Abstract
The control and management of fungal diseases is a worldwide problem. A variety of microbial pigments have excellent antibacterial effects, and naturally occurring bacterial pigments may help in tackling fungal diseases. In order to explore the basic properties and biological functions of the pink pigment produced by Erwinia persicina Cp2, we used organic solvents to extract the pink pigment, analyzed the physicochemical properties of the pigment, determined the chemical composition using ultra-high performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS), and selected five pathogenic fungi to study the inhibitory effects of the pink pigment. The results showed that the main component of the pink pigment was usambarensine, which had a good light stability and a good temperature stability at room temperature (<40 °C), but the influence of the oxidant on its activity was greater than that of the reductant; simultaneously, we found that strong acids, strong alkalis, Cu2+, and Zn2+ all greatly affect the stability of the pink pigment, while Fe2+ and Fe3+ made the pigment darker. Meanwhile, the pigment could exert a good inhibitory effect against four plant pathogenic fungi: Alternaria solani, Sclerotinia sclerotiorum, Rhizoctonia solani, and Fusarium proliferatum. However, the inhibition of Fusarium oxysporum. f. sp cucumerinum decreased significantly in the later stages. This study had detected the purification process and antifungal activity on five fungi of the pink pigment of Erwinia persicina Cp2. It lays a theoretical and practical foundation for the production of related biological agents.
1. Introduction
A total of 40% of the world’s arable land is used for agriculture, but only 1.5% of this area is used for organic agriculture [1,2]. Soil-borne diseases are an important constraint on green agricultural production and result in the reduced productivity and lower yields. Common soil-borne pathogens include Fusarium oxysporum, Rhizoctonia solani, Phytophthora spp., Pythium spp., and Verticillium dahliae [3]. These pathogens are widely found in areas of intensively planted soil and cause devastating diseases such as fusarium wilt, root rot, early blight, and bacterial wilt, which affect maize, cotton, wheat, and other food crops, reducing yields by 50–75% or even wiping out crops entirely [4]. To prevent and control such diseases, the most widely used methods in traditional agricultural production are the cultivation of disease-resistant crop varieties and the use of chemical fungicides [5,6]. However, disease-resistant crop varieties have a long cultivation cycle and remain vulnerable to pathogens enriched in the soil. Fungicides are typically inexpensive and convenient to use, but because of the restrictions on the types of agricultural chemicals used in green and organic agriculture, as well as increasing concerns for food safety and the environment in recent years, greener and environmentally friendly biological control methods without chemical residues have become a hot spot for research at home and abroad [7].
Researchers have found that using biological control measures to inoculate artificially selected antagonistic bacteria in the soil, or applying the fermentation products or secondary metabolites of certain strains to plants, could effectively reduce the incidence of soil-borne diseases and increase crop yields [8,9]. Secondary metabolites of bacteria mainly include lipopolysaccharide (LPS), extracellular polysaccharide (EPS), bacterial pigment, and protease [10,11]. As one of the secondary metabolites of bacteria, pigments have been widely used in industries such as dyes [12], food additives [13], and medicine [14]. The biological functions of pigments used in such applications include the interference in the electron transfer of host cells [15], regulation of immune mechanisms [16], bactericidal effects [17] and bacteriostatic effects [18]. However, due to the different characteristics of various bacteria, the chemical components of the bacterial pigments produced are also different. Some chemical substances found in bacterial pigments, including phenazine carboxylic acid, pyocyanin, pyoluteorin, and pyrrolnitrin, may exhibit better biocontrol effects than others [19,20]. Ohmori et al. [21] also reported that the bacterial pigment pyoluteorin could be used as a new type of biological pesticide for the control of wilt disease in cotton.
Erwinia persicina is a Gram-negative rod-shaped bacterium of the Enterobacteriaceae family. Hao et al. [22] first reported and named this new pathogen isolated from cucumber, banana, and tomato plants in 1990. Since then, researchers have found that Erwinia persicina can cause diseases in plants [23,24], animals [25], and edible fungi [26] at certain levels. The pathogen is milky white on the NA medium, with glossy edges and a diameter of 1–4 mm. However, it grows rapidly on many nutrient media and produces a pink water-soluble pigment after it is connected into a piece, and it also causes a trend of the pink pigment spreading in the roots, stems, leaves, and other tissues of the invaded plants, including onion and parsley [27,28]. However, the recent study [29] pointed out that Erwinia persicina also exhibits the potential as a biological control agent, as an inhibitor of Salmonella pollution in the industrial cultivation of sprouts.
We also observed, during the experiment. that Erwinia persicina Cp2 produced a large amount of pink pigment under aging and extreme environmental conditions, and had certain antibacterial properties. Therefore, Erwinia persicina Cp2 was used as the tested strain, the bacterial pink pigment was extracted with organic solvents, the physicochemical properties and chemical composition of the pink pigment were studied, and the typical disease pathogens included: Alternaria solani, Sclerotinia sclerotiorum, Rhizoctonia solani, Fusarium oxysporum. f. sp cucumerinum, and Fusarium proliferatum were used as representative strains to verify the antibacterial activity of the pink pigment, to explore the particularity and potential biocontrol function of the pigment material, and to lay the foundation for further research into pesticides with biocontrol functions obtained from microorganisms.
2. Materials and Methods
2.1. Test Strains
The tested bacteria was Erwinia persicina Cp2 (ID: 756944) isolated from alfalfa seeds [30]. Alternaria solani (ID: 3106919), Sclerotinia sclerotiorum (ID: 17372726), Rhizoctonia solani (ID: 14572378), Fusarium oxysporum. f. sp cucumerinum (ID: 306247), and Fusarium proliferatum (ID: 17735672) were donated by the College of Plant Protection of Gansu Agricultural University. All strains were stored in the Pasture Pathology Laboratory of Gansu Agricultural University, at a temperature of −80 °C (LanZhou, China).
2.2. Test Agars
We used a nutrient agar (NA) for the cultivation and short-term storage of the Cp2 strains, and used King’s B medium for the cultivation and enrichment of the pink pigment of Cp2. In addition, we used a potato dextrose agar (PDA) for the cultivation of pathogenic fungi and the control of antibacterial tests. To the PDA base, we added pink pigment to a level of 20% of the total volume (the absorbance of the pink pigment was 0.785) to make pink pigment PDA (PPPDA), then used this PPPDA to study the antibacterial activity of the five kinds of pathogenic fungi.
2.3. Preparation of the Pink Pigment of Erwinia persicina Cp2
We used an inoculating loop to scrape the bacteria from the NA, then inoculated these bacteria in King’s B medium using the single-line method and left them to culture in the dark at 28 °C for about 72 h until they contained a large amount of pigment. Then, we took bacterial samples for experimental use using medicine spoons.
2.4. Extraction and Purification of the Pink Pigment
For the crude extraction and purification of the pink pigment, we used organic solvent extraction methods [31]. We collected bacteria in a culture plate, scraped a sample of 1.0 g and placed it in a 10 mL centrifuge tube. Then, we added 5 mL of distilled water, chloroform, ethyl acetate, isoamyl alcohol, petroleum ether, glacial acetic acid, n-butanol, and absolute ethanol, respectively, to the centrifuge tube with bacteria and soaked it for 2 h (During this process, the tube was shaken with a vortex oscillator for 1 min every 30 min). Following the centrifugation at 12,000 rpm for 15 min, the supernatant was collected [31]. Finally, we observed the dissolution status of the pink pigment in various solvents and selected the best leaching agent and extraction solution to obtain a pure pigment.
2.5. The Absorbance Spectrum of the Pink Pigment
Following the method used by Venil et al. [32], we used the spectral scanning mode of the UV spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) to scan the wavelengths of 200–800 nm at the interval of 1 nm in the pure pink pigment at room temperature in order to determine the maximum wavelength.
2.6. Components Detection of the Pink Pigment
2.6.1. Collection and Processing of the Pink Pigment Sample
We removed the sample from the −80 °C refrigerator, left it to thaw on ice, and then shook it with the vortex for 10 s. We mixed 50 μL of the sample and 150 μL of a 20% acetonitrile methanol internal standard extractant. We vortexed the mixture for 3 min and centrifuged it (12,000 rpm, 4 °C) for 10 min. Then we transferred 150 μL of the supernatant and left it to stand at −20 °C for 30 min. Finally, we centrifuged it again for 3 min (12,000 rpm, 4 °C) and removed the supernatant for analysis.
2.6.2. Analysis of the Metabolites of the Pink Pigment
The sample extracts were analyzed using an LC-ESI-MS/MS system (UHPLC, ExionLC AD, https://sciex.com.cn/ (accessed on 15 November 2021); MS, QTRAP® System, https://sciex.com/cn/ (accessed on 25 November 2021)). The analytical conditions were as follows: for the UPLC, the column was Waters ACQUITY UPLC HSS T3 C18 (1.8 μm, 2.1 mm × 100 mm); the column temperature was 40 °C; the flow rate was 0.35 mL/min; the injection volume was 5 μL; the solvent system was water (0.1% formic acid): acetonitrile (0.1% formic acid); and the gradient program was 95:5 v/v at 0 min, 10:90 v/v at 10.0 min, 10:90 v/v at 11.0 min, 95:5 v/v at 11.1 min, and 95:5 v/v at 14.0 min.
The Quadrupole Time-of-Flight mass spectrometer was used for its ability to acquire MS/MS spectra on an information-dependent basis (IDA) during an LC/MS experiment. In this mode, the acquisition software (TripleTOF 6600, AB SCIEX) continuously evaluated the full scan survey MS data as it collected and triggered the acquisition of the MS/MS spectra depending on the preselected criteria. For each cycle, 12 precursor ions whose intensity was greater than 100 were chosen for the fragmentation at a collision energy of 30 V (12 MS/MS events with the product ion accumulation time of 50 msec each). The electron spray ion (ESI) source conditions were set as the following: the ion source gas I (GSI) as 50 Psi; the ion source gas II (GSII) as 50 Psi; the curtain gas as 25 Psi; the source temperature 500 °C; the ion spray voltage floating (ISVF) was 5500 V or −4500 V in the positive or negative modes, respectively.
Linear ion trap (LIT) and triple quadrupole (QQQ) scans were acquired on a triple quadrupole-linear ion trap mass spectrometer (QTRAP), QTRAP® LC-MS/MS System, equipped with an ESI Turbo ion-spray interface, operating in the positive and negative ion modes and controlled by Analyst 1.6.3 software (Lincoln, Dearborn, MI, USA). The ESI source operation parameters were as follows: the source temperature was 500 °C; the ISVF was 5500 V (positive), −4500 V (negative); the ion source GSI, GSII, curtain gas were set at 50, 50, and 25.0 Psi, respectively; and the collision gas was high. Instrument tuning and mass calibration were performed with 10 and 100 μmol/L polypropylene glycol solutions in QQQ and LIT modes, respectively. For each period, we monitored a specific set of multiple reaction monitoring (MRM) transitions, according to the metabolites eluted within the period concerned.
2.7. The Stability of the Pink Pigment
2.7.1. The Light Stability of the Pink Pigment
The pigment solutions were taken and placed under natural light and dark conditions at room temperature, and the samples were taken after 0, 2, 4, 6, and 8 h of treatment to determine their absorbance at the characteristic wavelengths. Three experimental replicates were performed.
2.7.2. The Temperature Stability of the Pink Pigment
The method was referenced from Zhang et al. [33] and further improved. The pigment solutions were taken and placed at temperatures of 4, 20, 40, 60, 80, 100, and 120 °C for 2 h. The absorbance of the pink pigments after 2 h were also measured using 0 h as a control. Three experimental replicates were performed.
2.7.3. The pH Stability of the Pink Pigment
The method of Ugwu et al. [34] was referred to and further improved. Specifically, the pH value of the deionized water to levels between 1 and 14 using HCl and NaOH. For each pH level, we took 4 mL of deionized water, added 1 mL of the pigment solution, and then mixed to observe how the pigment color changed at the different pH conditions. We separately measured the absorbance after standing periods of 0 h and 2 h. Three experimental replicates were performed.
2.7.4. The Metal Ions Stability of the Pink Pigment
We adopted and improved upon the method used by Li et al. [35]. That is, we prepared 100 mmol/L solutions of Na+, K+, Ca2+, Mg2+, Mn2+, Cu2+, Zn2+, Fe2+, and Fe3+, we took 0.5 mL of each ion solution and separately added this to 4.5 mL of the pigment solution to give a final concentration of 10 mmol/L. Once it was mixed uniformly, we measured the absorbance after standing for 0 h and 2 h, then calculated the preservation rate. Three experimental replicates were performed.
Preservation Rate (%) = A2/A0 × 100%;
A0 and A2 were the absorbance of the pigment at 0 h and 2 h with metal ions, respectively.
2.7.5. The Redox Stability of the Pink Pigment
We took 4 mL of the pink pigment solution and added 2%, 4%, 6%, 8%, and 10% H2O2 solution (10% v/v) or Na2SO3 solution (100 g/L), separately. Once it was uniformly mixed, we determined the absorbance after standing for 0 h and 2 h. Three experimental replicates were performed.
2.8. The Inhibitory Effect of the Pink Pigment on the Soil-Borne Pathogenic Fungi
We referred to the research method of You et al. [36], and we used agar punch with a diameter of 0.5 cm to punch mycelial cakes on the same circumference as the edge of the cultured colony, and connected it to the center of the PDA (control) and the PPPDA medium. Three replicates were set up for each group of treatments. All were incubated in the dark at a constant 25 °C maintained by means of a thermostat. We took the time when the edge of the colony of the control group was close to the wall of the dish as the time point. During this period, the diameter of the colony of each treatment was measured using the cross method continuously every day, and the growth rate, and the growth inhibition rate were calculated simultaneously. Three experimental replicates were performed.
Growth inhibition rate (%) = (Diameter of control colony − Diameter of treated colony)/(Diameter of control colony − Diameter of mycelial cake) × 100%.
2.9. Statistical Analysis
All experiments were carried out at least three times while the results were expressed and presented in tables as mean, with error bars expressed in the form of ±standard error (SE). One-way ANOVA, Duncan’s new multiple range method and an independent sample t-test were used for the significant analysis of variance. The software we used was Analyst 1.6.3 (Lincoln, Dearborn, MI, USA) to process the metabolomic data.
3. Results
3.1. The Extraction and Purification of the Pink Pigment
The results showed that the pink pigment was extremely soluble in glacial acetic acid, followed by anhydrous ethanol and distilled water. This phenomenon made the pink pigment water-soluble. We also found that the pigment was soluble in n-butanol and slightly soluble in ethyl acetate; however, it was insoluble in chloroform, isoamyl alcohol and petroleum ether (Table 1). Therefore, after a thorough consideration of the solubility and characteristics of the solvents, we chose ethanol as the leaching agent and chloroform as the extractant for our experiment.

Table 1.
The solubility of the pink pigment in organic solvents.
The pink pigment could be dissolved under various ethanol concentration gradients, with a maximum solubility at an ethanol concentration of 75% (Table 2).

Table 2.
The solubility of the pink pigment in ethanol solutions.
The leaching agent was 75% ethanol and the extractant chloroform were selected to extract and purify the pink pigment. In this process, there would be three situations due to the change of the ratio of the two solvents. A, B, and C were the diagrams of the chloroform and anhydrous ethanol ratios set as close to 1:1, greater than 1:1, and less than 1:1, respectively (Figure 1). In this test, chloroform was added to the crude pigment extract containing 75% ethanol and left to stand for a few moments until the effect of A was achieved and then collected the “pigment + water” phase.
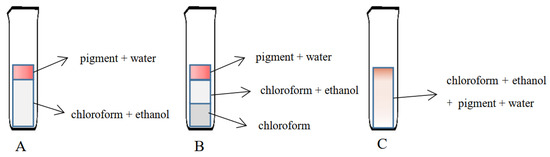
Figure 1.
Schematic diagram of the purifying pink pigment with different ratios of the leaching agent and extractant. (A) indicates chloroform and anhydrous ethanol ratios close to 1:1; (B) indicates chloroform and anhydrous ethanol ratios greater than 1:1; (C) indicates chloroform and anhydrous ethanol ratios less than 1:1.
3.2. The Wavelength Scanning of the Pink Pigment
We scanned the wavelength of the pure pigment under 200–800 nm and found that the maximum absorbance of 0.943 was achieved when the wavelength was 340 nm (Supplementary Materials Figure S1).
3.3. Results of the Composition Test of the Pink Pigment
We used Analyst 1.6.3 software to process the mass spectrometry data, we obtained the total ions current (TIC) of the pink pigment sample (Figure 2), and used the multiple reaction monitoring (MRM) to collect the pink pigment sample date (Supplementary Materials Figure S2). At the same time, the MRM scanning was used to obtain the peak area of each compound. In order to compare the material content difference of each metabolite in the sample among all of the detected metabolites, we corrected the mass spectrum peaks according to the information of the metabolites retention time and peak type. By such means, we ensured the accuracy of the qualitative quantification (Supplementary Materials Figure S3) and obtained a final figure for the metabolites detected in the pink pigment solution (Supplementary Materials Table S1).
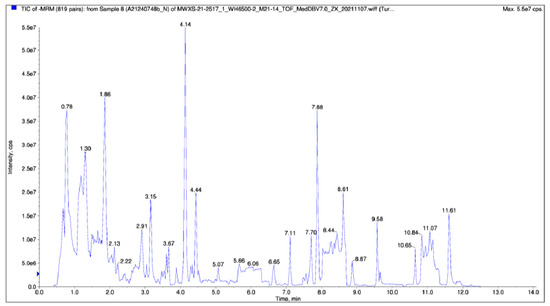
Figure 2.
The total ions current (TIC) for the sample mass spectrometry. The abscissa was the retention time of the metabolite detection, and the ordinate was the ion current intensity of the ion detection (the intensity unit is count per second, cps).
According to the MRM scan results, the ten substances most prevalent in the pink pigment samples were usambarensine, isopentenyl pyrophosphate, eicosanoyl EA, and various small molecule substances and polypeptides (Table 3).

Table 3.
Top ten compounds of the pink pigment.
3.4. The Stability of the Pink Pigment
3.4.1. Effect of the Light on the Stability of the Pink Pigment
The results showed no significant differences in the absorbance of the pink pigment between light and dark conditions (Figure 3A).
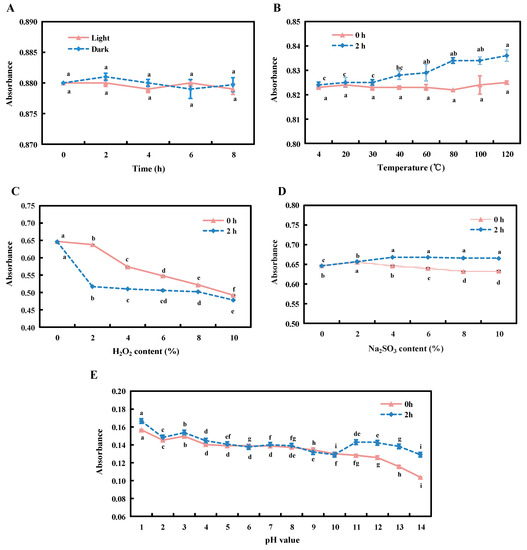
Figure 3.
Effects of the different conditions on the stability of the pink pigment. The pink solid line and the blue dotted line in the figure indicate the changing trends of the absorbance of the pink pigment at 0 h and 2 h treatment, respectively. Light stability (A), temperature stability (B), oxidants stability (C), reducing agent on the stability (D) and pH stability (E) were measured. Different letters indicate significant differences in the pink pigment under different durations of the treatment at p < 0.05, and the error bars were expressed in the form of ±standard error (SE).
3.4.2. Effect of the Temperature on the Stability of the Pink Pigment
At 0 h, there was no significant difference in absorbance of the pink pigment under the eight temperature treatments. With the increase in temperature, the absorbance values showed an overall increasing trend after 2 h, but there was no significant difference in the absorbance of the pink pigment under the temperature conditions between 4 and 30 °C; after 40 °C, the difference was significant under each temperature treatment (p < 0.05); at 120 °C, the maximum value was reached but only 1.01 times that of 4 °C (Figure 3B).
3.4.3. Effect of Oxidants on the Stability of the Pink Pigment
With the increase of the 10% H2O2 content, the absorbance of the pink pigment decreased to varying degrees at 0 h and 2 h. At 0 h, the absorbance of the pink pigment was significantly different from the control (10% H2O2 content was 0) when the content was between 2 and 10% (p < 0.05). At 2 h, when the content was 2%, the absorbance of the pink pigment was 80% of the control, afterwards, although the absorbance continued to decrease, the overall trend did not change much (Figure 3C).
3.4.4. Effect of the Reducing Agent on the Stability of the Pink Pigment
With the increased percentage of the 100 g/L Na2SO3 content, at 0 h, the absorbance of the pink pigment first increased and then decreased, and reached the maximum value when the Na2SO3 content was 2%. At 8%, it reached the minimum value of 0.98 times that of the control. At 2 h, with the increasing percentages of the reducing agent, the absorbance of the pink pigment gradually increased, but with no significant difference between 4% and 10% (Figure 3D).
3.4.5. Effect of the pH on the Stability of the Pink Pigment
When the pH value was between 1 and 10, the absorbance of the pink pigment showed a downward trend at 0 and 2 h, but the difference was not significant at the pH values between 5 and 9. However, the absorbance of the pink pigment significantly increased under acidic conditions at pH 1. Under alkaline conditions, the pH value showed a downward trend at 0 h, but at 2 h, it first decreased and then increased, with a pH value of 14 at 0 h, the absorbance levels were 80% of those recorded at 2 h (Figure 3E).
3.4.6. Effect of the Metal Ions on the Stability of the Pink Pigment
The nine metal ions had little effect on the absorbance of the pink pigment at 0 h, compared to the control (CK), but after 2 h, the pink pigment changed color in some of the metal ions and there were differences in the same absorbance. Among them, the absorbance of the pink pigment added with Na+, K+, Mn2+, and Ca2+ decreased after 2 h, but the reduction was only small, observation of the pink pigment solution revealed that the color only slightly lightened. Mg2+ produced a certain decolorizing effect on the pink pigment, both Fe2+ and Fe3+ deepened the color, and the color enhancement effect of Fe2+ ions was more than 1.86 times that of CK. Fe3+ ions deepened the color of the pink pigment while also producing a flocculent precipitation. Cu2+ and Zn2+ ions caused the pink pigment to turn grassy green and light green, respectively after 2 h (Table 4).

Table 4.
Effect of the metal ions on the stability of the pink pigment.
3.5. Inhibition of the Soil-Borne Pathogenic Fungi by the Pink Pigment
3.5.1. The Inhibition of Alternaria solani by the Pink Pigment
On the 8th day, the colony of the CK control group was close to the wall of the dish (Figure 4A). The growth rate of the CK colony showed an upward trend with the increase in treatment time from the 1st to 8th day, and the difference was extremely significant at different times (p < 0.05). The growth inhibition rate of the treatment group PM against Alternaria solani, reached 100% on the 1st to 3rd days, and the growth inhibition rate was still above 90% on the 4th to the 5th day. Subsequently, the inhibitory effect of PM decreased significantly, but on the 8th day, the growth rate of the bacterial circle was only 0.45 times that of the CK control group, and the growth inhibition rate was as high as 58.29% (Figure 4B).
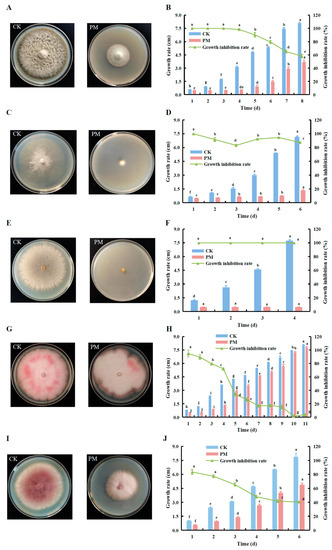
Figure 4.
Inhibition of the soil-borne pathogenic fungi by the pink pigment. The inhibition rates of the pink pigment on Alternaria solani (A,B), Sclerotinia sclerotiorum (C,D), Rhizoctonia solani (E,F), Fusarium oxysporum. f. sp cucumerinum (G,H), and Fusarium proliferatum (I,J) were determined. CK is the control group without pink pigment; PM is the treatment group with pink pigment. Different letters indicate significant differences in the pink pigment under different treatments at p < 0.05, and error bars were expressed in the form of ±standard error (SE).
3.5.2. The Inhibition of Sclerotinia sclerotiorum by the Pink Pigment
On the 6th day, the colony of the CK control group was close to the wall of the dish (Figure 4C). The growth rate for both the CK control and PM treatment groups exhibited an upward trend from the 1st to the 6th day. However, the growth rate of the colonies on the PM treatment had no significant difference between the 1st–2nd day and the 3rd–5th day. The growth inhibition rate of the PM treatment group on Sclerotinia sclerotiorum showed a trend of first a decline and then an increase, and reached the minimum value of 83.14% on the 3rd day, the growth inhibition rate was over 90% on the 4th to the 5th day, and the growth inhibition rate was as high as 87.36% on the 6th day and the colony growth rate was only 0.19 times that of the control group (Figure 4D).
3.5.3. The Inhibition of Rhizoctonia solani by the Pink Pigment
On the 4th day, the colony on the CK control group was close to the wall of the dish, but the mycelial cake on the PM treatment group did not grow further (Figure 4E). The growth rate of the colony on the control group CK showed a gradual increase to 7.76 cm on the 4th day, but the colony on the PM treatment did not grow any further and the inhibition of the growth of Rhizoctonia solani remained at 100% during this period (Figure 4F).
3.5.4. The Inhibition of Fusarium oxysporum. f. sp cucumerinum by the Pink Pigment
On the 11th day, the colonies in the CK control group and the PM treatment group were close to the wall of the dish, but the colony in the PM treatment was smaller in diameter and lighter in color than the CK group (Figure 4G). From the 1st to 11th days, the growth rate of the CK control and PM treatment groups showed an upward trend, and the difference at each time was extremely significant (p < 0.05). On the 1st day, the maximum growth inhibition rate of the treatment group PM on Fusarium oxysporum. f.sp cucumerinum was 89.45%, and the growth inhibition rate was as high as 70% in the first 4 days, and the growth inhibition rate continued to decline after the 4th day (Figure 4H).
3.5.5. The Inhibition of Fusarium proliferatum by the Pink Pigment
On the 6th day, the colony of the CK control group was close to the dish wall (Figure 4I). The growth rate of the colonies in both the CK control and the PM treatment group showed an increasing trend from the 1st to 6th days, with highly significant differences at all times (p < 0.05). The growth inhibition rate of the colony in PM, remained above 60% from 1st to 3rd days, then decreased but remained above 40%. On the 6th day, the growth inhibition rate reached a minimum of 40.46% and the growth rate of the colony in the PM treatment group was 0.62 times higher than that of the CK control group (Figure 4J).
4. Discussion
Bacterial pigments offer new potential solutions for the biological control of plant diseases. For the extraction of pigments, a common method is to use organic solvents to extract the crude pigments, and then use other organic solvents to prepare the mobile phases according to a certain ratio to separate and obtain the purified pigments [32], because the carrier involves at least three or four kinds of organic solvents, the process of removing organic solvents is cumbersome and various organic solvents may affect the activity of the pigment. Therefore, this experiment was based on a review study on the extraction process of the bacterial pigments by Muhammad et al. [31]. We measured the solubility of the pigment-containing bacteria in several organic solvents, then used a 75% ethanol solution as a leaching agent to obtain a crude pigment solution, and we used chloroform as an extractant for purification purposes, thereby ensuring the pink pigment of the highest activity and quality. In this study, we found the maximum absorption wavelength of the pink pigment to be 340 nm. However, previous studies of the maximum absorption wavelengths of the bacterial pigments prodigiosin, carotenoids and pyocyanin were around 530 nm [37], 480 nm [38], and 250 nm [39], respectively. These findings may be closely related to the specific compounds of bacterial pigments. In our study, after analyzing the compounds of the pink pigment, we found that usambarensine, isopentenyl pyrophosphate, eicosanoyl EA were included. Among them, Dassonneville et al. [40] showed that usambarensine, as a bis-indole compound, displayed potent antiamoebic activities and showed antigardial, antimalarial, and cytotoxic effects. Therefore, the antifungal activity of the pink pigment in this study may be closely related to this substance.
The antibacterial substances contained in the fermentation broth of the strains include alkaloids, anthraquinones, phenazines, and lipoic acids [41,42,43,44]. Chang et al. [39] identified the most important pigment components of Pseudomonas aeruginosa as three phenazine substances: dihydroxyphenazine-1-carboxylic acid, phenazine-1-carboxylic acid, and oxychloroquinoline. Therefore, the antibacterial activity of the fermentation broth is likely to be related to the bacterial pigment produced by the strain during the cultivation process. Tanya et al. [45] also found that prodigiosin had an inhibitory effect on crop pathogens. This study found that the growth inhibition rate of the pink pigment on Alternaria solanacearum was as high as 100% in early stages. Samen et al. [46] used PDA plates containing the commonly used fungicides difenocarb and chlorothalonil, similar to those used in this study, for susceptibility testing against Alternaria solanacearum in tomato plants and found that these fungicides inhibited some pathogens by only about 30%. Rapeseed sclerotinia is a fungal disease caused by Sclerotinia sclerotiorum [47]. In this experiment, we found that the growth inhibition rate of the pink pigment against Sclerotinia sclerotiorum reached more than 80% between the 1st and 6th days. Potato sclerotia, also known as mole disease or rhizoctonia canker, is a globally occurring soil disease caused by Rhizoctonia solani [48], which causes disease mainly by infesting plants through secreted proteins, effectors, secondary metabolites, and cell wall degrading enzymes [49]. In this study, we found that the growth inhibition rate of the plate containing the pink pigment on Rhizoctonia solani reached 100% between the 1st and 4th days. Cucumber wilt is a type of fungal disease caused by Fusarium oxysporum. f. sp cucumerinum invading the vascular bundles of melons, it is particularly serious during the flowering and fruiting stages [50]. Our study found that the pink pigment exhibited good antibacterial activity against Fusarium oxysporum. f. sp cucumerinum in the early stages, but its effectiveness decreased with time. Tuli et al. [51] pointed out that the bacterial pigments were biodegradable and thus completely non-toxic in the environment. In addition, this study found that the growth inhibition rate of the pink pigment against the pathogen of alfalfa root rot (Fusarium oxysporum) was higher than 40% from the 1st to 6th days, which was higher than the corresponding inhabitation rate against Fusarium oxysporum. f. sp cucumerinum for the same period.
In summary, the water-soluble pink pigment of Erwinia persicina Cp2 showed a good inhibition of a variety of soil-borne pathogens. Although the pink pigment showed a reduced growth inhibition rate of some strains in the later stage, it further showed that the biological activity of the pink pigment can be reduced in the environment with the increase of time. Moreover, the study reported that the bacterial pigments could promote plant development and inhibit the growth of pathogenic fungi [52]. Therefore, the sensitivity of the five pathogenic fungi to the pink pigment in this study proved that pink pigment has the potential as a biocontrol agent, or a new biological agent and may be less polluting to the environment as a new bioreagent. However, the effect of the pink pigment on plants still needs further research.
5. Conclusions
In this study, we studied the physical and chemical properties of the pink pigment of Erwinia persicina Cp2 and found that the best leaching agent solvent for the pink pigment was 75% ethanol, while the best extractant was chloroform. We determined the maximum absorption wavelength was 340 nm; and the main component of the pink pigment was usambarensine. In addition, we study found that the pink pigment exerted a good inhibitory effect on four types of soil-borne disease pathogens: Alternaria solani, Sclerotinia sclerotiorum, Rhizoctonia solani, and Fusarium proliferatum. Among them, the inhibitory effect of R. solani was most notable, however, the inhibition of Fusarium oxysporum. f. sp cucumerinum decreased significantly with time.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture12101641/s1, Figure S1: The wavelength scanning map of pink pigment; Figure S2: Multimodal Plot for Multiple reaction monitoring (MRM) metabolite detection; Figure S3: Integral correction diagram for metabolite quantitative analysis; Table S1: Data for metabolites detected in the pink pigment solution.
Author Contributions
Y.Z. performed the experiments, analyzed the data. X.L. (Xiaoni Liu), X.L. (Xiangyang Li), L.Z., H.Z., Q.J. and B.Y. made helpful comments on our work and manuscript. Z.Z. conceived and supervised the project. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (32060396), Natural Science Foundation of Gansu Province, China (20JR10RA562), Science and Technology Planning Project of Lanzhou City (2019-RC-116), Youth Tutor Fund of Gansu Agricultural University (GAU-QDFC-2019-08), and Key Laboratory of Grassland Ecosystem of the Ministry of Education (KLGE202203).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature. 2011, 478, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Larsen, A.E.; Powers, L.C.; Mccomb, S. Identifying and characterizing pesticide use on 9000 fields of organic agriculture. Nat. Commun. 2021, 12, 5461. [Google Scholar] [CrossRef] [PubMed]
- Daguerre, Y.; Siegel, K.; Edel-Hermann, V.; Steinberg, C. Fungal proteins and genes associated with biocontrol mechanisms of soil-borne pathogens: A review. Fungal Biol. Rev. 2014, 28, 97–125. [Google Scholar] [CrossRef]
- Mihajlovic, M.; Rekanovic, E.; Hrustic, J.; Grahovac, M.; Tanovic, B. Methods for management of soilborne plant pathogens. Pestic. I Fitomedicina. 2017, 32, 9–24. [Google Scholar] [CrossRef]
- Mowlick, S.; Inoue, T.; Takehara, T.; Kaku, N.; Ueki, K.; Uekiet, A. Changes and recovery of soil bacterial communities influenced by biological soil disinfestation as compared with chloropicrin-treatment. AMB Express. 2013, 3, 46. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Phillips, D.; Verheyen, G.; Li, H.; Barbetti, M.J. Yields and resistance of strawberry cultivars to crown and root diseases in the field, and cultivar responses to pathogens under controlled environment conditions. Phytopathol. Mediterr. 2012, 51, 69–84. [Google Scholar] [CrossRef]
- Shim, M.Y.; Starr, J.L.; Keller, N.P.; Woodard, K.E.; Lee, T.A., Jr. Distribution of isolates of Sclerotium rolfsii tolerant to pentachloronitrobenzene in texas peanut fields. Plant Dis. 1998, 82, 103–106. [Google Scholar] [CrossRef][Green Version]
- Xu, T.; Li, Y.; Zeng, X.D.; Yang, X.L.; Yang, Y.Z.; Yuan, S.S.; Hu, X.C.; Zeng, J.R.; Wang, Z.Z.; Liu, Q.; et al. Isolation and evaluation of endophytic Streptomyces endus OsiSh-2 with potential application for biocontrol of rice blast disease. J. Sci. Food Agric. 2017, 97, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Hagberg, I.; Novitsky, L.; Hadj-Moussa, H.; Avis, T.J. Interaction of antimicrobial cyclic lipopeptides from Bacillus subtilis influences their effect on spore germination and membrane permeability in fungal plant pathogens. Fungal Biol. 2014, 118, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.C. Pseudomonas aeruginosa in cystic fibrosis: Pathogenesis and persistence. Paediatr. Respir. Rev. 2002, 3, 128–134. [Google Scholar] [CrossRef]
- Ali, J.; Rafiq, Q.A.; Ratcliffe, E. Antimicrobial resistance mechanisms and potential synthetic treatments. Future Sci. OA 2018, 4, FSO290. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.; Koehler, P.E. Production of red water-soluble Monascus pigments. J. Food Sci. 2010, 48, 1200–1203. [Google Scholar] [CrossRef]
- Dufossé, L. Microbial production of food grade pigments. Food Technol. Biotechnol. 2006, 44, 313–321. [Google Scholar]
- Prince, L.R.; Bianchi, S.M.; Vaughan, K.M.; Bewley, M.A.; Marriott, H.M.; Walmsley, S.R.; Taylor, G.W.; Buttle, D.J.; Sabroe, I.; Dockrell, D.H.; et al. Subversion of a lysosomal pathway regulating neutrophil apoptosis by a major bacterial toxin, pyocyanin. J. Immunol. 2008, 180, 3502–3511. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, G.F.; Sartorio, R.; Lee, S.H.; Rogers, C.J.; Meijler, M.M.; Moss, J.A.; Clapham, B.; Brogan, A.P.; Dickerson, T.J.; Janda, K.D. Revisiting quorum sensing: Discovery of additional chemical and biological functions for 3-Oxo-N-acylhomoserine lactones. Proc. Natl. Acad. Sci. USA 2005, 102, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Rada, B.; Gardina, P.; Myers, T.G.; Leto, T.L. Reactive oxygen species mediate inflammatory cytokine release and EGFR-dependent mucin secretion in airway epithelial cells exposed to Pseudomonas pyocyanin. Mucosal Immunol. 2011, 4, 158–171. [Google Scholar] [CrossRef]
- Ge, P.; Scholl, D.; Prokhorov, N.S.; Avaylon, J.; Shneider, M.M.; Browning, C.; Buth, S.A.; Plattner, M.; Chakraborty, U.; Ding, K.; et al. Action of a minimal contractile bactericidal nanomachine. Nature. 2020, 580, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Toohey, J.I.; Nelson, C.D.; Krotkov, G. Toxicity of phenazine carboxylic acid to some bacteria, algae, higher plants, and animals. Can. J. Bot. 2011, 43, 1151–1155. [Google Scholar] [CrossRef]
- Maurhofer, M.; Keel, C.; Haas, D.; Défago, G. Influence of plant species on disease suppression by Pseudomonas fluorescens strain CHAO with enhanced antibiotic production. Plant Pathol. 1995, 44, 40–50. [Google Scholar] [CrossRef]
- Jiang, G.C.; Lin, Y.C.; Zhou, S.N.; Vrijmoed, L.; Jones, E. Studies on the secondary metabolites of mangrove fungus No.1403 from the South China Sea. Acta Sci. Nat. Univ. Sunyatseni. 2000, 39, 68–72. [Google Scholar] [CrossRef]
- Ohmori, T.; Hagiwara, S.I.; Ueda, A.; Minoda, Y.; Yamada, K. Production of pyoluteorin and its derivatives from n-paraffin by Pseudomonas aeruginosa S10B2. Agric. Biol. Chem. 2014, 42, 2031–2036. [Google Scholar] [CrossRef]
- Hao, M.V.; Brenner, D.J.; Steigerwalt, A.G.; Kosako, Y.; Komagata, K. Erwinia persicinus, a new species isolated from plants. Int. J. Syst. Evol. Microbiol. 1990, 40, 379–383. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Nan, Z.B. Erwinia persicina, a possible new necrosis and wilt threat to forage or grain legumes production. Eur. J. Plant Pathol. 2014, 139, 349–358. [Google Scholar] [CrossRef]
- Orel, D.C. Erwinia persicina as the new causal agent of lettuce soft rot. Eur. J. Plant Pathol. 2020, 158, 223–235. [Google Scholar] [CrossRef]
- Walaa, I.M.; Zhang, Z.F.; Ibrahim, M.H.; Shi, S.L. Experimental infection in mice with Erwinia persicina. Microb. Pathog. 2019, 130, 38–43. [Google Scholar] [CrossRef]
- Yan, J.J.; Lin, Z.Y.; Wang, R.Q.; Liu, F.; Tong, Z.J.; Jiang, Y.J.; Mukhtar, I.; Xie, B.G. First report of Erwinia persicina causing pink disease in Flammulina velutipes (Enoki Mushroom) in China. Plant Dis. 2018, 103, 1–5. [Google Scholar] [CrossRef]
- Cho, H.; Park, J.Y.; Kim, Y.K.; Sohn, S.H.; Park, D.S.; Kwon, Y.S.; Kim, C.W.; Back, C.G. Whole-genome sequence of Erwinia persicina B64, which causes pink soft rot in onions. Microbiol. Resour. Announc. 2019, 8, 1–3. [Google Scholar] [CrossRef]
- Nechwatal, J.; Theil, S. Erwinia persicina associated with a pink rot of parsley root in Germany. J. Plant Dis. Prot. 2019, 126, 161–167. [Google Scholar] [CrossRef]
- Kim, W.; Choi, S.Y.; Han, I.; Cho, S.K.; Lee, Y.; Kim, S.; Kang, B.; Choi, O.; Kim, J. Inhibition of Salmonella enterica growth by competitive exclusion during early alfalfa sprout development using a seed-dwelling Erwinia persicina strain EUS78. Int. J. Food Microbiol. 2020, 312, 1–7. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Nan, Z.B. First report of Erwinia persicinus causing wilting of Medicago sativa sprouts in China. Plant Dis. 2012, 96, 454. [Google Scholar] [CrossRef]
- Numan, M.; Bashir, S.; Mumtaz, R.; Tayyab, S.; Rehman, N.U.; Khan, A.L.; Shinwari, Z.K.; Al-Harrasi, A. Therapeutic applications of bacterial pigments: A review of current status and future opportunities. Biotech. 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Venil, C.K.; Zakaria, Z.A.; Usha, R.; Ahmad, V.A. Isolation and characterization of flexirubin type pigment from Chryseobacterium sp. UTM-3 T. Biocatal. Agric. Biotechnol. 2014, 3, 103–107. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Zhao, X.Y.; Ma, Y.; Jiang, Y.; Wang, D.; Liang, H. Comparison of blue discoloration in radish root among different varieties and blue pigment stability analysis. Food Chem. 2021, 340, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ugwu, C.T.; Ogbonna, C.N.; Ogbonna, J.C.; Aoyagi, H. Production and stability of pigments by Talaromyces purpurogenus LC128689 in an alternating air phase-liquid phase cultivation system. Biotechnol. Appl. Biochem. 2021, 69, 1–10. [Google Scholar] [CrossRef]
- Li, S.H.; Li, T.P.; Fu, W.N.; Yu, X.J.; Zhao, Z.S.; Jia, Y.F. Effects of metal ions on the stability of purple sweet potato pigment. Adv. Mater. Res. 2011, 396-398, 1325–1328. [Google Scholar] [CrossRef]
- You, C.; Zhang, C.; Feng, C.; Wang, J.; Kong, F. Myroides odoratimimus, a biocontrol agent from the rhizosphere of tobacco with potential to control Alternaria alternata. BioControl 2015, 60, 555–564. [Google Scholar] [CrossRef]
- Kang, S.G.; Jin, W.; Bibb, M.; Lee, K.J. Actinorhodin and undecylprodigiosin production in wild-type and relA mutant strains of Streptomyces coelicolor A3 (2) grown in continuous culture. FEMS Microbiol. Lett. 1998, 168, 221–226. [Google Scholar] [CrossRef]
- Deli, J.; Molnár, P.; Matus, Z.; Tóth, G.; Steck, A. Reisolation of carotenoid 3,6-epoxides from red paprika (Capsicum annuum). Helv. Chim. Acta 1996, 79, 1435–1443. [Google Scholar] [CrossRef]
- Chang, P.C.; Blackwood, A.C. Simultaneous production of three phenazine pigments by Pseudomonas aeruginosa Mac 436. Can. J. Microbiol. 1969, 15, 439–444. [Google Scholar] [CrossRef]
- Dassonneville, L.; Wattez, N.; Mahieu, C.; Colson, P.; Houssier, C.; Frederich, M.; Tits, M.; Angenot, L.; Bailly, C. The plant alkaloid usambarensine intercalates into DNA and induces apoptosis in human HL60 leukemia cells. Anticancer Res. 1999, 19, 5245–5250. [Google Scholar]
- Han, J.H.; Shim, H.; Shin, J.H.; Kim, K.S. Antagonistic activities of Bacillus spp. strains isolated from tidal flat sediment towards anthracnose pathogens Colletotrichum acutatum and C. gloeosporioides in south Korea. Plant Pathol. J. 2015, 31, 165–175. [Google Scholar] [CrossRef]
- Abriouel, H.; Franz, C.; Omar, N.B.; Gálvez, A. Diversity and applications of Bacillus bacteriocins. FEMS Microbiol. Rev. 2011, 35, 201–232. [Google Scholar] [CrossRef]
- El-Beih, A.A.; Kato, H.; Ohta, T.T. CYP3A4 inhibitors isolated from a marine derived fungus Penicillium species. J. Nat. Med. 2007, 61, 175–177. [Google Scholar] [CrossRef]
- Isabel, M.; Jordi, C.; Emilio, M. Cyclic lipopeptide biosynthetic genes and products, and inhibitory activity of plant-associated bacillus against phytopathogenic bacteria. PLoS ONE 2015, 10, 1–21. [Google Scholar] [CrossRef]
- Tanya, C.; Marina, R.; Thando, N.; Sehaam, K.; Wesaal, K. A Metabolomics and molecular networking approach to elucidate the structures of secondary metabolites produced by Serratia marcescens strains. Front. Chem. 2021, 72, 633870. [Google Scholar] [CrossRef]
- Samen, F.A.E.; Goussous, S.J.; Al-Shudifat, A.; Makhadmeh, I. Reduced sensitivity of tomato early blight pathogen (Alternaria solani) isolates to protectant fungicides, and implication on disease control. Arch. Phytopathol. Plant Prot. 2016, 49, 1–17. [Google Scholar] [CrossRef]
- Kirkegaard, J.A.; Robertson, M.J.; Hamblin, P.; Sprague, S.J. Effect of blackleg and sclerotinia stem rot on canola yield in the high rainfall zone of southern New South Wales, Australia. Aust. J. Agric. Res. 2006, 57, 201–212. [Google Scholar] [CrossRef]
- Suwannarach, N.; Kumla, J.; Bussaban, B.; Lumyong, S. Biocontrol of Rhizoctonia solani AG-2, the causal agent of damping-off by Muscodor cinnamomi CMU-Cib 461. World J. Microbiol. Biotechnol. 2012, 28, 3171–3177. [Google Scholar] [CrossRef]
- Li, D.Y.; Li, S.A.; Wei, S.H.; Sun, W.X. Strategies to manage rice sheath blight: Lessons from interactions between rice and Rhizoctonia solani. Rice 2021, 14, 21. [Google Scholar] [CrossRef]
- Chen, F.; Wang, M.; Zheng, Y.; Luo, J.M.; Yang, X.R.; Wang, X.L. Quantitative changes of plant defense enzymes and phytohormone in biocontrol of cucumber fusarium wilt by Bacillus subtilis B579. World J. Microbiol. Biotechnol. 2010, 26, 675–684. [Google Scholar] [CrossRef]
- Tuli, H.S.; Chaudhary, P.; Beniwal, V.; Sharma, A.K. Microbial pigments as natural color sources: Current trends and future perspectives. J. Food Sci. Technol. 2015, 52, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Levy, E.; Eyal, Z.; Chet, I.; Hochman, A. Resistance mechanisms of Septoria tritici to antifungal products of Pseudomonas. Physiol. Mol. Plant Pathol. 1992, 40, 163–171. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).