3% v/v), aqueous sucrose solution (20% w/v) and the EO in serial concentrations was deposited in a glass vial (200 mL). For I. verum EO, concentrations between 0.1–100 μL mL−1 were used, for M. fragrans EO the range was 5–50 μL mL−1 and S. molle 10–150 μL mL−1. As a negative control, a 2% v/v DMSO solution was used. For each repetition, twenty-five non-sexed, same-age insects were introduced into the glass bottles, and the bottles were closed with foam plugs and kept in a BOD incubator at 24 ± 2 °C and 50% RH, with a 12 h L:12 h D photoperiod. After 24 h, mortality was assessed visually. Flies that showed no movement even after stimulation with a fine brush were considered dead.
2.4. Acetylcholinesterase Inhibitory Activity of Essential Oils
The AChE inhibitory activity of the three EOs was assessed using a modified version of Ellman’s method [47]. The method used consists of monitoring the rate of formation of the chromophore compound 5-thio-2-nitrobenzoate produced from the reaction of Ellman’s Reagent (5,5-dithiobis-2-nitrobenzoic acid or its ionized form) with thiocholine. Thiocholine is obtained by the hydrolysis of acetylthiocholine (substrate) catalyzed by the enzyme acetylcholinesterase (AChE) (0.04 U mL−1; type VI-S) from Electrophorus electricus.
In a test tube, 2970 µL of the Tris-HCl buffer (50 mmol L−1), pH 8, was firstly mixed with 254 µL of the acetylthiocholine solution (1000 U mL−1) and incubated at 37 °C for 15 min. Then, 25 µL of the EO diluted in ethanol at concentrations of 0.25; 0.50; 1.0; 5; 10; 50 and 100 mg mL−1 was added to the mixture followed by the addition of 100 µL of 10 mmol L−1 Ellman’s reagent and 80 µL of the 0.02 mol L−1 substrate solution. The mixture was incubated again at 37 °C for 15 min, and the absorbance was measured on a spectrophotometer at 412 nm. As a blank, 3.2 mL of Tris-HCl buffer was used.
For comparison purposes, carvacrol was used as a positive control at the same concentrations used for EOs. To consider the spontaneous hydrolysis of acetylthiocholine, non-enzymatic controls were performed for each concentration of oil tested, replacing the enzyme with a Tris-HCl buffer. The negative control contained all reagents except the EO, which was replaced by ethanol. The tests were performed in three repetitions and the percentage of enzyme inhibition was calculated according to the following equation:
where I(%) = percent of enzyme inhibition; AT = absorbance of the treatment containing the essential oil/positive control; AC = non-enzymatic control absorbance; and AO = negative control absorbance.
2.5. Evaluation of the Effects of Exposure to Low Doses of Illicium verum Essential Oil on Females’ Histological Structures
Based on the results of toxicity and enzyme inhibition bioassays (see Section 3), the EO of I. verum was chosen to assess potential structural alterations caused by exposure to low concentrations on the fat body, midgut and muscular tissues of female flies.
2.5.1. Histopathological Analysis
For histopathological analysis, D. suzukii pupae were exposed to I. verum EO solutions (LC20 and LC50), determined in the toxicology experiment, and control (water and DMSO aqueous solution at 3% v/v) by submersion for 30 s. Afterward, they were dried with absorbent paper and stored in a container sealed with voile tissue. After emergence, which took approximately five days, five adult females were collected for each treatment and fixed in a 4% paraformaldehyde solution for 72 h and transferred to 70% ethanol solution. Females were chosen for this analysis due to their importance for the propagation of the species and possible greater resistance to insecticidal treatment compared to males [48,49]. The insects were, then, dehydrated using serial concentrations of ethanol (70, 80, 90 and 95%) and soaked in Leica historesin for 24 h at 4 °C before being transferred to plastic molds for inclusion. Subsequently, slides of 4 µm thickness were prepared using a microtome (Luptec MRP09) and stained with Hematoxylin and Eosin [50]. A minimum of two histological glasses containing at least six sections were obtained for each sample. Finally, the midgut, thoracic muscle tissue and fat body of the insects were evaluated under conventional light microscopy to verify possible histopathological changes. We decided to evaluate these tissues due to their abundance in the female’s body as well as their physiological importance.
2.5.2. Histochemical Analysis
To assess changes in factors such as the presence, frequency and distribution of proteins and polysaccharides in the female flies emerging from exposed and unexposed pupae, Periodic Acid-Schiff (polysaccharides) and Bromophenol Blue Stain (proteins) techniques were used. Briefly, for polysaccharide detection, slides containing six sections for each treatment were immersed for 30 min in 0.5% periodic acid, washed with distilled water for 20 min and stained with Schiff’s reagent for 30 min in the dark before being submerged in water for 30 min and dried at room temperature. After drying, the slides were mounted in Entellan® [50]. For protein detection, slides containing six sections for each treatment were stained with bromophenol blue for 2 h at room temperature. Then washed with 0.5% acetic acid for five minutes and rinsed in distilled water for 15 min, quickly immersed in tertiary butyl alcohol, left to dry at room temperature and mounted in Entellan® [51].
2.5.3. Morphometric Analysis
For morphometric analysis, histological sections were photographed using a trinocular image capture system (Olympus Optical Ltd. Brazil, São Paulo, SP, Brazil) and camera (SCOS Color for light microscopy). Measurements of thoracic muscular structures, midgut thickness and fat body area were performed using Image J (NIH) software (National Institutes of Health, Bethesda, MD, USA). For thorax muscles, longitudinal histological sections in which it was possible to visualize the junction of the insect’s head with the thorax were selected. In these sections, for each insect, ten measurements of the thickness of the muscle fibers arranged horizontally and of the space between each fiber were taken. Five insects were used for each treatment. Three measurements of the horizontal thoracic diameter were also performed on each insect. To assess the midgut thickness, ten measurements of the portion identified as the midgut were taken per insect and five insects were used for each treatment. In addition, finally, to determine the average area of fat cells (trophocytes) of the fat body, 50 measurements were performed per insect, five insects for each treatment, of histological sections of the abdominal portion.
2.6. Statistical Analysis
Dose-mortality data were subjected to probit analysis (SAS Institute, Cary, NC, USA), and 95% confidence intervals for toxicity ratios were estimated following Robertsen et al. [52]; the values were considered significant if the range did not include the value 1. In enzyme inhibition test, the values obtained for IC50 of EOs and the pattern of carvacrol were compared to each other by analysis of variance by the Scott–Knott test at the level of 5% probability, using the Sisvar software (UFLA, Lavras, Brazil). Morphometric data were submitted to the Shapiro–Wilk normality test. Then, they were compared by analysis of variance (ANOVA), followed by Tukey’s post-hoc test (α < 0.05) using the Graph Pad Prism 7.00 software (GraphPad Software, San Diego, CA, USA).
3. Results
3.1. Chemical Characterization of Extracted Essential Oils
The chemical characterization showed that ten and fourteen different constituents were found in M. fragrans and S. molle EOs, respectively. The phenylpropanoid (E)-anethole was the major (99.61% of the mix) compound identified in the I. verum oil (Table 1 and Supplementary Figure S1). In the oil of M. fragrans, the four major constituents belonged to monoterpenic hydrocarbons and were sabinene (27.39%), limonene (25.51%), β-pinene (17.95%) and α-pinene (17.77%). In the EO of S. molle, two major components were monoterpenic hydrocarbons (limonene: 25.55% and sabinene: 19.66%) and the two others were sesquiterpenic hydrocarbons (bicyclogermacrene: 22.93% and trans-β-caryophyllene: 12.74%).

Table 1.
Moisture content, yield and chemical composition of essential oils of Illicium verum, Myristica fragrans and Schinus molle.
3.2. Insecticidal Activity
The mortality levels obtained in the dose-mortality bioassays were satisfactorily described by the probit model (goodness-of-fit tests exhibiting low χ2-values (<3) and high p-values (>0.05)). The toxicity ratios (TR) were estimated relative to the I. verum EO as it presented the lowest LC50 (1.9 μL mL−1). The toxicities of the M. fragrans (LC50 = 26.5 μL mL−1) and S. molle (LC50 =58.7 μL mL−1) EOs were similar and were, respectively, 14- and 31-fold less lethal to the D. suzukii flies compared to the I. verum EO (Table 2).

Table 2.
Toxicological performance of essential oils on Drosophila suzukii.
3.3. Acetylcholinesterase Inhibitory Activity of Essential Oils
The three EOs presented enzyme inhibitory activity that was concentration-dependent (Supplementary Figure S2). The S. molle EO promoted considerable enzyme inhibition at lower concentrations, when compared to the other oils and to carvacrol used as a comparison standard (Table 3). The I. verum oil presented a higher value, (IC50 = 0.117 mg mL−1), differing statistically (F = 16.04; df = 3; p = 0.0009) from the others and, thus, showing the least enzyme inhibitory activity among the tested EOs. There was no statistical difference among the IC50 values for carvacrol (IC50 = 0.029 mg mL−1) and the oils of M. fragrans (IC50 = 0.057 mg mL−1) and S. molle (IC50 = 0.047 mg mL−1).

Table 3.
Estimated IC50 values for acetylcholinesterase inhibition.
3.4. Histology of Thoracic Muscles, Midgut and Fat Body of Unexposed Adult Female of Drosophila suzukii
Figure 1A presents a longitudinal histological section of an unexposed (control) adult female of D. suzukii subjected to the periodic acid–Schiff technique. Figure 1B shows details of histological sections of thoracic muscle (I, II and III), midgut (IV, V and VI) and fat body (VII, VIII and IX) stained with Hematoxylin and Eosin (HE) (I, IV and VII), Bromophenol blue (BB) (II, V and VIII) and the periodic acid–Schiff technique (PAS) (III, VI and IX).
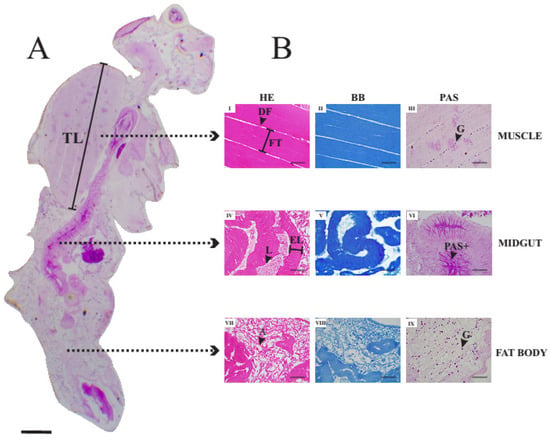
Figure 1.
(A)—Longitudinal histological section of adult Drosophila suzukii female of the Control treatment subjected to the periodic acid–Schiff technique. Scale bar: 200 μm. (B)—Histological sections of muscle (I, II and III), midgut (IV, V and VI) and fat body (VII, VIII and IX) stained with Hematoxylin and Eosin (HE) (I, IV and VII), Bromophenol blue (BB) (II, V and VIII) and periodic acid–Schiff technique (PAS) (III, VI and IX) of adult D. suzukii female belonging to Control treatment. Scale bars: 50 μm. Legends: A—adipocytes; DF—distance between muscle fibers; EL—epithelium length; FT—muscle fiber thickness; G—glycogen granules; L—midgut lumen; PAS+—PAS positive region; TL—thorax length.
In sections allowing to visualize the junction between the insect’s head and thorax, on average, six muscle fibers arranged longitudinally were observed above the esophagus. Fibers were connected from one end of the exoskeleton to the other. Differences in thickness between fibers were verified, but the thickness was constant in the same fiber. Small spacings between fibers characterized by straight regions not reactive to hematoxylin and eosin were verified. In each muscle fiber, an average of five to eight myofibrils were observed, arranged in a straight line. In sections where the junction between the head and the thorax was not visualized, the fibers normally occupied the entire thoracic region and were arranged in different directions. In addition, many fibers in these regions were attached to one end of the thorax and extend to the center of that compartment, where a small spacing was found connecting one fiber to another. For bromophenol blue staining, proteins were evidenced by an intense blue stain, homogeneously arranged in the tissue. In the PAS technique, the tissue is weakly and homogeneously stained. Strongly reactive points (glycogen) distributed in a spaced fashion at the end of the muscle fibers were observed.
In the abdominal section, the midgut is evidenced as a tube that extends to approximately half of the abdomen followed by a thinner tube representing the hindgut. The tube could be viewed both transversely and longitudinally, depending on the position of the histological section. The midgut has columnar cells with nuclei positioned at approximately equal heights and all cells appeared to adhere to the basal membrane. The nucleus of the cells was not very evident, and the nucleolus was not observed. The thickness of the midgut epithelium was practically constant. The apical part of the epithelium has small spaces between the cells, which extend to the middle of the epithelium. The staining with bromophenol blue allowed to observe uniform distribution of proteins in the intestine, being evidenced by a medium-intensity blue stain. The PAS technique strongly stained the intestinal contents as well as the apical part of the epithelial cells. In addition, thin extracellular rectilinear regions reactive to PAS were observed. These regions were evenly distributed in the midgut and connect the apical part to the basal pole of the cells.
The fat body represents about 20 to 30% of the constitution of the abdomen of the adult female fly. This tissue is mainly present at the ends of the abdomen, particularly in the final portion of this compartment and characterized by the presence of adipose cells evidenced by regions not stained by hematoxylin and eosin (HE). The droplets have a shape ranging from circular to oval, with small variations in area. The droplets were surrounded by eosinophilic matrix, which is also homogeneously stained by bromophenol blue. Strongly reactive points stained in purple were observed to be uniformly distributed throughout the fat body. The PAS-stained dots were about twenty times smaller than the lipid droplets and were also present in smaller numbers compared to the unstained droplets.
3.5. Histopathological Alterations Caused by Exposure to Illicium verum Essential Oil
The exposure of pupae to LC20 and LC50 of I. verum EO caused clear histological and morphological alterations in the structures analyzed (i.e., midgut, fat body and thoracic muscle fibers). These alterations were concentration-dependent.
3.5.1. Histological Alterations in the Midgut
The midgut of the LC20-treated individuals presented a greater space between cells (Figure 2D–F). The intestinal lumen was strongly stained with PAS but epithelial cells appeared weakly stained at their apical poles, being non-reactive in their interior. No changes were revealed using bromophenol blue staining. Similar but more severe alterations in the midgut were encountered for the LC50-treated individuals (Figure 2G–I). The cells appeared to be more elongated and spaced farther apart. The epithelium appeared thicker with hyperplasia of the cells in the intestinal wall. Although the PAS technique revealed strongly stained intestinal contents, the apical part of the cells was stained purple very faintly. No changes in intestinal protein content were seen by staining with bromophenol blue.
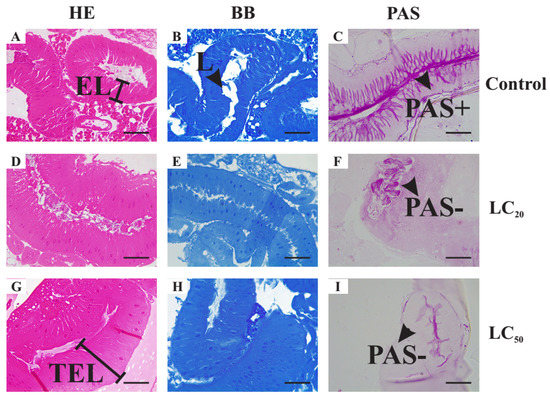
Figure 2.
Histological sections of midgut of adult Drosophila suzukii females stained with Hematoxylin and Eosin (HE) (A–G), Bromophenol blue (BB) (B,E,H) and periodic acid–Schiff technique (PAS) (C,F,I). Control Group II (C II) (A–C)—3% DMSO solution. Treatment group I (D–F)—1 μL mL−1 (LC20) of the essential oil of Illicium verum diluted in 3% DMSO. Treatment group II (G–I)—2 μL mL−1 (LC50) of the essential oil of I. verum diluted in 3% DMSO. Scale bars: 50 μm. Legends: EL—epithelium length; L—midgut lumen; PAS−—PAS negative region (please note that the intestinal content is always PAS-positive, but the enterocytes are PAS negative in treatment groups); PAS+—PAS-positive region; TEL—thicker epithelium length.
3.5.2. Histological Alterations in the Fat Body
The proportion of fat body in the abdomen of EO-treated insects was smaller. It represented about 20% of the LC20-treated insects’ abdomen and about 10% of LC50-treated ones. The fat body had apparently smaller trophocytes (Figure 3D–I) when compared to control (Figure 3A–C). The staining with bromophenol blue showed that the color of matrix surrounding the lipid droplets is non-homogeneous.
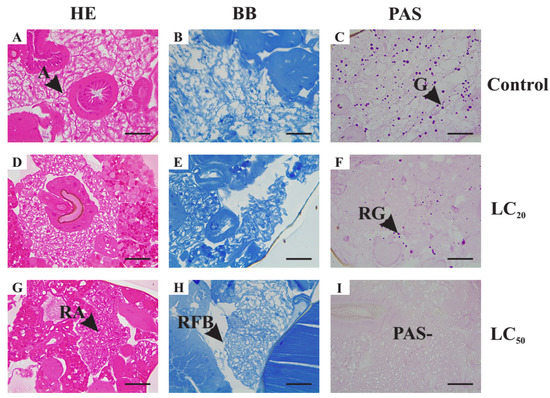
Figure 3.
Histological sections of fat body of adult Drosophila suzukii females stained with Hematoxylin and Eosin (HE) (A,D,G), Bromophenol blue (BB) (B,E,H) and periodic acid–Schiff technique (PAS) (C,F,I). Control Group II (C II) (A–C)—3% DMSO solution. Treatment group I (D–F)—1 μL mL−1 (LC20) of the essential oil of Illicium verum diluted in 3% DMSO. Treatment group II (G–I)—2 μL mL−1 (LC50) of the essential oil of I. verum diluted in 3% DMSO. Scale bars: 50 μm. Legends: A—adipocytes; G—glycogen granules; PAS−—PAS negative region; RA—reduced adipocytes area; RFB—reduced fat-body area; RG—reduced number of glycogen granules.
Regarding the PAS technique, 60% of LC20 treated insects have a non-reactive fat body with only 40% of visible pink-stained spots (Figure 3F), representing less than 50% of the total number observed in the controls (Figure 3C). The fat body of none of the LC50-treated insects was reactive to PAS (Figure 3I). Finally, large spaces between organs, such as intestine and ovary, were observed in regions that, in the control groups, were filled by the fat body. Such regions do not show reactivity to any of the dyes used.
3.5.3. Histological Alterations in the Thoracic Muscle Fibers
The treated insects showed considerable morphological changes in their muscle fibers (Figure 4D–I) compared to the control (Figure 4A–C). Intense variations in fiber thickness, as well as greater spacing between them, are observed. In 40% of LC20-treated insects, myofibrils were also more clearly spaced and about 10% of these myofibrils had a torn appearance. In LC50-treated insects, about half of the fibers seem not to be attached to the exoskeleton, with an eosinophilic acellular matrix being observed in this region. Tears in muscle fibers were also observed, with unstained regions being evidenced. Non-homogeneous color with bromophenol blue was seen in both treatments. In addition, glycogen granules were drastically reduced in LC20-treated insects, while no glycogen reserves were observed in any of the LC50-treated ones.
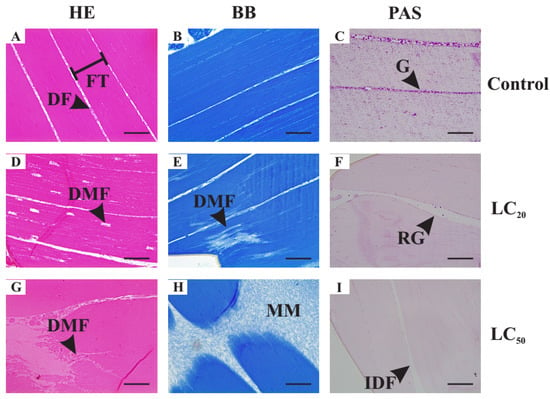
Figure 4.
Histological sections of muscle of adult Drosophila suzukii females stained with Hematoxylin and Eosin (HE) (A,D,G), Bromophenol blue (BB) (B,E,H) and periodic acid–Schiff technique (PAS) (C,F,I). Control Group II (C II) (A–C)—3% DMSO solution. Treatment group I (D–F)—1 μL mL−1 (LC20) of the essential oil of Illicium verum diluted in 3% DMSO. Treatment group II (G–I)—2 μL mL−1 (LC50) of the essential oil of I. verum diluted in 3% DMSO. Scale bars: 50 μm. Legends: DF—distance between muscle fibers; DMF—disruption of muscle fibers; FT—muscle fiber thickness; G—glycogen granules; IDF—increased distance between muscle fibers; MM—extracellular muscle matrix; RG—reduced amount of glycogen granules.
3.5.4. Morphometric Analysis of the Histological Alterations
The alterations found in the histological analysis were confirmed by the results of the morphometric analysis of the thickness and distance between thorax muscle fibers, thorax diameter, thickness of the midgut epithelium and the average area of lipid droplets of fat body (Table 4). Significant differences between the measured parameters for LC20- and LC50-treated insects and the control were observed except for the thorax diameter (Table 4). The thickness of thorax muscle fibers as well as the average area of lipid droplets of fat body decreased with increase in the concentrations used. Conversely, the distance between thorax muscle fibers and thickness of the midgut epithelium increases with the concentrations.

Table 4.
Morphometric measurements of thorax, midgut and fat body structures of Drosophila suzukii unexposed (water + DMSO) and exposed to LC20 and LC50 of Illicium verum essential oil.
4. Discussion
Here, we demonstrated that I. verum EO acts on insect’ internal morphology, such as the thorax, midgut and fat-body tissues, which can contribute to its toxic efficacy against insect pests. Our findings revealed that in addition to its satisfactory toxicological performance against D. suzukii, the exposure to low concentrations of I. verum EO promoted drastic concentration-dependent histological and structural alterations in the female adults of this fly.
As for the major constituents, the I. verum EO was found to present a lesser chemical diversity of constituents compared to the two other EOs (i.e., M. fragrans and S. molle). The oil of I. verum is majorly constituted by (E)-anethole (99.61%). In other studies, (E)-anethole was also found as the major compound for this species, but it was quantified in different proportions [37,53]. For M. fragrans EO, the major ones were sabinene (27.39%), limonene (25.51%), β-pinene (17.95%) and α-pinene (17.77%). Kapoor et al. (2013) identified sabinene (29.4%), β-pinene (10.6%), α-pinene (10.2%) and terpinen-4-ol (9.6%) as the majority [54]. For S. molle EO, the major compounds were limonene (25.55%), bicyclogermacrene (22.93%), sabinene (19.66%) and trans-β-caryophyllene (12.74%). Do Prado et al. (2019) identified β-pinene (25.33%), epi-α-cadinol (21.29%), α-pinene (18.72%), and myrcene (11.54%) as major compounds [55]. The variations observed in the chemical composition of these oils depend on several factors, including the geographic locations where the plants are growing, seasons, availability of nutrients and water, pathogen- and herbivore-attacks pressure, pollution, solar radiation and genetic factors [56,57].
The EO of I. verum exhibited the best toxicological action on D. suzukii, with the lowest LC50 (1.9 μL mL−1) being 4 and 31-fold more lethal to the D. suzukii flies compared to M. fragrans and S. molle EOs, respectively. Such differential insecticidal activity shown by these EOs can be explained by their chemical diversity of constituents, as it can mirror different mechanisms of action [58,59,60].
However, the toxicological results are in contrast with the inhibitory activity of the acetylcholinesterase (AChE) enzyme of these oils considering that the inhibition of the AChE enzyme is one of the mechanisms widely recognized to be involved in the insecticidal activity of EOs [58]. Indeed, the star anise oil presented an enzymatic inhibition concentration (IC50 = 0.117 mg mL−1) twice as high as the other oils. The higher IC50 values jointly with the lower LC50 found for the I. verum EO compared to the two others suggest that the AChE inhibition is not the main mechanism involved in I. verum EO toxicity to D. suzukii flies. The oil of I. verum is basically constituted of (E)-anethole (99.61%), allowing to infer that the insecticidal activity of this oil is due to the action of this phenylpropanoid, and the occurrence of synergism with the other constituents is unlikely. The compound (E)-anethole is derived from p-coumaric acid, a phenolic compound, which undergoes reduction and has a methyl group replacing the hydroxyl hydrogen [61]. The replacement of hydroxyl by a methoxyl gives the compound greater stability against oxidation [62].
It is noteworthy that the insects were exposed to EOs through direct contact, fumigation and, mainly, ingestion, allowing the toxic action of EO to affect different target organs and causing damage to other tissues of the exposed flies. In fact, the histological and histochemical analyses verified alterations in the epithelial thickness and in the distribution of carbohydrates in the midgut of D. suzukii exposed to low concentrations of I. verum EO. Additionally, in the fat body, there was a drastic reduction in the area of fat cells as well as carbohydrate reserves. In the thorax, muscle fibers became thinner, more spaced apart and prone to ruptures in some cases, and glycogen reserves were absent. Through the analysis of these changes arising from exposure to sublethal concentrations, it is possible to infer that an alternative mechanism of toxic action of I. verum oil may be happening.
Recently, similar morphological changes in insects’ digestive system caused by EOs were reported by Hashem et al. (2018), who found that sublethal doses of the nanoemulsified EO of Pimpinella anisum, with (E)-anethole content equal to 81.2%, caused alterations in the integument and midgut of Tribolium castaneum [63]. Our results with the PAS technique demonstrate that the intestine of insects exposed to anise oil was severely damaged, especially in the treatment with LC50, suggesting that carbohydrate absorption in the flies’ intestine may be compromised. Carbohydrate metabolism is essential for the maintenance of energy balance in animals, and in insects the main energy sources are trehalose sugars and glycogen [64,65]. Trehalose is the primary carbohydrate in insects, playing a fundamental role in several physiological processes. This sugar is synthesized by trehalose-6-phosphate synthase (TPS) and trehalose-6-phosphatase, and is rapidly hydrolyzed by trehalase (TRE) to glucose for energy [66].
Alternatively, insects use lipids as a secondary source of energy. In this sense, the fat body of insects, where triglycerides, glycogen and proteins are stored, is characterized as the main energy reserve structure. In addition to storing biomolecules that provide energy, the fat body of insects acts in the biosynthesis of several proteins and other metabolites, and participates in the detoxification of xenobiotics [67,68,69,70]. Here, the positive PAS results in the control (water and DMSO exposed flies) confirmed the occurrence of glycogen in the fat body of D. suzukii flies. However, in LC20, the carbohydrate content decreased and in LC50 was inexistent. Simultaneously, a concentration-dependent reduction in the area of lipid droplets was observed in LC20 and LC50 when compared to control (water- and DMSO-exposed) flies. Thus, it can be inferred that when subjected to I. verum EO, the probable difficulty in absorbing carbohydrates from the diet may have led the animals to consume their glycogen and lipid reserves.
Moreover, when stored carbohydrate is consumed, there may also be protein degradation for energy [71,72]. Our findings showed clearly that the individuals in the control groups had glycogen deposits in the thoracic muscle regions. In contrast, stored glycogen was absent in individuals exposed to LC50 and some fragmented muscle fibers were observed. Furthermore, in both LC20 and LC50 flies, a significant reduction in the thickness of muscle fibers and greater spacing between them were also observed. Such damage can strongly impact the insects’ ability to move and fly, implying difficulty in obtaining food, mating and finding substrate for oviposition, and so effectively reducing the ability of this pest to prosper and generate damage.
The use of low concentrations of I. verum EO drastically affected the morphophysiology of D. suzukii. Probably, this oil does not act by inhibiting the acetylcholinesterase enzyme, since the results obtained suggest a possible inhibition of digestive enzymes or cell-membrane receptors that caused the non-absorption of carbohydrates from the food by the insect’s intestine. Bromophenol blue staining indicated a constant protein content in the midgut for controls and exposed flies but with possible inhibition of digestive enzymes, insects cannot obtain energy from the ingested food. Such changes can be credited to (E)-anethole, the predominant compound in star anise EO. Thus, I. verum oil appears as an alternative for the long-term control of D. suzukii, as it has a high lethality rate for this insect and causes severe damage to those who survive the exposure. However, more studies are warranted to assess its toxicity to non-target organisms and develop products for field application.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture12101667/s1. Supplementary Figure S1: Chromatograms obtained by chemical characterization of essential oils of Illicium verum, Myristica fragrans and Schinus molle. Supplementary Figure S2: Acetylcholinesterase inhibition by essential oils of Illicium verum, Myristica fragrans, Schinus molle and carvacrol.
Author Contributions
L.d.S.: Investigation, formal analysis, writing—original draft; M.d.G.C.: Resources, conceptualization, methodology, validation, supervision, writing—original draft; I.F.M.K.: Methodology, investigation, writing—original draft; V.R.F.F.: Methodology, investigation, writing—original draft; A.R.S.C.: Investigation, data curation; G.A.C.: Investigation, data curation; K.H.: Resources, conceptualization, methodology, validation, supervision, formal analysis, writing—original draft. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Council of Scientific and Technological Development (CNPq), Minas Gerais State Foundation for Research Aid (FAPEMIG, APQ-02230-21) and Coordination for the Improvement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Capes, Brazil) (Finance code 001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
This work was supported by the National Council of Scientific and Technological Development (CNPq), Minas Gerais State Foundation for Research Aid (FAPEMIG, APQ-02230-21) and Coordination for the Improvement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Capes, Brazil) (Finance code 001).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Cantrell, C.L.; Dayan, F.E.; Duke, S.O. Natural Products As Sources for New Pesticides. J. Nat. Prod. 2012, 75, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Cloonan, K.R.; Abraham, J.; Angeli, S.; Syed, Z.; Rodriguez-Saona, C. Advances in the Chemical Ecology of the Spotted Wing Drosophila (Drosophila suzukii) and Its Applications. J. Chem. Ecol. 2018, 44, 922–939. [Google Scholar] [CrossRef]
- Campolo, O.; Giunti, G.; Russo, A.; Palmeri, V.; Zappalà, L. Essential Oils in Stored Product Insect Pest Control. J. Food Qual. 2018, 2018, 6906105. [Google Scholar] [CrossRef]
- Haddi, K.; Turchen, L.M.; Viteri Jumbo, L.O.; Guedes, R.N.C.; Pereira, E.J.G.; Aguiar, R.W.S.; Oliveira, E.E. Rethinking Biorational Insecticides for Pest Management: Unintended Effects and Consequences. Pest Manag. Sci. 2020, 76, 2286–2293. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. Botanical Insecticides, Deterrents, and Repellents in Modern Agriculture and an Increasingly Regulated World. Annu Rev Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential Oils in Insect Control: Low-Risk Products in a High-Stakes World. Annu Rev Entomol. 2012, 57, 405–424. [Google Scholar] [CrossRef]
- Isman, M.B.; Grieneisen, M.L. Botanical Insecticide Research: Many Publications, Limited Useful Data. Trends Plant Sci. 2014, 19, 140–145. [Google Scholar] [CrossRef]
- Gonzales Correa, Y.D.C.; Faroni, L.R.A.; Haddi, K.; Oliveira, E.E.; Pereira, E.J.G. Locomotory and Physiological Responses Induced by Clove and Cinnamon Essential Oils in the Maize Weevil Sitophilus zeamais. Pestic BioChem. Physiol. 2015, 125, 31–37. [Google Scholar] [CrossRef]
- Freitas, R.C.P.; Faroni, L.R.D.; Haddi, K.; Viteri Jumbo, L.O.; Oliveira, E.E. Allyl Isothiocyanate Actions on Populations of Sitophilus zeamais Resistant to Phosphine: Toxicity, Emergence Inhibition and Repellency. J. Stored Prod. Res. 2016, 69, 257–264. [Google Scholar] [CrossRef]
- Massango, H.G.L.L.; Faroni, L.R.A.; Haddi, K.; Heleno, F.F.; Viteri Jumbo, L.O.; Oliveira, E.E. Toxicity and Metabolic Mechanisms Underlying the Insecticidal Activity of Parsley Essential Oil on Bean Weevil, Callosobruchus maculatus. J. Pest Sci. 2017, 90, 723–733. [Google Scholar] [CrossRef]
- Lourenço, A.M.; Haddi, K.; Ribeiro, B.M.; Corrêia, R.F.T.; Tomé, H.V.V.; Santos-Amaya, O.; Pereira, E.J.G.; Guedes, R.N.C.; Santos, G.R.; Oliveira, E.E.; et al. Essential Oil of Siparuna guianensis as an Alternative Tool for Improved Lepidopteran Control and Resistance Management Practices. Sci. Rep. 2018, 8, 7215. [Google Scholar] [CrossRef] [PubMed]
- Borges, J.C.M.; Haddi, K.; Oliveira, E.E.; Silva Andrade, B.; Nascimento, V.L.; Silva Melo, T.; Didonet, J.; Carvalho, J.C.T.; Cangussu, A.S.; Soares, I.M.; et al. Mosquiticidal and Repellent Potential of Formulations Containing Wood Residue Extracts of a Neotropical Plant, Tabebuia Heptaphylla. Ind. Crops Prod. 2019, 129, 424–433. [Google Scholar] [CrossRef]
- Ferreira, T.P.; Haddi, K.; Corrêa, R.F.T.; Zapata, V.L.B.; Piau, T.B.; Souza, L.F.N.; Santos, S.-M.G.; Oliveira, E.E.; Jumbo, L.O.V.; Ribeiro, B.M.; et al. Prolonged Mosquitocidal Activity of Siparuna guianensis Essential Oil Encapsulated in Chitosan Nanoparticles. PLoS Negl. Trop. Dis. 2019, 13, e0007624. [Google Scholar] [CrossRef] [PubMed]
- Shaaya, E.; Rafaeli, A. Essential Oils as Biorational Insecticides–Potency and Mode of Action. In Insecticides design using advanced technologies; Ishaaya, I., Horowitz, A.R., Nauen, R., Eds.; Springer Science & Business Media: Berlin, Germany, 2007; pp. 249–261. [Google Scholar]
- Jankowska, M.; Rogalska, J.; Wyszkowska, J.; Stankiewicz, M. Molecular Targets for Components of Essential Oils in the Insect Nervous System-A Review. Molecules 2017, 23, 34. [Google Scholar] [CrossRef]
- Osman, S.; Swidan, M.; Kheirallah, D.; Nour, F. Histological Effects of Essential Oils, Their Monoterpenoids and Insect Growth Regulators on Midgut, Integument of Larvae and Ovaries of Khapra Beetle, Trogoderma granarium Everts. J. Biol. Sci. 2016, 16, 93–101. [Google Scholar] [CrossRef]
- Alves, K.F.; Caetano, F.H.; Pereira Garcia, I.J.; Santos, H.L.; Silva, D.B.; Siqueira, J.M.; Tanaka, A.S.; Alves, S.N. Baccharis dracunculifolia (Asteraceae) Essential Oil Toxicity to Culex quinquefasciatus (Culicidae). Environ. Sci. Pollut. Res. 2018, 25, 31718–31726. [Google Scholar] [CrossRef]
- Chaaban, A.; Richardi, V.S.; Carrer, A.R.; Brum, J.S.; Cipriano, R.R.; Martins, C.E.N.; Silva, M.A.N.; Deschamps, C.; Molento, M.B. Insecticide Activity of Curcuma longa (Leaves) Essential Oil and Its Major Compound α-Phellandrene against Lucilia cuprina Larvae (Diptera: Calliphoridae): Histological and Ultrastructural Biomarkers Assessment. Pestic. BioChem. Physiol. 2019, 153, 17–27. [Google Scholar] [CrossRef]
- Dutra, K.A.; Wanderley Teixeira, V.; Cruz, G.S.; Silva, C.T.S.; D Assunção, C.G.; Ferreira, C.G.M.; Monteiro, A.L.B.; Agra Neto, A.C.; Lapa Neto, C.J.C.; Teixeira, A.A.C.; et al. Morphological and Immunohistochemical Study of the Midgut and Fat Body of Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) Treated with Essential Oils of the Genus Piper. Biotech. HistoChem. 2019, 94, 498–513. [Google Scholar] [CrossRef]
- França, L.P.; Amaral, A.C.F.; Ramos, A.d.S.; Ferreira, J.L.P.; Maria, A.C.B.; Oliveira, K.M.T.; Araujo, E.S.J.; Branches, A.D.S.; Silva, J.N.; Silva, N.G.; et al. Piper Capitarianum Essential Oil: A Promising Insecticidal Agent for the Management of Aedes aegypti and Aedes albopictus. Environ. Sci. Pollut. Res. Int. 2021, 28, 9760–9776. [Google Scholar] [CrossRef]
- Asplen, M.K.; Anfora, G.; Biondi, A.; Choi, D.-S.; Chu, D.; Daane, K.M.; Gibert, P.; Gutierrez, A.P.; Hoelmer, K.A.; Hutchison, W.D.; et al. Invasion Biology of Spotted Wing Drosophila (Drosophila suzukii): A Global Perspective and Future Priorities. J. Pest Sci. 2015, 88, 469–494. [Google Scholar] [CrossRef]
- Tait, G.; Mermer, S.; Stockton, D.; Lee, J.; Avosani, S.; Abrieux, A.; Anfora, G.; Beers, E.; Biondi, A.; Burrack, H.; et al. Drosophila suzukii (Diptera: Drosophilidae): A Decade of Research towards a Sustainable Integrated Pest Management Program. J. Econ. Entomol. 2021, 114, 1950–1974. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Bruck, D.J.; Dreves, A.J.; Ioriatti, C.; Vogt, H.; Baufeld, P. In Focus: Spotted Wing Drosophila, Drosophila suzukii, across Perspectives. Pest Manag. Sci. 2011, 67, 1349–1351. [Google Scholar] [CrossRef] [PubMed]
- Hamby, K.A.; Bellamy, D.E.; Chiu, J.C.; Lee, J.C.; Walton, V.M.; Wiman, N.G.; York, R.M.; Biondi, A. Biotic and Abiotic Factors Impacting Development, Behavior, Phenology, and Reproductive Biology of Drosophila Suzukii. J. Pest Sci. 2016, 89, 605–619. [Google Scholar] [CrossRef]
- Deprá, M.; Poppe, J.L.; Schmitz, H.J.; de Toni, D.C.; Valente, V.L.S. The First Records of the Invasive Pest Drosophila suzukii in the South American Continent. J. Pest Sci. 2014, 87, 379–383. [Google Scholar] [CrossRef]
- Walsh, D.B.; Bolda, M.P.; Goodhue, R.E.; Dreves, A.J.; Lee, J.; Bruck, D.J.; Walton, V.M.; O’Neal, S.D.; Zalom, F.G. Drosophila suzukii (Diptera: Drosophilidae): Invasive Pest of Ripening Soft Fruit Expanding Its Geographic Range and Damage Potential. J. Integr. Pest Manag. 2011, 2, G1–G7. [Google Scholar] [CrossRef]
- Karageorgi, M.; Bräcker, L.B.; Lebreton, S.; Minervino, C.; Cavey, M.; Siju, K.P.; Grunwald Kadow, I.C.; Gompel, N.; Prud’homme, B. Evolution of Multiple Sensory Systems Drives Novel Egg-Laying Behavior in the Fruit Pest Drosophila suzukii. Curr. Biol. 2017, 27, 847–853. [Google Scholar] [CrossRef]
- van Timmeren, S.; Isaacs, R. Control of Spotted Wing Drosophila, Drosophila suzukii, by Specific Insecticides and by Conventional and Organic Crop Protection Programs. Crop Prot. 2013, 54, 126–133. [Google Scholar] [CrossRef]
- Erland, L.; Rheault, M.; Mahmoud, S. Insecticidal and Oviposition Deterrent Effects of Essential Oils and Their Constituents against the Invasive Pest Drosophila suzukii (Matsumura) (Diptera: Drosophilidae). Crop Prot. 2015, 78, 20–26. [Google Scholar] [CrossRef]
- Renkema, J.M.; Wright, D.; Buitenhuis, R.; Hallett, R.H. Plant Essential Oils and Potassium Metabisulfite as Repellents for Drosophila suzukii (Diptera: Drosophilidae). Sci. Rep. 2016, 6, 21432. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, J.; Zhou, L.; Wang, J.; Gong, Y.; Chen, X.; Guo, Z.; Wang, Q.; Jiang, W. Antifungal Activity of the Essential Oil of Illicium verum Fruit and Its Main Component Trans-Anethole. Molecules 2010, 15, 7558–7569. [Google Scholar] [CrossRef]
- Gupta, A.D.; Bansal, V.K.; Babu, V.; Maithil, N. Chemistry, Antioxidant and Antimicrobial Potential of Nutmeg (Myristica fragrans Houtt). J. Genet. Eng. Biotechnol. 2013, 11, 25–31. [Google Scholar] [CrossRef]
- Muhsinah, A.B.; Maqbul, M.S.; Mahnashi, M.H.; Jalal, M.M.; Altayar, M.A.; Saeedi, N.H.; Alshehri, O.M.; Shaikh, I.A.; Khan, A.A.L.; Shakeel Iqubal, S.M.; et al. Antibacterial Activity of Illicium verum Essential Oil against MRSA Clinical Isolates and Determination of Its Phyto-Chemical Components. J. King Saud. Univ. Sci. 2022, 34, 101800. [Google Scholar] [CrossRef]
- Morales-Rabanales, Q.N.; Coyotl-Pérez, W.A.; Rubio-Rosas, E.; Cortes-Ramírez, G.S.; Sánchez Ramírez, J.F.; Villa-Ruano, N. Antifungal Properties of Hybrid Films Containing the Essential Oil of Schinus Molle: Protective Effect against Postharvest Rot of Tomato. Food Control 2022, 134, 108766. [Google Scholar] [CrossRef]
- Nikolic, V.; Nikolic, L.; Dinic, A.; Gajic, I.; Urosevic, M.; Stanojevic, L.; Stanojevic, J.; Danilovic, B. Chemical Composition, Antioxidant and Antimicrobial Activity of Nutmeg (Myristica fragrans Houtt.) Seed Essential Oil. J. Essent. Oil Bear. Plants 2021, 24, 218–227. [Google Scholar] [CrossRef]
- López, A.; Castro, S.; Andina, M.J.; Ures, X.; Munguía, B.; Llabot, J.M.; Elder, H.; Dellacassa, E.; Palma, S.; Domínguez, L. Insecticidal Activity of Microencapsulated Schinus Molle Essential Oil. Ind. Crops Prod. 2014, 53, 209–216. [Google Scholar] [CrossRef]
- Matos, L.F.; da Cruz Lima, E.; de Andrade Dutra, K.; Navarro, D.M.D.A.F.; Alves, J.L.R.; Silva, G.N. Chemical Composition and Insecticidal Effect of Essential Oils from Illicium verum and Eugenia Caryophyllus on Callosobruchus maculatus in Cowpea. Ind. Crops Prod. 2020, 145, 112088. [Google Scholar] [CrossRef]
- Gomes da Rocha Voris, D.; dos Santos Dias, L.; Alencar Lima, J.; dos Santos Cople Lima, K.; Pereira Lima, J.B.; dos Santos Lima, A.L. Evaluation of Larvicidal, Adulticidal, and Anticholinesterase Activities of Essential Oils of Illicium verum Hook. f., Pimenta Dioica (L.) Merr., and Myristica fragrans Houtt. against Zika Virus Vectors. Environ. Sci. Pollut. Res. Int. 2018, 25, 22541–22551. [Google Scholar] [CrossRef]
- Emiljanowicz, L.M.; Ryan, G.D.; Langille, A.; Newman, J. Development, Reproductive Output and Population Growth of the Fruit Fly Pest Drosophila suzukii (Diptera: Drosophilidae) on Artificial Diet. J. Econ. Entomol. 2014, 107, 1392–1398. [Google Scholar] [CrossRef]
- Andreazza, F.; Bernardi, D.; Marangon, R.; Scheunemann, T.; Botton, M.; Nava, D. Técnica de Criação de Drosophila Suzukii (Matsumura, 1931) (Diptera: Drosophilidae) Em Dieta Artificial; Boletim de Pesquisa e Desenvolvimento/Embrapa Clima Temperado. 2016. [Google Scholar]
- Mendonca, L.d.P.; Oliveira, E.E.; Andreazza, F.; Rezende, S.M.; Faroni, L.R.D.; Guedes, R.N.C.; Haddi, K. Host Potential and Adaptive Responses of Drosophila suzukii (Diptera: Drosophilidae) to Barbados Cherries. J. Econ. Entomol. 2019, 112, 3002–3006. [Google Scholar] [CrossRef]
- Brasil. Farmacopéia Brasileira, 5th ed.; Agência Nacional de Vigilância Sanitária: Brasília, Brazil, 2010.
- Pimentel, F.A.; Cardoso, M.D.G.; Salgado, A.P.S.; Aguiar, P.M.; Silva, V.D.F.; Morais, A.R.D.; Nelson, D.L. A Convenient Method for the Determination of Moisture in Aromatic Plants. Quim Nova 2006, 29, 373–375. [Google Scholar] [CrossRef]
- van den Dool, H.; Kratz, P. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas—Liquid Partition Chromatography. J. Chromatogr A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy. Carol. Stream 2005, 16, 65–120. [Google Scholar]
- Insecticide Resistance Action Committee (IRAC). Susceptibility Test Methods Series: Method 026. 2011. Available online: https://irac-online.org/methods/musca-domestica-adults/ (accessed on 23 July 2020).
- Ellman, G.L.; Courtney, K.D.; Andres, V.J.; Feather-Stone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. BioChem. Pharm. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Smirle, M.J.; Zurowski, C.L.; Ayyanath, M.-M.; Scott, I.M.; MacKenzie, K.E. Laboratory Studies of Insecticide Efficacy and Resistance in Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) Populations from British Columbia, Canada. Pest Manag. Sci. 2017, 73, 130–137. [Google Scholar] [CrossRef]
- Blouquy, L.; Mottet, C.; Olivares, J.; Plantamp, C.; Siegwart, M.; Barrès, B. How Varying Parameters Impact Insecticide Resistance Bioassay: An Example on the Worldwide Invasive Pest Drosophila suzukii. PLoS ONE 2021, 16, e0247756. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, L.C.; Junqueira, L. Basic Techniques of Cytology and Histology; Bookstore Santos: São Paulo, Brazil, 1983. [Google Scholar]
- Pearse, A.G.E. Histochemistry Theoretical and Applied; Churchill Livingstone: Edinburgh, UK, 1985. [Google Scholar]
- Robertson, J.; Jones, M.; Olguin, E.; Alberts, B. Bioassays with Arthropods, 3rd ed.; CRC: Boca Raton, FL, USA, 2017; ISBN 9781315373775. [Google Scholar]
- Li, Y.; Wang, Y.; Kong, W.; Yang, S.; Luo, J.; Yang, M. Illicium verum Essential Oil, a Potential Natural Fumigant in Preservation of Lotus Seeds from Fungal Contamination. Food Chem. Toxicol. 2020, 141, 111347. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, I.P.S.; Singh, B.; Singh, G.; de Heluani, C.S.; de Lampasona, M.P.; Catalan, C.A.N. Chemical Composition and Antioxidant Activity of Essential Oil and Oleoresins of Nutmeg (Myristica fragrans Houtt.) Fruits. Int. J. Food Prop 2013, 16, 1059–1070. [Google Scholar] [CrossRef]
- do Prado, A.C.; Garces, H.G.; Bagagli, E.; Rall, V.L.M.; Furlanetto, A.; Fernandes Junior, A.; Furtado, F.B. Schinus Molle Essential Oil as a Potential Source of Bioactive Compounds: Antifungal and Antibacterial Properties. J. Appl Microbiol. 2019, 126, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Gobbo-Neto, L.; Lopes, N. Plantas Medicinais: Fatores de Influência No Conteúdo de Metabólitos Secundários. Quim. Nova 2007, 30, 374–381. [Google Scholar] [CrossRef]
- Blank, A.; Souza, E.; Paula, J.; Alves, P. Comportamento Fenotípico e Genotípico de Populações de Manjericão. Hortic Bras 2010, 28, 305–310. [Google Scholar] [CrossRef]
- Blenau, W.; Rademacher, E.; Baumann, A. Plant Essential Oils and Formamidines as Insecticides/Acaricides: What Are the Molecular Targets? Apidologie 2012, 43, 334–347. [Google Scholar] [CrossRef]
- Ikbal, C.; Pavela, R. Essential Oils as Active Ingredients of Botanical Insecticides against Aphids. J. Pest Sci. 2019, 92, 971–986. [Google Scholar] [CrossRef]
- Isman, M.B. Commercial Development of Plant Essential Oils and Their Constituents as Active Ingredients in Bioinsecticides. Phytochem. Rev. 2020, 19, 235–241. [Google Scholar] [CrossRef]
- Dewick, P.M. The Shikimate Pathway: Aromatic Amino Acids and Phenylpropanoids. Med. Nat. Prod. 2009, 137, 86. [Google Scholar]
- Solomons, T.W.G.; Fryhle, C.B.; Snyder, S.A. Organic Chemistry, 13th ed.; Wiley: Hoboken, NJ, USA, 2021. [Google Scholar]
- Hashem, A.S.; Awadalla, S.S.; Zayed, G.M.; Maggi, F.; Benelli, G. Pimpinella Anisum Essential Oil Nanoemulsions against Tribolium Castaneum-Insecticidal Activity and Mode of Action. Environ. Sci. Pollut. Res. Int. 2018, 25, 18802–18812. [Google Scholar] [CrossRef]
- Mattila, J.; Hietakangas, V. Regulation of Carbohydrate Energy Metabolism in Drosophila Melanogaster. Genetics 2017, 207, 1231–1253. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.-K.; Wang, S.; Wang, S.-G.; Zhang, L.; Xu, Y.-X.; Guo, X.-J.; Zhang, F.; Tang, B. Effects of Starvation on the Carbohydrate Metabolism in Harmonia Axyridis (Pallas). Biol. Open 2017, 6, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Zhang, L.; Xiong, X.; Wang, H.; Wang, S. Advances in trehalose metabolism and its regulation of insect chitin synthesis. Sci. Agric. Sin. 2018, 51, 697–707. [Google Scholar]
- Canavoso, L.E.; Jouni, Z.E.; Karnas, K.J.; Pennington, J.E.; Wells, M.A. Fat Metabolism in Insects. Annu. Rev. Nutr. 2001, 21, 23–46. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.T.; Soulages, J.L.; Hariharasundaram, B.; Arrese, E.L. Activation of the Lipid Droplet Controls the Rate of Lipolysis of Triglycerides in the Insect Fat Body. J. Biol. Chem. 2005, 280, 22624–22631. [Google Scholar] [CrossRef] [PubMed]
- Arrese, E.L.; Soulages, J.L. Insect Fat Body: Energy, Metabolism, and Regulation. Annu Rev Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef] [PubMed]
- Roma, G.C.; Bueno, O.C.; Camargo-Mathias, M.I. Morpho-Physiological Analysis of the Insect Fat Body: A Review. Micron 2010, 41, 395–401. [Google Scholar] [CrossRef] [PubMed]
- McCue, M.D.; Guzman, R.M.; Passement, C.A.; Davidowitz, G. How and When Do Insects Rely on Endogenous Protein and Lipid Resources during Lethal Bouts of Starvation? A New Application for 13C-Breath Testing. PLoS ONE 2015, 10, e0140053. [Google Scholar] [CrossRef]
- Welch, K.C.J.; Péronnet, F.; Hatch, K.A.; Voigt, C.C.; McCue, M.D. Carbon Stable-Isotope Tracking in Breath for Comparative Studies of Fuel Use. Ann. N. Y. Acad. Sci. 2016, 1365, 15–32. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).