Using RGB Imaging, Optimized Three-Band Spectral Indices, and a Decision Tree Model to Assess Orange Fruit Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Physical Characteristics
2.2.1. Chlorophyll Content in the Peel
2.2.2. Peel Color
2.3. Chemical Characteristics
2.3.1. Total Soluble Solids (TSS)
2.3.2. Total Soluble Solids/Titratable Acidity Ratio (TSS/Acid)
2.4. Digital RGB Imaging
2.5. Measuring Spectral Reflectance and Selection of SRIs of Orange Fruits
| SRIs | Formula | Reference |
|---|---|---|
| Published SRIs | ||
| Normalized difference index (NDI570, 540) | (R570-R540)/(R570 + R540) | [11] |
| Normalized difference index (NDI686, 620) | (R686-R620)/(R686 + R620) | [11] |
| Anthocyanin index (NAI) | (R760-R720)/(R760 + R720) | [54] |
| NDI780, 550 | (R780-R550)/(R780 + R550) | [55] |
| Greenness index (GI) | R554/R677 | [56] |
| Pigment-sensitive ripening monitoring index (PRMI) | (R750-R678)/R550 | [14] |
| Normalized chlorophyll index (NCI) | (R750-R678)/(R750 + R678) | [14] |
| Normalized difference index (NDI 800, 640) | (R800-R640)/(R800 + R640) | [12] |
| Normalized difference index (NDI 826, 670) | (R826-R670)/(R826 + R670) | [12] |
| Normalized difference index (NDI 970, 670) | (R970-R670)/(R970 + R670) | [12] |
| New three-band SRIs | ||
| Normalized difference index (NDI574,592,724) | (R574-R592- R724)/(R574 + R592 + R724) | This work |
| Normalized difference index (NDI572,584,724) | (R572-R584-R724)/(R572 + R584 + R724) | |
| Normalized difference index (NDI574,722,590) | (R574-R722-R590)/(R574 + R722 + R590) | |
| Normalized difference index (NDI526,664,700) | (R526-R664-R700)/(R526 + R664 + R700) | |
| Normalized difference index (NDI524,700,664) | (R524-R700-R664)/(R524 + R700 + R664) | |
| Normalized difference index (NDI628,412,694) | (R628-R412-R694)/(R628 + R412 + R694) | |
| Normalized difference index (NDI628,410,694) | (R628-R410-R694)/(R628 + R410 + R694) | |
| Normalized difference index (NDI580,568,594) | (R580-R568-R594)/(R580 + R568 + R594) | |
| Normalized difference index (NDI578,590,566) | (R578-R590-R566)/(R578 + R590 + R566) | |
| Normalized difference index (NDI620,616,630) | (R620-R616-R630)/(R620 + R616 + R630) | |
| Normalized difference index (NDI568,550,600) | (R568-R550-R600)/(R568 + R550 + R600) | |
| Normalized difference index (NDI596,598,594) | (R596-R598-R594)/(R596 + R598 + R594) | |
| Normalized difference index (NDI624,632,620) | (R624-R632-R620)/(R624 + R632 + R620) | |
| Normalized difference index (NDI596,588,604) | (R596-R588-R604)/(R596 + R588 + R604) |
2.6. Decision Tree (DT)
2.6.1. Implementation of the Planned Approaches
2.6.2. Datasets and Software for Data Analysis
2.6.3. Model Evaluation
3. Results and Discussion
3.1. Physical and Chemical Parameter Variation and Correlation Analysis for Orange Fruits
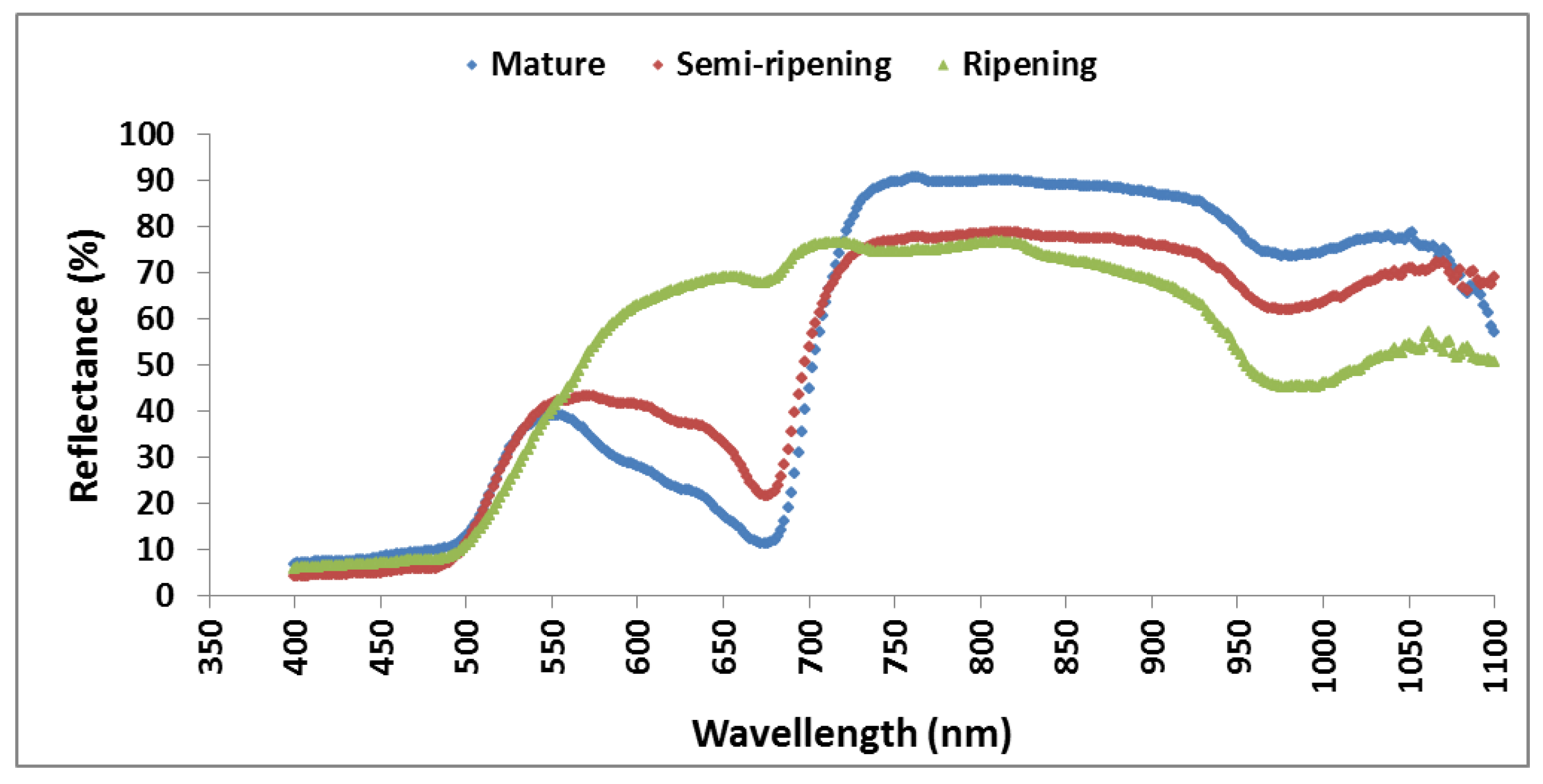
3.2. Variation of RGB Indices and Spectral Indices of Orange Fruits at Different Ripening Degrees
3.3. Evaluation of RGB Indices and Published and Newly Developed Three-Band SRIs to Assess the Physical and Chemical Parameters
3.4. Performance of Decision Tree Model Based on RGB Indices and Published and Newly Developed Three-Band SRIs for Predicting the Fruit Quality Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Food and Agricultural Organization of the United Nations. 2022. Available online: http://faostat.fao.org (accessed on 5 August 2022).
- Egyptian Ministry of Agriculture and Land Reclamation. Bulletin of the Agricultural Statistics Part (2); Summer & Nile Crops: Cairo, Egypt, 2019. [Google Scholar]
- Huang, Y.; Lu, R.; Chen, K. Assessment of tomato soluble solids content and pH by spatially-resolved and conventional Vis/NIR spectroscopy. J. Food Eng. 2018, 236, 19–28. [Google Scholar] [CrossRef]
- Ahmed, Z.F.R.; Taha, E.M.A.; Abdelkareem, N.A.A.; Mohamed, W.M. Postharvest properties of unripe bananas and the potential of producing economic nutritious. Int. J. Fruit Sci. Prod. 2020, 20, 995–1014. [Google Scholar] [CrossRef]
- Bureau, S.; Scibisz, I.; le Bourvellec, C.; Renard, C.M. Effect of sample preparation on the measurement of sugars, organic acids, and polyphenols in apple fruit by mid-infrared spectroscopy. J. Agric. Food. Chem. 2012, 60, 3551–3563. [Google Scholar] [CrossRef]
- Tadeo, J.L.; Ortiz, J.M.; Estelles, A. Sugar changes in Clementine and orange fruit during ripening. J. Hortic. Sci. 1987, 62, 531–537. [Google Scholar] [CrossRef]
- Iglesias, D.J.; Cercós, M.; Colmenero-Flores, J.M.; Naranjo, M.A.; Ríos, G.; Carrera, E.; Ruiz-Rivero, O.; Lliso, I.; Morillon, R.; Tadeo, F.R.; et al. Physiology of citrus fruiting. Braz. J. Plant Physiol. 2007, 19, 333–362. [Google Scholar] [CrossRef]
- Mbogo, G.; Mubofu, E.; Othman, C. Post harvest changes in physico-chemical properties and levels of some inorganic elements in off vine ripened orange (Citrus sinensis) fruits cv (Navel and Valencia). Afr. J. Biotechnol. 2010, 9, 1809–1815. [Google Scholar] [CrossRef]
- Chan, L.M.; Tan, R.; Thio, G. Design of visual- based colour classification system. Research papers. JASA 2007, 2, 30–33. [Google Scholar]
- Wang, H.; Peng, J.; Xie, C.; Bao, Y.; He, Y. Fruit quality evaluation using spectroscopy technology: A Review. Sensors 2015, 15, 11889–11927. [Google Scholar] [CrossRef]
- Elsayed, S.; Galal, H.; Allam, A.P.; Schmidhalter, U. Passive reflectance sensing anddigital image analysis for assessing quality parameters of mango fruits. Sci. Hortic. 2016, 212, 136–147. [Google Scholar] [CrossRef]
- Elsayed, S.; El-Gozayer, K.; Allam, A.; Schmidhalter, U. Passive reflectance sensing using regression and multivariate analysis to estimate biochemical parameters of different fruits kinds. Sci. Hortic. 2019, 243, 21–33. [Google Scholar] [CrossRef]
- Galal, H.; Elsayed, S.; Allam, A.; Farouk, M. Indirect quantitative analysis of biochemical parameters in banana using spectral reflectance indices combined with machine learning modeling. Horticulturae 2022, 8, 438. [Google Scholar] [CrossRef]
- Nagy, A.; Riczu, P.; Tamás, J. Spectral evaluation of apple fruit ripening and pigment content alteration. Sci. Hortic. 2016, 201, 256–264. [Google Scholar] [CrossRef]
- Sirisomboon, P. NIR Spectroscopy for Quality Evaluation of Fruits and Vegetable. Mater. Today Proc. 2018, 5, 22481–22486. [Google Scholar]
- He, J.G.; Luo, Y.; Liu, G.S.; Xu, S.; Si, Z.H.; He, X.G.; Wang, S.L. Prediction of soluble solids content of jujube fruit using hyperspectral reflectance imaging. In Mechatronics and Intelligent Materials III, Pts 1–3; Chen, R., Sung, W.P., Kao, J.C.M., Eds.; Trans. Tech. Publications: XiShuangBanNa, China, 2013; Volume 706–708, pp. 201–204. [Google Scholar]
- Mc Clure, W.F. Near-infrared spectroscopy–the giant is running strong. Anal. Chem. 1994, 66, 43–53. [Google Scholar] [CrossRef]
- Hashim, N.; Onwude, D.I.; Osman, M.S. Evaluation of chilling injury in mangoes using multispectral imaging. J. Food Sci. 2018, 83, 1271–1279. [Google Scholar] [CrossRef]
- Lu, R.; Guyer, D.E.; Beaudry, R.M. Determination of firmness and sugar content of apples using near-infrared diffuse reflectance. J. Texture Stud. 2000, 31, 615–630. [Google Scholar] [CrossRef]
- Luz, R. Near-infrared multispectral scattering. J. Texture Stud. 2004, 35, 263–276. [Google Scholar]
- Qing, Z.; Ji, B.; Zude, M. Wavelength selection for predicting physicochemical properties of apple fruit based on near-infrared spectroscopy. J. Food Qual. 2007, 30, 511–526. [Google Scholar] [CrossRef]
- Włodarska, K.; Szulc, J.; Khmelinskii, I.; Sikorska, E. Non-destructive determination of strawberry fruit and juice quality parameters using ultraviolet, visible, and near-infrared spectroscopy. J. Sci. Food Agric. 2019, 99, 5953–5961. [Google Scholar] [CrossRef]
- Elsayed, S.; El-Hendawy, S.; Khadr, M.; Elsherbiny, O.; Al-Suhaibani, N.; Alotaibi, M.; Tahir, M.U.; Darwish, W. Combining thermal and RGB imaging indices with multivariate and data-driven modeling to estimate the growth, water status, and yield of potato under different drip irrigation regimes. Remote Sens. 2021, 13, 1679. [Google Scholar] [CrossRef]
- Elsherbiny, O.; Zhou, L.; Feng, L.; Qiu, Z. Integration of Visible and Thermal Imagery with an Artificial Neural Network Approach for Robust Forecasting of Canopy Water Content in Rice. Remote Sens. 2021, 13, 1785. [Google Scholar] [CrossRef]
- del Valle, J.C.; Gallardo-López, A.; Buide, M.L.; Whittall, J.B.; Narbona, E. Digital photography provides a fast, reliable, and noninvasive method to estimate anthocyanin pigment concentration in reproductive and vegetative plant tissues. Ecol. Evol. 2018, 8, 3064–3076. [Google Scholar] [CrossRef]
- Ismail, W.I.W.; Razali, M.H. Machine vision to determine agricultural crop maturity. In Trends in Vital Food and Control Engineering; IntechOpen: London, UK, 2012; pp. 115–124. [Google Scholar]
- Liu, Y.; Ouyang, A.; Luo, J.; Chen, X. Near infrared diffuse reflectance spectroscopy for rapid analysis of soluble solids content in navel orange. Spectrosc. Spect. Anal. 2007, 27, 2190–2192. [Google Scholar]
- Fouda, T.; Derbala, A.; Elmetwalli, A.; Salah, S. Detection of orange color using Imaging analysis. AgroLife Sci. J. 2013, 2, 181–184. [Google Scholar]
- Domingo, L.D.; Serrano, P.B.; Serrano, P.E.; del Rosario, E.J. Digital photometric method for Determining degree of Harvest maturity and ripeness of Sinta Papava (Carica papaya L.) fruits. Philipp. Agric. Sci. 2012, 3, 252–259. [Google Scholar]
- Choi, D.; Lee, W.S.; Schueller, J.K.; Ehsani, R.; Roka, F.; Diamond, J. A Performance Comparison of RGB, NIR, and Depth Images in Immature Citrus Detection Using Deep Learning Algorithms for Yield Prediction. In 2017 ASABE Annual International Meeting; American Society of Agricultural and Biological Engineers: St. Joseph Charter Township, MI, USA, 2017; Volume 1. [Google Scholar]
- Gomes-Junior, F.G.; Arruda, N.; Marcos-Filho, J. Swingle citrumelo seed vigor and storability associated with fruit maturity classes. Sci. Agric. 2017, 74, 357–363. [Google Scholar] [CrossRef]
- Beltrán, N.H.; Duarte-Mermoud, M.A.; Salah, S.A.; Bustos, M.A.; Peña-Neira, A.I.; Loyola, E.A.; Jalocha, J.W. Feature selection algorithms using Chilean wine chromatograms as examples. J. Food Eng. 2005, 67, 483–490. [Google Scholar] [CrossRef]
- Guyon, I.; Elisseeff, A. An Introduction to Variable and Feature Selection. J. Mach. Learn. Res. 2003, 3, 1157–1182. [Google Scholar]
- Schuize, F.H.; Wolf, H.; Jansen, H.; Vander, V.P. Applications of artificial neural networks in integrated water management: Fiction or future? Water Sci. Technol. 2005, 52, 21–31. [Google Scholar] [CrossRef]
- EIMasry, G.; Sun, D.W.; Allen, P. Near-infrared hyperspectral imaging for predicting colour, pH and tenderness of fresh beef. J. Food Eng. 2012, 110, 127–140. [Google Scholar] [CrossRef]
- Strobl, C.; Boulesteix, A.-L.; Kneib, T.; Augustin, T.; Zeileis, A. Conditional variable importance for random forests. BMC Bioinform. 2008, 9, 307. [Google Scholar] [CrossRef] [PubMed]
- Glorfeld, L.W. A methodology for simplification and interpretation of backpropagation-based neural network models. Expert Syst. Appl. 1996, 10, 37–54. [Google Scholar] [CrossRef]
- Melis, G.; Dyer, C.; Blunsom, P. On the state of the art of evaluation in neural language models. arXiv 2017, arXiv:1707.05589. [Google Scholar]
- Bergstra, J.; Yamins, D.; Cox, D. Making a science of model search: Hyperparameter optimization in hundreds of dimensions for vision architectures. In Proceedings of the 30th International Conference on Machine Learning, PMLR, Atlanta, GA, USA, 16–21 June 2013; pp. 115–123. [Google Scholar]
- Wu, J.; Chen, X.-Y.; Zhang, H.; Xiong, L.-D.; Lei, H.; Deng, S.-H. Hyperparameter optimization for machine learning models based on Bayesian optimization. J. Electron. Sci. Technol. 2019, 17, 26–40. [Google Scholar]
- Mc Guire, R.G. Reporting of objective color measurements. Hortscience 1992, 27, 1254–1255. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 13th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1980. [Google Scholar]
- Fauzi, M.H.; Tjandrasa, H. Implementasi thresholding citra menggunakan algoritma hybrid optimal estimation. In Teknologi Informasi; Institut Teknologi Sepuluh Nopember: Surabaya, Indonesia, 2010. [Google Scholar]
- Yossya, E.H.; Pranata, J.; Wijaya, T.; Hermawan, H.; Budiharto, W. Mango Fruit Sortation System using Neural Network and Computer Vision. Procedia Comput. Sci. 2017, 116, 569–603. [Google Scholar] [CrossRef]
- Kumaseh, M.R.; Luther, L.; Nainggolan, N. Segmentasi Citra Digital Ikan Menggunakan Metode Thresholding. J. Ilm. Sains 2013, 13, 74–79. [Google Scholar] [CrossRef][Green Version]
- Verrelst, J.; Schaepman, M.E.; Koetz, B.; Kneubuhler, M. Angular sensitivity analysis of vegetation indices derived from 726 CHRIS/PROBA data. Remote Sens. Environ. 2008, 112, 2341–2353. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Woebbecke, D.; Meyer, G.; von Bargen, K.; Mortensen, D. Plant species identification, size, and enumeration using machine vision techniques on near-binary images. Int. Soc. Opt. Photonics 1993, 1836, 208–219. [Google Scholar]
- Mao, W.; Wang, Y.; Wang, Y. Real-time detection of between-row weeds using machine vision. In Proceedings of the ASAE Annual Meeting, Las Vegas, NV, USA, 27–30 July 2003; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2003; p. 1. [Google Scholar]
- Hague, T.; Tillett, N.; Wheeler, H. Automated crop and weed monitoring in widely spaced cereals. Precis. Agric. 2006, 7, 21–32. [Google Scholar] [CrossRef]
- Saberioon, M.M.; Amin, M.S.M.; Anuar, A.R.; Gholizadeh, A.; Wayayok, A.; Khairunniza- Bejo, S. Assessment of rice leaf chlorophyll content using visible bands at different growth stages at both the leaf and canopy scale. Int. J. Appl. Earth Obs. Geoinf. 2014, 32, 35–45. [Google Scholar] [CrossRef]
- Guijarro, M.; Pajares, G.; Riomoros, I.; Herrera, P.J.; Burgos-Artizzu, X.P.; Ribeiro, A. Automatic segmentation of relevant textures in agricultural images. Comput. Electron. Agric. 2011, 75, 75–83. [Google Scholar] [CrossRef]
- Elsayed, S.; Ibrahim, H.; Hussein, H.; Elsherbiny, O.; Elmetwalli, A.H.; Moghanm, F.S.; Ghoneim, A.M.; Danish, S.; Datta, R.; Gad, M. Assessment of water quality in Lake Qaroun using ground-based remote sensing data and artificial neural networks. Water 2021, 13, 3094. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W.; Harlan, J.C. Monitoring the Vernal Advancement of Retro Gradation of Natural Vegetation; Final Report; NASA/GSFC, Type lll: Greenbelt, MD, USA, 1974. [Google Scholar]
- Gutierrez, M.; Reynolds, M.P.; Raun, W.R.; Stone, M.L.; Klatt, A.R. Spectral water indices for assessing yield in elite bread wheat genotypes in well irrigated, water stressed, and high temperature conditions. Crop Sci. 2010, 50, 197–214. [Google Scholar] [CrossRef]
- Acharya, U.K.; Subedi, P.P.; Walsh, K.B.; McGlasson, W.B. Estimation of fruit maturation and ripening using spectral indices. Acta Hortic. 2016, 1119, 265–272. [Google Scholar] [CrossRef]
- Han, J.; Kamber, M. Data Mining: Concepts and Techniques; Morgan Kaufmann: San Francisco, CA, USA, 2001. [Google Scholar]
- Wang, C.; Nie, S.; Xi, X.H.; Luo, S.Z.; Sun, X.F. Estimating the biomass of maize with hyperspectral and LiDAR data. Remote Sens. 2017, 9, 11. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, Z.H.; Sun, H.; Wang, G.X. Mapping forest ecosystem biomass density for Xiangjiang river basin by combining plot and remote sensing data and comparing spatial extrapolation methods. Remote Sens. 2017, 9, 241. [Google Scholar] [CrossRef]
- Malone, B.P.; Styc, Q.; Minasny, B.; McBratney, A.B. Digital soil mapping of soil carbon at the farm scale: A spatial downscaling approach in consideration of measured and uncertain data. Geoderma 2017, 290, 91–99. [Google Scholar] [CrossRef]
- Saggi, M.K.; Jain, S. Reference evapotranspiration estimation and modeling of the Punjab Northern India using deep learning. Comput. Electron. Agric. 2019, 156, 387–398. [Google Scholar] [CrossRef]
- Choo, W.S. Fruit Pigment Changes during Ripening, Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Khodabakhshian, R.; Khojastehpour, M. Characteristics changes of date fruits during ripening period on-palm. Agric. Eng. Int. CIGR J. 2021, 23, 243–255. [Google Scholar]
- Maduwanthi, S.D.T.; Marapana, R.A.U.J. Induced ripening agents and their effect on fruit quality of banana. Int. J. Food Sci. 2019, 2019, 2520179. [Google Scholar] [CrossRef]
- Rooban, R.; Shanmugam, M.; Venkatesan, T.; Tamilmani, C. Physiochemical changes during different stages of fruit ripening of climacteric fruit of mango (Mangifera indica L.) and non-climacteric of fruit cashew apple (Anacardium occidentale L.). J. Appl. Adv. Res. 2016, 1, 53–58. [Google Scholar] [CrossRef]
- Lammertyn, J.; Peirs, A.; De Baerdemaeker, J.; Nicolai, B. Light penetration properties of NIR radiation in fruit with respect to non-destructive quality assessment. Postharvest Biol. Technol. 2000, 18, 121–132. [Google Scholar] [CrossRef]
- Rutkowski, K.P.; Michalczuk, B.; Konopacki, P. Nondestructive determination of ‘golden delicious’ apple quality and harvest maturity. J. Fruit Ornam. Plant Res. 2008, 16, 39–52. [Google Scholar]
- Abbaspour-Gilandeh, Y.; Sabzi, S.; Hernández-Hernández, M.; Hernández-Hernández, J.L.; Azadshahraki, F. Nondestructive estimation of the chlorophyll b of apple fruit by color and spectral features using different methods of hybrid artificial neural network. Agronomy 2019, 9, 735. [Google Scholar] [CrossRef]
- Abbott, J.A. Quality measurement of fruits and vegetables. Postharvest Biol. Technol. 1999, 15, 207–225. [Google Scholar] [CrossRef]
- Solovchenko, A.E.; Chivkunova, O.B.; Merzlyak, M.N.; Gudkovsky, V.A. Relationship between chlorophyll and carotenoid pigments during on-and off- tree ripening of apple fruits as revealed non-destructively with reflectance spectroscopy. Postharvest Biol. Technol. 2005, 38, 9–17. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Solovchenko, A.E.; Gitelson, A.A. Reflectance spectral features and non-destructive estimation of chlorophyll, carotenoid and anthocyanin content in apple fruit. Postharvest Biol. Technol. 2003, 27, 197–211. [Google Scholar] [CrossRef]
- Qin, J.W.; Thomas, F.B.; Zhao, X.H.; Nikhil, N.; Mark, A.R. Development of a two-band spectral imaging system for real-time citrus canker detection. J. Food Eng. 2012, 108, 87–93. [Google Scholar] [CrossRef]
- Pourdarbani, R.; Sabzi, S.; Hernández-Hernández, M.; Hernández-Hernández, J.L.; Gallardo-Bernal, I.; Herrera-Miranda, I. Non-destructive estimation of total chlorophyll content of apple fruit based on color feature, spectral data and the most effective wavelengths using hybrid artificial neural network-imperialist competitive algorithm. Plants 2020, 9, 1547. [Google Scholar] [CrossRef] [PubMed]
- Ringer, T.; Blanke, M. Non-invasive, real time in-situ techniques to determine the ripening stage of banana. J. Food Meas. Charact. 2021, 15, 4426–4437. [Google Scholar] [CrossRef]
- Elsherbiny, O.; Fan, Y.; Zhou, L.; Qiu, Z. Fusion of feature selection methods and regression algorithms for predicting the canopy water content of rice based on hyperspectral data. Agriculture 2021, 11, 51. [Google Scholar] [CrossRef]

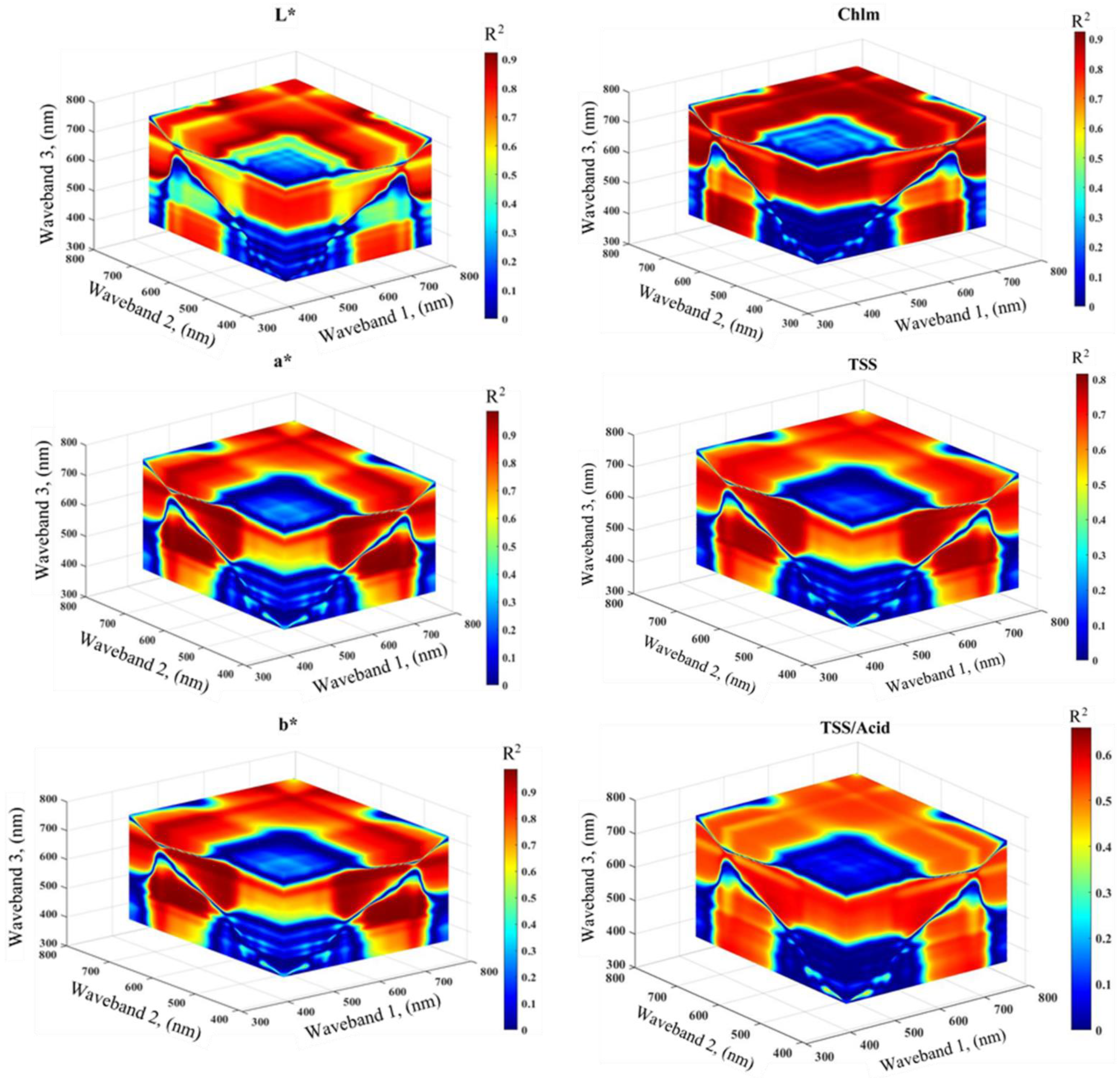

| RGB Indices | Formula | References |
|---|---|---|
| Red pixel percentage (R %) | R/(R + G + B) | [45] |
| Green pixel percentage (G %) | G/(R + G + B) | [45] |
| Blue pixel percentage (B %) | B/(R + G + B) | [45] |
| Green blue ratio index (GBRI) | G/B | [46] |
| Woebbecke index (WI) | (G − B)/(R − G) | [47] |
| Excess green vegetation index (ExG) | 2 × G − R − B | [48] |
| Excess blue vegetation index (ExB) | 1.4 × bn − gn | [49] |
| Excess green minus excess red index (ExGR) | ExG − ExR | [50] |
| Vegetative index (VEG) | G/(Ra × B(1 − a)), a = 0.667 | [51] |
| Combination (COM) | 0.25 × ExG + 0.3 × ExGR + 0.33 × CIVE + 0.12 × VEG | [52] |
| Degree | Statis. | L* | a* | b* | Chlm | TSS | TSS/acid |
|---|---|---|---|---|---|---|---|
| Mature | Min | 49.54 | −14.25 | 33.87 | 24.30 | 6.85 | 4.28 |
| Max | 61.04 | −9.15 | 46.75 | 45.60 | 8.60 | 9.51 | |
| Mean | 54.37 b | −11.24 c | 39.37 c | 36.42 a | 7.36 c | 7.16 c | |
| SD | 3.61 | 1.36 | 4.18 | 6.94 | 0.41 | 1.45 | |
| Semi-ripening | Min | 59.44 | −6.45 | 49.83 | 4.95 | 6.70 | 6.67 |
| Max | 70.45 | 6.85 | 63.95 | 17.10 | 11.65 | 16.20 | |
| Mean | 65.91 a | −0.15 b | 58.53 b | 10.14 b | 8.94 b | 11.64 b | |
| SD | 2.53 | 3.91 | 3.75 | 3.61 | 1.14 | 2.61 | |
| Ripening | Min | 60.92 | 15.27 | 62.83 | 0.00 | 8.70 | 8.57 |
| Max | 73.35 | 31.06 | 71.89 | 0.00 | 13.95 | 19.89 | |
| Mean | 67.58 a | 22.09 a | 67.23 a | 0.00 c | 11.50 a | 13.51 a | |
| SD | 3.20 | 4.75 | 2.55 | 0.00 | 1.15 | 2.76 |
| L* | a* | b* | Chlm | TSS | TSS/Acid | |
|---|---|---|---|---|---|---|
| L* | 1 | |||||
| a* | 0.67 ** | 1 | ||||
| b* | 0.94 ** | 0.86 ** | 1 | |||
| Chlm | −0.88 ** | −0.85 ** | −0.94 ** | 1 | ||
| TSS | 0.64 ** | 0.90 ** | 0.81 ** | −0.78 ** | 1 | |
| TSS/Acid | 0.60 ** | 0.74 ** | 0.74 ** | −0.73 ** | 0.83 ** | 1 |
| Degree | Statis. | R% | G% | B% | GBRI | WI | EXG | EXB | EXGR | VEG | COM |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mature | Min | 0.058 | 0.423 | 0.371 | 1.043 | −0.250 | 0.271 | 0.096 | 0.407 | 1.722 | 6.596 |
| Max | 0.205 | 0.481 | 0.462 | 1.142 | −0.047 | 0.443 | 0.166 | 0.844 | 4.408 | 7.093 | |
| Mean | 0.141 a | 0.450 a | 0.408 c | 1.104 a | −0.144 c | 0.350 a | 0.122 c | 0.602 a | 2.372 a | 6.753 a | |
| SD | 0.036 | 0.014 | 0.022 | 0.028 | 0.051 | 0.043 | 0.018 | 0.108 | 0.578 | 0.111 | |
| Semi-ripening | Min | 0.087 | 0.383 | 0.410 | 0.787 | −0.012 | 0.149 | 0.162 | 0.340 | 1.766 | 6.559 |
| Max | 0.177 | 0.434 | 0.500 | 1.010 | 0.408 | 0.301 | 0.299 | 0.585 | 3.046 | 6.816 | |
| Mean | 0.140 a | 0.403 b | 0.456 b | 0.889 b | 0.200 b | 0.210 b | 0.235 b | 0.418 b | 2.004 b | 6.618 b | |
| SD | 0.020 | 0.013 | 0.022 | 0.062 | 0.118 | 0.038 | 0.038 | 0.060 | 0.301 | 0.060 | |
| Ripening | Min | 0.126 | 0.319 | 0.505 | 0.594 | 0.742 | −0.045 | 0.356 | 0.070 | 1.415 | 6.379 |
| Max | 0.149 | 0.352 | 0.539 | 0.695 | 1.264 | 0.055 | 0.432 | 0.224 | 1.740 | 6.488 | |
| Mean | 0.138 a | 0.341 c | 0.521 a | 0.654 c | 0.900 a | 0.021 c | 0.389 a | 0.168 c | 1.585 c | 6.446 c | |
| SD | 0.006 | 0.008 | 0.010 | 0.026 | 0.123 | 0.024 | 0.021 | 0.034 | 0.063 | 0.023 |
| Degree | Statis. | NDI570, 540 | NDI686, 620 | NAI | NDI780, 550 | GI | PRMI | NCI | NDI800, 640 | NDI826, 670 | NDI970, 670 | NDI574,592, 724 | NDI572,584,724 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mature | Min | −0.062 | −0.227 | 0.082 | 0.360 | 3.055 | 1.818 | 0.748 | 0.558 | 0.742 | 0.692 | −0.652 | −0.646 |
| Max | −0.017 | −0.093 | 0.179 | 0.597 | 4.702 | 3.634 | 0.867 | 0.799 | 0.872 | 0.845 | −0.501 | −0.499 | |
| Mean | −0.039 c | −0.185 c | 0.120 a | 0.477 a | 3.769 a | 2.589 a | 0.827 a | 0.709 a | 0.831 a | 0.795 a | −0.577 b | −0.573 b | |
| SD | 0.009 | 0.030 | 0.023 | 0.051 | 0.405 | 0.377 | 0.025 | 0.048 | 0.027 | 0.031 | 0.031 | 0.030 | |
| Semi-ripening | Min | 0.014 | −0.178 | 0.014 | 0.228 | 1.114 | 0.742 | 0.297 | 0.178 | 0.298 | 0.189 | −0.485 | −0.484 |
| Max | 0.098 | −0.046 | 0.069 | 0.357 | 2.557 | 1.653 | 0.669 | 0.479 | 0.672 | 0.599 | −0.418 | −0.419 | |
| Mean | 0.056 b | −0.128 b | 0.036 b | 0.286 b | 1.803 b | 1.240 c | 0.527 b | 0.342 b | 0.526 b | 0.438 b | −0.449 a | −0.450 a | |
| SD | 0.023 | 0.036 | 0.014 | 0.034 | 0.399 | 0.246 | 0.105 | 0.087 | 0.104 | 0.114 | 0.019 | 0.019 | |
| Ripening | Min | 0.088 | −0.079 | −0.011 | 0.182 | 0.389 | 0.035 | 0.011 | 0.024 | 0.017 | −0.195 | −0.507 | −0.508 |
| Max | 0.347 | 0.061 | 0.021 | 0.509 | 1.230 | 0.914 | 0.367 | 0.226 | 0.365 | 0.259 | −0.389 | −0.390 | |
| Mean | 0.225 a | 0.031 a | 0.000 c | 0.329 b | 0.612 c | 0.173 b | 0.050 c | 0.058 c | 0.059 c | −0.071 c | −0.440 a | −0.441 a | |
| SD | 0.068 | 0.028 | 0.006 | 0.090 | 0.173 | 0.179 | 0.074 | 0.041 | 0.072 | 0.082 | 0.031 | 0.031 | |
| Degree | Statis. | NDI574,722,590 | NDI526,664,700 | NDI524,700,664 | NDI628,412,694 | NDI628,410,694 | NDI580,568,594 | NDI578,590,566 | NDI620,616,630 | NDI568,550,600 | NDI596,598,594 | NDI624,632,620 | NDI596,588,604 |
| Mature | Min | −0.642 | −0.316 | −0.341 | −0.499 | −0.337 | −0.353 | −0.353 | −0.332 | −0.305 | −0.334 | −0.334 | −0.336 |
| Max | −0.496 | −0.271 | −0.293 | −0.250 | −0.203 | −0.341 | −0.339 | −0.331 | −0.290 | −0.333 | −0.332 | −0.333 | |
| Mean | −0.570 b | −0.289 a | −0.312 a | −0.379 c | −0.282 c | −0.348 c | −0.347 c | −0.331 a | −0.295 a | −0.333 b | −0.333 a | −0.334 c | |
| SD | 0.029 | 0.013 | 0.013 | 0.047 | 0.025 | 0.002 | 0.003 | 0.000 | 0.003 | 0.000 | 0.000 | 0.001 | |
| Semi-ripening | Min | −0.481 | −0.521 | −0.536 | −0.249 | −0.204 | −0.337 | −0.335 | −0.334 | −0.332 | −0.333 | −0.335 | −0.333 |
| Max | −0.417 | −0.356 | −0.374 | −0.099 | −0.092 | −0.328 | −0.325 | −0.332 | −0.307 | −0.333 | −0.334 | −0.331 | |
| Mean | −0.447 a | −0.436 b | −0.454 b | −0.157 b | −0.137 b | −0.332 b | −0.329 b | −0.333 b | −0.318 b | −0.333 a | −0.334 b | −0.332 b | |
| SD | 0.018 | 0.049 | 0.049 | 0.037 | 0.028 | 0.002 | 0.003 | 0.001 | 0.007 | 0.000 | 0.000 | 0.000 | |
| Ripening | Min | −0.504 | −0.841 | −0.849 | −0.107 | −0.097 | −0.329 | −0.326 | −0.338 | −0.378 | −0.333 | −0.336 | −0.332 |
| Max | −0.389 | −0.514 | −0.529 | −0.077 | −0.070 | −0.324 | −0.321 | −0.333 | −0.328 | −0.333 | −0.334 | −0.329 | |
| Mean | −0.439 a | −0.718 c | −0.730 c | −0.093 a | −0.085 a | −0.326 a | −0.323 a | −0.336 c | −0.349 c | −0.333 a | −0.335 c | −0.331 a | |
| SD | 0.031 | 0.078 | 0.076 | 0.008 | 0.007 | 0.001 | 0.001 | 0.001 | 0.012 | 0.000 | 0.000 | 0.001 |
| RGB Index | L* | a* | b* | Chlm | TSS | TSS/Acid |
|---|---|---|---|---|---|---|
| R% | 0.05 | 0.00 | 0.03 | 0.00 | 0.01 | 0.00 |
| G% | 0.53 *** | 0.92 *** | 0.78 *** | 0.80 *** | 0.74 *** | 0.54 *** |
| B% | 0.61 *** | 0.83 *** | 0.83 *** | 0.72 *** | 0.74 *** | 0.48 *** |
| GBRI | 0.67 *** | 0.91 *** | 0.89 *** | 0.84 *** | 0.78 *** | 0.55 *** |
| WI | 0.53 *** | 0.94 *** | 0.79 *** | 0.73 *** | 0.78 *** | 0.50 *** |
| EXG | 0.53 *** | 0.92 *** | 0.78 *** | 0.80 *** | 0.74 *** | 0.54 *** |
| EXB | 0.62 *** | 0.92 *** | 0.86 *** | 0.80 *** | 0.79 *** | 0.53 *** |
| EXGR | 0.45 *** | 0.85 *** | 0.67 *** | 0.74 *** | 0.66 *** | 0.50 *** |
| VEG | 0.16 * | 0.44 *** | 0.28 ** | 0.42 *** | 0.29 ** | 0.27 ** |
| COM | 0.37 *** | 0.75 *** | 0.56 *** | 0.67 *** | 0.56 *** | 0.44 *** |
| NDI570, 540 | 0.42 *** | 0.95 *** | 0.71 *** | 0.70 *** | 0.79 *** | 0.55 *** |
| NDI686, 620 | 0.38 *** | 0.90 *** | 0.63 *** | 0.62 *** | 0.70 *** | 0.42 *** |
| NAI | 0.81 *** | 0.73 *** | 0.92 *** | 0.89 *** | 0.63 *** | 0.54 *** |
| NDI780, 550 | 0.77 *** | 0.15 * | 0.55 *** | 0.52 *** | 0.13 * | 0.17 * |
| GI | 0.69 *** | 0.83 *** | 0.88 *** | 0.88 *** | 0.71 *** | 0.59 *** |
| PRMI | 0.76 *** | 0.82 *** | 0.91 *** | 0.88 *** | 0.70 *** | 0.52 *** |
| NCI | 0.59 *** | 0.92 *** | 0.81 *** | 0.78 *** | 0.75 *** | 0.49 *** |
| NDI800, 640 | 0.74 *** | 0.86 *** | 0.91 *** | 0.89 *** | 0.72 *** | 0.54 *** |
| NDI 826, 670 | 0.60 *** | 0.92 *** | 0.82 *** | 0.79 *** | 0.75 *** | 0.49 *** |
| NDI970, 670 | 0.60 *** | 0.92 *** | 0.82 *** | 0.80 *** | 0.75 *** | 0.51 *** |
| NDI574,592,724 | 0.90 *** | 0.44 *** | 0.83 *** | 0.80 *** | 0.39 *** | 0.38 *** |
| NDI572,584,724 | 0.90 *** | 0.43 *** | 0.83 *** | 0.79 *** | 0.38 *** | 0.38 *** |
| NDI574,722,590 | 0.90 *** | 0.44 *** | 0.83 *** | 0.80 *** | 0.39 *** | 0.38 *** |
| NDI526,664,700 | 0.45 *** | 0.97 *** | 0.73 *** | 0.71 *** | 0.79 *** | 0.54 *** |
| NDI524,700,664 | 0.44 *** | 0.97 *** | 0.72 *** | 0.71 *** | 0.79 *** | 0.53 *** |
| NDI628,412,694 | 0.83 *** | 0.69 *** | 0.93 *** | 0.91 *** | 0.62 *** | 0.55 *** |
| NDI628,410,694 | 0.83 *** | 0.73 *** | 0.94 *** | 0.92 *** | 0.65 *** | 0.56 *** |
| NDI580,568,594 | 0.82 *** | 0.75 *** | 0.94 *** | 0.92 *** | 0.65 *** | 0.56 *** |
| NDI578,590,566 | 0.80 *** | 0.76 *** | 0.94 *** | 0.92 *** | 0.66 *** | 0.57 *** |
| NDI620,616,630 | 0.49 *** | 0.93 *** | 0.76 *** | 0.73 *** | 0.80 *** | 0.58 *** |
| NDI568,550,600 | 0.48 *** | 0.94 *** | 0.75 *** | 0.75 *** | 0.81 *** | 0.56 *** |
| NDI596,598,594 | 0.52 *** | 0.41 *** | 0.62 *** | 0.59 *** | 0.43 *** | 0.56 *** |
| NDI624,632,620 | 0.55 *** | 0.75 *** | 0.77 *** | 0.74 *** | 0.70 *** | 0.65 *** |
| NDI596,588,604 | 0.59 *** | 0.75 *** | 0.80 *** | 0.76 *** | 0.71 *** | 0.66 *** |
| V | Indices | Optimal Features | Parameters (Md, Ms, Mln) | Training | Cross-Validation | Testing | |||
|---|---|---|---|---|---|---|---|---|---|
| R2 | RMSE | R2 | RMSE | R2 | RMSE | ||||
| L* | Spectral | NDI970, 670, PRMI, NDI526,664,700, NDI524,700,664, NDI800, 640, NDI596,598,594, NDI686, 620, NDI572,584,724, NDI574,722,590, NDI574,592,724 | (5, 2, 10) | 0.965 | 1.233 | 0.908 | 1.499 | 0.780 | 3.205 |
| RGB | ExB | (5, 4, 10) | 0.898 | 2.106 | 0.805 | 2.238 | 0.880 | 2.371 | |
| Total | NDI524,700,664, VEG, PRMI, NDI596,598,594, NDI686, 620, NDI574,592,724, NDI572,584,724, ExB | (5, 2, none) | 0.988 | 0.730 | 0.904 | 1.470 | 0.779 | 3.208 | |
| a* | Spectral | PRMI, NDI578,590,566, NDI826, 670, GI, NDI568,550,600, NDI800, 640, NDI686, 620, NDI620,616,630, NDI524,700,664, NDI628,412,694, NDI780, 550, NAI, NDI628,410,694 | (5, 6, 10) | 0.987 | 1.641 | 0.972 | 1.553 | 0.940 | 3.362 |
| RGB | bn, VEG, COM, ExB, ExG, GBRI, WI, ExGR | (3, 2, none) | 0.984 | 1.852 | 0.956 | 1.903 | 0.959 | 2.774 | |
| Total | NDI526,664,700, NDI826, 670, rn, ExB, PRMI, NDI628,410,694, NDI596,598,594, VEG, NDI686, 620, NCI, NDI568,550,600, NDI800, 640, NDI572,584,724, NDI970, 670, NDI578,590,566, ExG, COM, bn, NDI580,568,594, gn, GI, ExGR, WI, NDI624,632,620, NDI524,700,664, NDI574,592,724, NDI628,412,694, NDI780, 550, NAI, GBRI | (7, 2, 40) | 0.999 | 0.514 | 0.986 | 1.121 | 0.964 | 2.604 | |
| b* | Spectral | rn, NDI574,592,724, GI, NDI970, 670 | (5, 2, 30) | 0.993 | 0.998 | 0.951 | 1.973 | 0.929 | 3.296 |
| RGB | ’ExGR, GBRI | (3, 4, none) | 0.968 | 2.162 | 0.949 | 2.057 | 0.951 | 2.739 | |
| Total | VEG, NDI628,410,694, NDI574,722,590, ExGR, NDI970, 670 | (7, 2, 40) | 0.998 | 0.551 | 0.957 | 1.713 | 0.929 | 3.309 | |
| Chlm | Spectral | NDI624,632,620, NDI596,588,604, NDI568,550,600, NDI578,590,566, NDI524,700,664, NDI628,412,694 | (7, 2, 20) | 0.996 | 0.974 | 0.949 | 2.038 | 0.944 | 3.762 |
| RGB | COM, GBRI, ExG, ExGR, gn, ExB, WI | (3, 2, 10) | 0.962 | 3.153 | 0.938 | 2.477 | 0.874 | 5.659 | |
| Total | NDI628,412,694, rn, NDI624,632,620, NDI568,550,600, NDI596,588,604, NDI578,590,566, NDI524,700,664, WI | (9, 2, none) | 0.998 | 0.722 | 0.952 | 1.801 | 0.922 | 4.442 | |
| TSS | Spectral | NDI628,410,694, NDI578,590,566 | (5, 2, 20) | 0.941 | 0.494 | 0.829 | 0.602 | 0.432 | 1.348 |
| RGB | COM, GBRI, ExG, bn, WI | (5, 4, 10) | 0.911 | 0.606 | 0.792 | 0.661 | 0.705 | 0.971 | |
| Total | bn, NDI 628,410,694,WI | (3, 6, none) | 0.898 | 0.648 | 0.828 | 0.595 | 0.674 | 1.022 | |
| TSS/Acid | Spectral | NDI580,568,594, NDI568,550,600, NDI596,598,594, NDI596,588,604, NDI628,410,694 | (5, 2, none) | 0.959 | 0.777 | 0.657 | 1.632 | 0.328 | 2.346 |
| RGB | VEG, GBRI, ExGR, bn, ExG | (7, 2, 10) | 0.846 | 1.503 | 0.578 | 1.800 | 0.173 | 2.603 | |
| Total | NDI568,550,600, NDI970, 670, NDI596,598,594, NDI596,588,604, NDI628,410,694 | (5, 2, 10) | 0.905 | 1.177 | 0.688 | 1.552 | 0.484 | 2.056 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galal, H.; Elsayed, S.; Elsherbiny, O.; Allam, A.; Farouk, M. Using RGB Imaging, Optimized Three-Band Spectral Indices, and a Decision Tree Model to Assess Orange Fruit Quality. Agriculture 2022, 12, 1558. https://doi.org/10.3390/agriculture12101558
Galal H, Elsayed S, Elsherbiny O, Allam A, Farouk M. Using RGB Imaging, Optimized Three-Band Spectral Indices, and a Decision Tree Model to Assess Orange Fruit Quality. Agriculture. 2022; 12(10):1558. https://doi.org/10.3390/agriculture12101558
Chicago/Turabian StyleGalal, Hoda, Salah Elsayed, Osama Elsherbiny, Aida Allam, and Mohamed Farouk. 2022. "Using RGB Imaging, Optimized Three-Band Spectral Indices, and a Decision Tree Model to Assess Orange Fruit Quality" Agriculture 12, no. 10: 1558. https://doi.org/10.3390/agriculture12101558
APA StyleGalal, H., Elsayed, S., Elsherbiny, O., Allam, A., & Farouk, M. (2022). Using RGB Imaging, Optimized Three-Band Spectral Indices, and a Decision Tree Model to Assess Orange Fruit Quality. Agriculture, 12(10), 1558. https://doi.org/10.3390/agriculture12101558









