Soil Microbial Community Driven by Soil Moisture and Nitrogen in Milk Vetch (Astragalus sinicus L.)–Rapeseed (Brassica napus L.) Intercropping
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Sampling
2.4. Soil Physico-Chemical Analysis
2.5. Soil Microbial Community
2.6. Statistical Analysis
3. Results
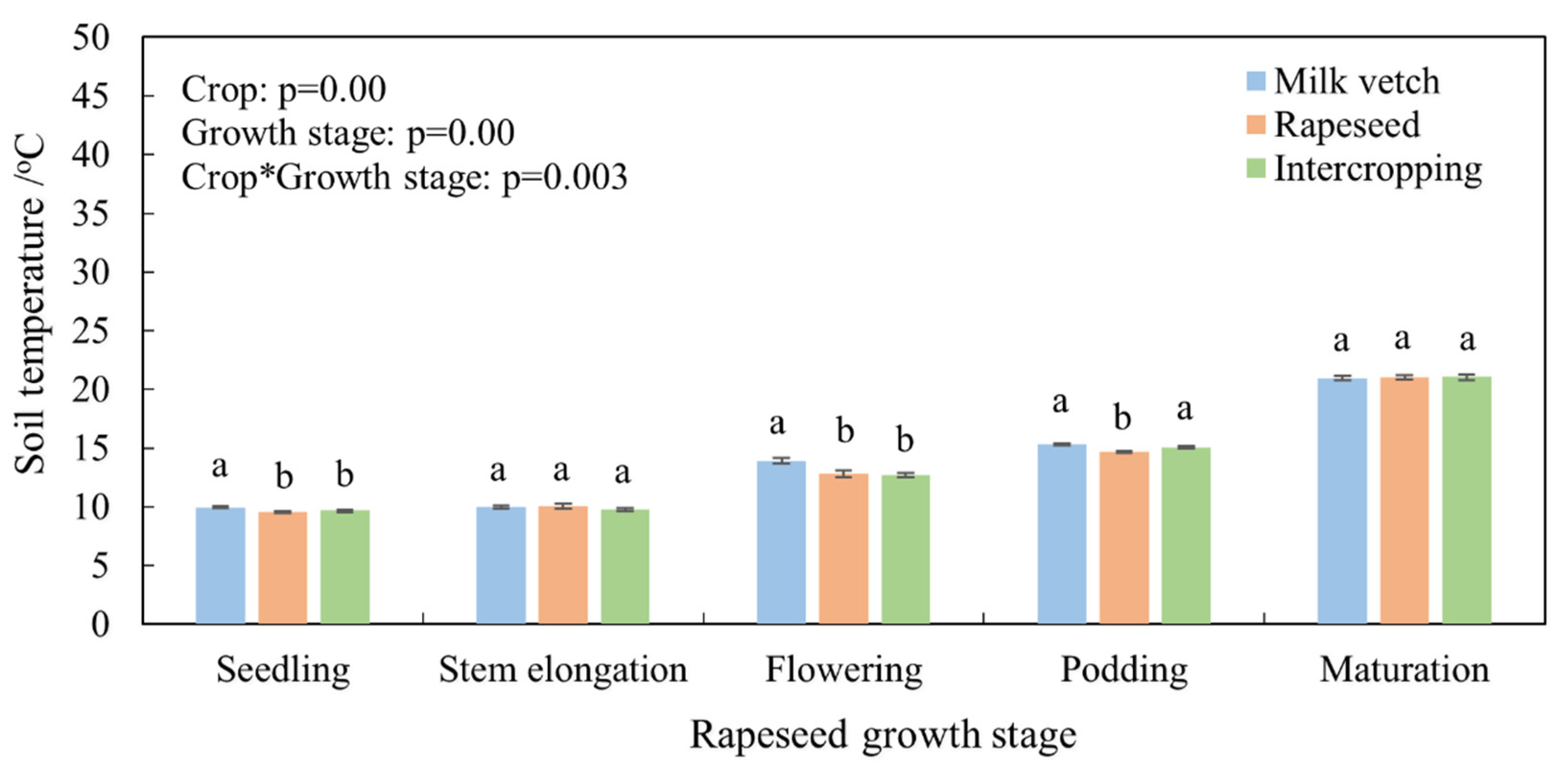
3.1. Soil Temperature
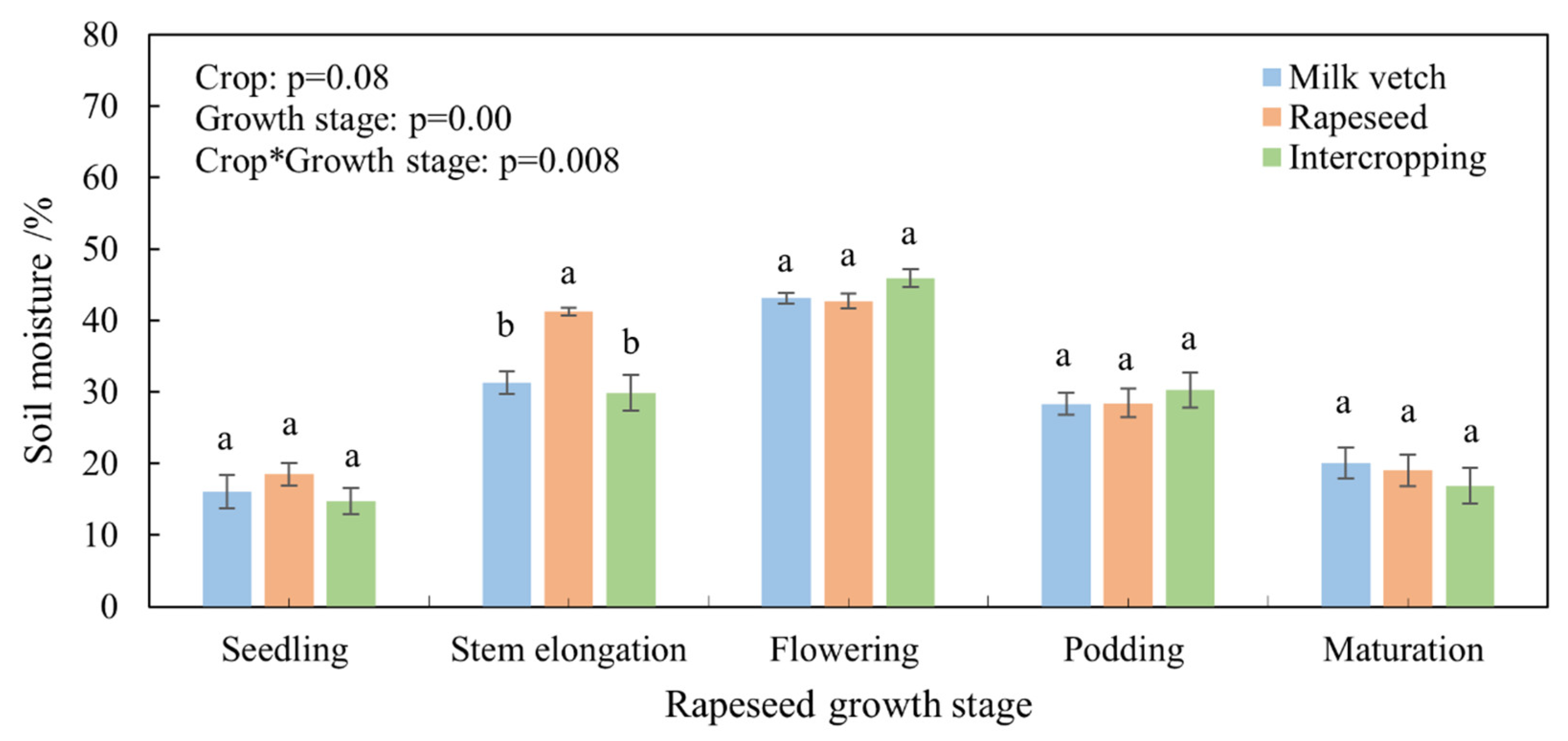
3.2. Soil Moisture
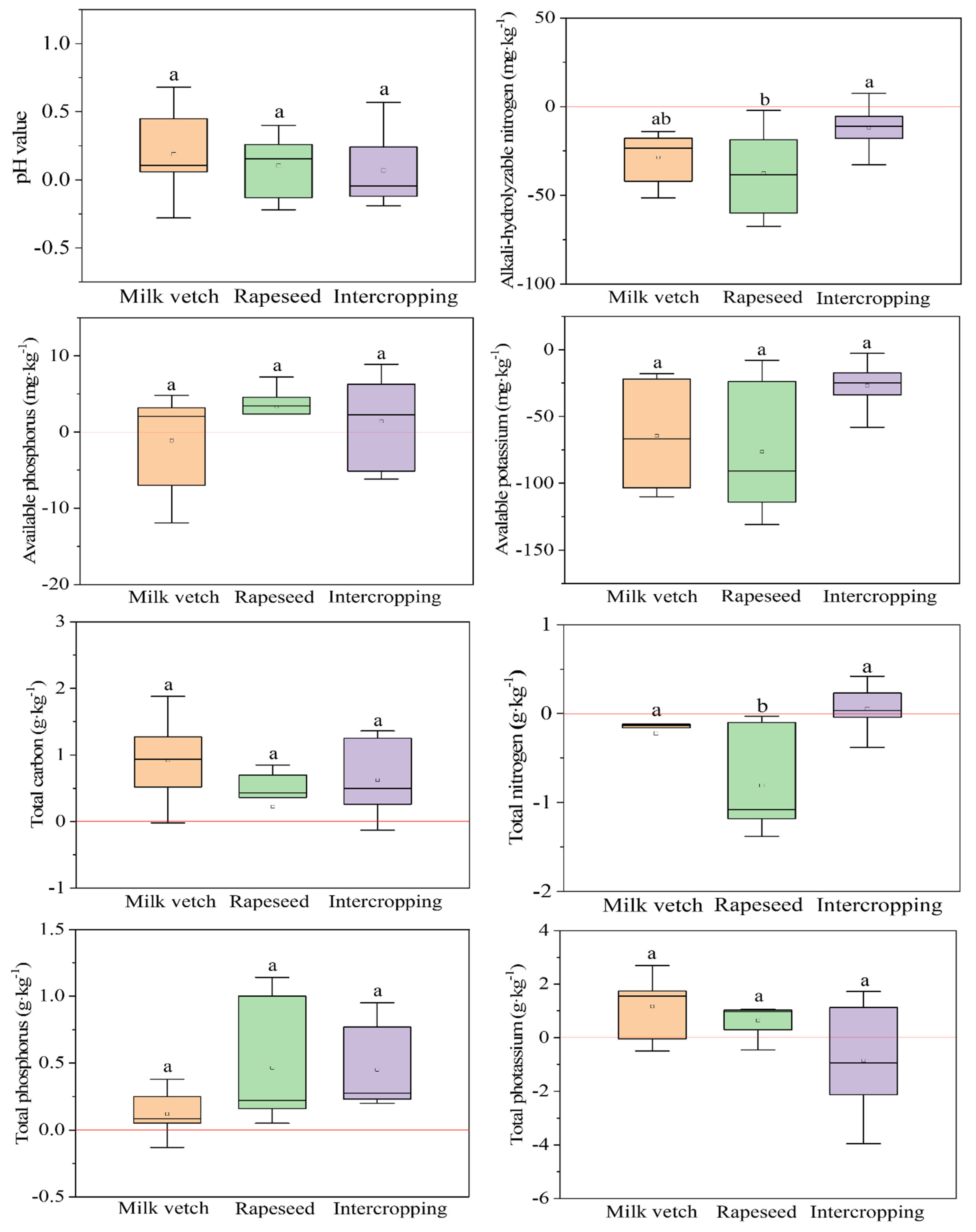
3.3. Soil Nutrients
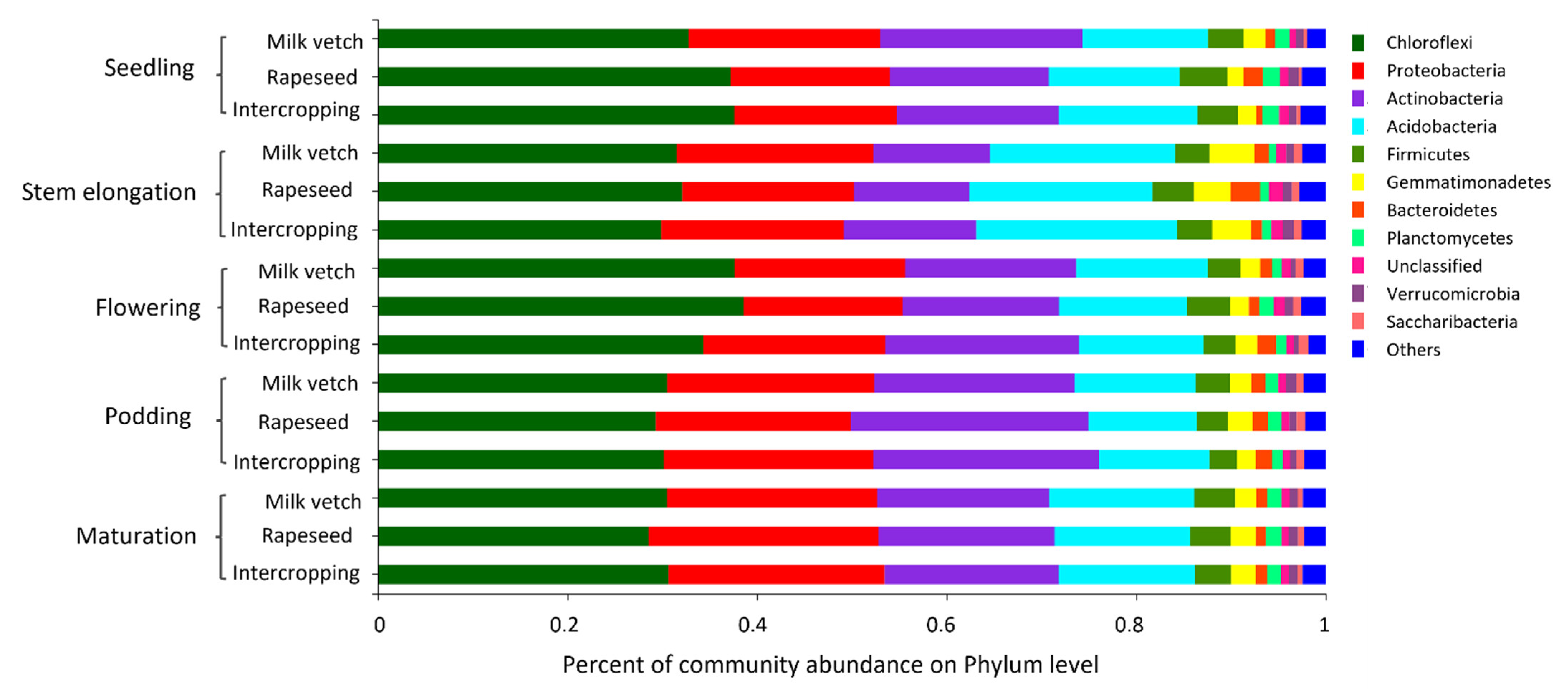
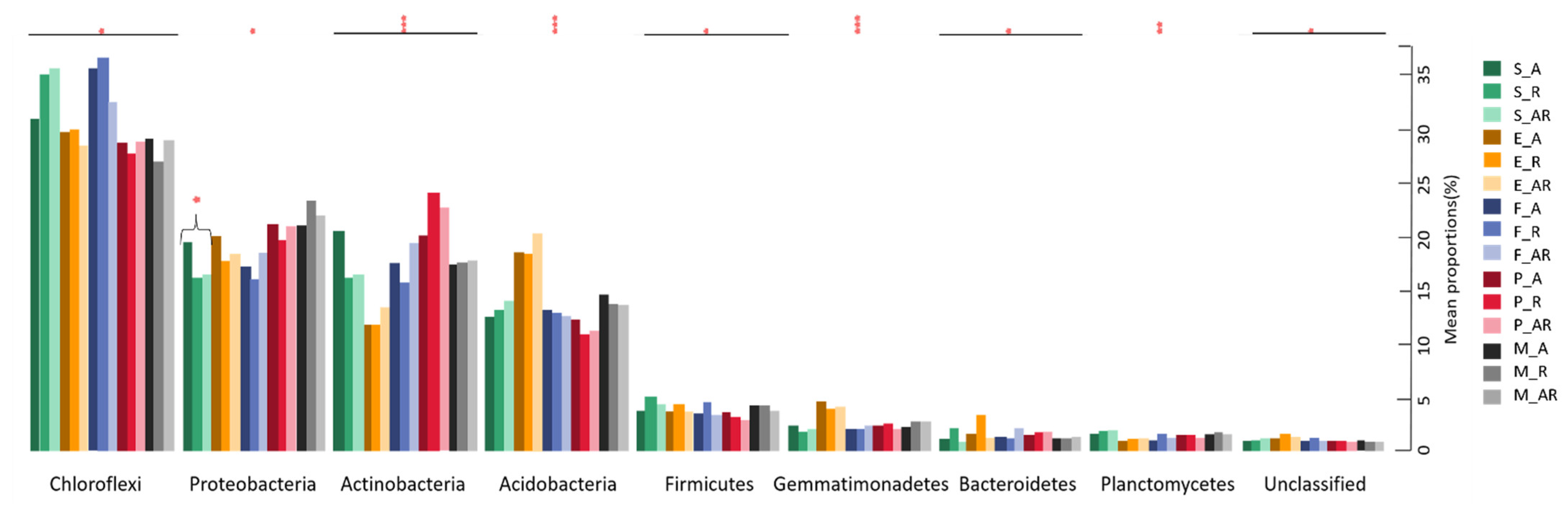
3.4. Soil Microbial Community Abundance
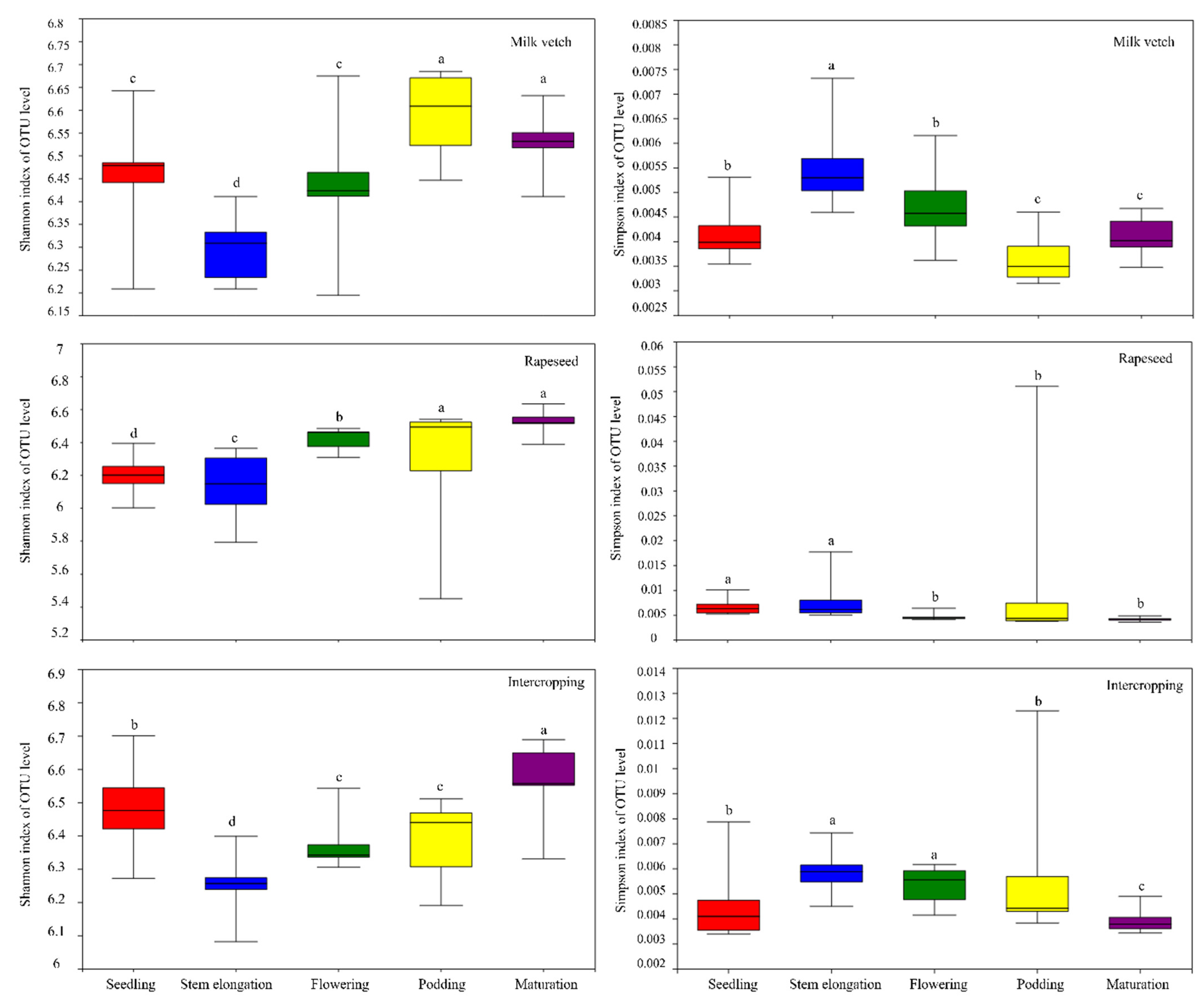
3.5. Soil Microbial Community Diversity
3.6. Correlation between Soil Microorganisms and Environmental Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pablo, G.P.; Vandegehuchte, M.L.; Shaw, E.A.; Marie, D. Are there links between responses of soil microbes and ecosystem functioning to elevated CO2, N deposition and warming? A global perspective. Glob. Chang. Biol. 2020, 21, 1590–6000. [Google Scholar] [CrossRef]
- Kumar, A.; Padhy, S.R.J.; Das, R.R. Elucidating relationship between nitrous oxide emission and functional soil microbes from tropical lowland rice soil exposed to elevated CO2: A path modelling approach. Agric. Ecosyst. Environ. 2021, 308, 107268. [Google Scholar] [CrossRef]
- Li, C.P.; Shi, W.C.; Wu, D. Biocontrol of potato common scab by Brevibacillus laterosporus BL12 is related to the reduction of pathogen and changes in soil bacterial community. Biol. Control 2021, 153, 104496. [Google Scholar] [CrossRef]
- A’Bear, A.D.; Jones, T.H.; Kandeler, E.; Boddy, L. Interactive effffects of temperature and soil moisture on fungal-mediated wood decomposition and extracellular enzyme activity. Soil Biol. Biochem. 2014, 70, 151–158. [Google Scholar] [CrossRef]
- Wang, D.M.; Marschner, P.; Solaiman, Z.; Rengel, Z. Growth, P uptake and rhizosphere properties of intercropped wheat and chickpea in soil amended with iron phosphate or phytate. Soil Biol. Biochem. 2006, 39, 249–256. [Google Scholar] [CrossRef]
- Ricardo, S.S.; Luis, A.P.L.N.; Jadson, E.L.A.; Ademir, S.F.A. Maize rhizosphere soil stimulates greater soil microbial biomass and enzyme activity leading to subsequent enhancement of cowpea growth. Environ. Sustain. 2019, 2, 89–94. [Google Scholar] [CrossRef]
- Bao, X.; Zhu, X.; Chang, X.; Wang, S.; Xu, B.; Luo, C. Effects of Soil Temperature and Moisture on Soil Respiration on the Tibetan Plateau. PLoS ONE 2016, 11, e0165212. [Google Scholar] [CrossRef]
- Luo, C.Y.; Xu, G.P.; Wang, Y.F.; Wang, S.P.; Lin, X.W.; Hu, Y.G. Effects of grazing and experimental warming on DOC concentrations in the soil solution on the Qinghai-Tibet plateau. Soil Biol. Biochem. 2009, 41, 2493–2500. [Google Scholar] [CrossRef]
- Luo, C.Y.; Xu, G.P.; Chao, Z.G.; Wang, S.P.; Lin, X.W.; Hu, Y.G. Effect of warming and grazing on litter mass loss and temperature sensitivity of litter and dung mass loss on the Tibetan plateau. Glob. Chang. Biol. 2010, 16, 1606–1617. [Google Scholar] [CrossRef]
- Curtin, D.; Beare, M.H.; Hernandez-Ramirez, G. Temperature and moisture effects on microbial biomass and soil organic matter mineralization. Soil Sci. Soc. Am. J. 2012, 76, 2055–2067. [Google Scholar] [CrossRef]
- Rivest, D.; Cogliastro, A.; Bradley, R.L.; Olivier, A. Intercropping hybrid poplar with soybean increases soil microbial biomass, mineral N supply and tree growth. Agrofor. Syst. 2010, 80, 33–40. [Google Scholar] [CrossRef]
- Li, X.P.; Mu, Y.H.; Cheng, Y.B.; Liu, X.G.; Nian, H. Effects of intercropping sugarcane and soybean on growth, rhizosphere soil microbes, nitrogen and phosphorus availability. Acta Physiol. Plant 2013, 35, 1113–1119. [Google Scholar] [CrossRef]
- Garbeva, P.; Van, V.J.; Van, E.J. Microbial diversity in soil: Selection of microbial populations by plant and soil type and implications for disease suppressiveness. Annu. Rev. Phytopathol. 2004, 42, 243–270. [Google Scholar] [CrossRef]
- Song, Y.N.; Zhang, F.S.; Marschner, P. Effect of intercropping on crop yield and chemical and microbiological properties in rhizosphere of wheat (Triticum aestivum L.), maize (Zea mays L.), and faba bean (Vicia faba L.). Biol. Fertil. Soils 2007, 43, 565–574. [Google Scholar] [CrossRef]
- Salles, J.F.; Van Veen, J.A.; van Elsas, J.D. Multivariate analyses of Burkholderia species in soil: Effect of crop and land use history. Appl. Environ. Microb. 2004, 70, 4012–4020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.C.; Wang, H.L.; Yu, X.F.; Hou, H.Z.; Fang, Y.J.; Ma, Y.F. The Study on the Effect of Potato and Beans Intercropping with Whole Field Plastics Mulching and Ridge-Furrow Planting on Soil Thermal-Moisture Status and Crop Yield on Semi-Arid Area. Sci. Agric. Sin. 2016, 49, 468–481. [Google Scholar]
- Xu, S.; Liu, X.W.; Cui, D.J.; Du, H.Y.; Zhao, Y.H. Effect of different fertilization treatments on cotton growth, soil microbes and enzyme activity in Saline field. J. Soil Water Conserv. 2015, 29, 316–332. [Google Scholar]
- Gong, X.W.; Liu, C.J.; Li, J.; Luo, Y.; Yang, Q.H.; Zhang, W.L.; Yang, P.; Feng, B.L. Responses of rhizosphere soil properties, enzyme activities and microbial diversity to intercropping patterns on the Loess Plateau of China. Soil Tillage Res. 2019, 195, 104355. [Google Scholar] [CrossRef]
- Veen, J.A.V.; Kuikman, P.J. Soil structural aspects of decomposition of organic matter by micro-organisms. Biogeochemistry 1990, 11, 213–233. [Google Scholar] [CrossRef]
- Blagodatskaya, E.; Kuzyakov, Y. Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: Critical review. Biol. Fertil. Soils 2008, 45, 115–131. [Google Scholar] [CrossRef]
- Xue, Y.; Xia, H.; Christie, P.; Zhang, Z.; Li, L.; Tang, C.X. Crop acquisition of phosphorus, iron and zinc from soil in cereal/legume intercropping systems: A critical review. Ann. Bot. 2016, 117, 363–377. [Google Scholar] [CrossRef]
- Jensen, E.S. Grain yield, symbiotic N2 fixation and interspecifific competition for inorganic N in pea-barley intercrops. Plant Soil 1996, 182, 25–38. [Google Scholar] [CrossRef]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef] [PubMed]
- Mulder, L.; Hogg, B.; Bersoult, A.; Cullimore, J.V. Integration of signalling pathways in the establishment of the legume-rhizobia symbiosis. Physiol. Plant 2005, 123, 207–218. [Google Scholar] [CrossRef]
- Guo, F.; Wang, M.; Si, T.; Wang, Y.F.; Zhao, H.J.; Zhang, X.J.; Yu, X.N. Maize-peanut intercropping led to an optimization of soil from the perspective of soil microorganism. Arch. Agron. Soil Sci. 2021, 67, 1986–1999. [Google Scholar] [CrossRef]
- Chen, J.; Arafat, Y.; Wu, L.K.; Xiao, Z.G.; Li, Q.S. Shifts in soil microbial community, soil enzymes and crop yield under peanut/maize intercropping with reduced nitrogen levels. Appl. Soil Ecol. 2018, 124, 327–334. [Google Scholar] [CrossRef]
- Dube, E.D.N.; Madanzi, T.; Kapenzi, A.; Masvaya, E. Root length density in maize/cowpea intercropping under a basin tillage system in a semi-arid area of Zimbabwe. Am. J. Plant Sci. 2014, 5, 1499–1507. [Google Scholar] [CrossRef][Green Version]
- Pelzer, E.; Hombert, N.; Jeuffroy, M.H.; Makowski, D. Meta-analysis of the effect of nitrogen fertilization on annual cereal–legume intercrop production. Agron. J. 2014, 106, 1775–1786. [Google Scholar] [CrossRef]
- Trail, P.; Abaye, O.; Thomason, W.E.; Thompson, T.L.; Gueye, F.; Diedhiou, I.; Diatta, M.B.; Faye, A. Evaluating intercropping (living cover) and mulching (desiccated cover) practices for increasing millet yields in Senegal. Agron. J. 2016, 108, 1742–1752. [Google Scholar] [CrossRef]
- Xiang, Y.C.; Guan, C.Y.; Huang, H. Effects of Intercropping on Accumulation of Cd and Pb in Oilseed Rapeseed. J. Soil Water Conserv. 2010, 24, 50–55. [Google Scholar]
- Zhou, Q.; Wang, L.C.; Ma, S.M. Influences of rapeseed intercropping with Chinese milk vetch and straw mulching on productive benefits in dryland of Southwest China. Acta Agron. Sin. 2018, 44, 431–441. [Google Scholar] [CrossRef]
- Dubach, M.; Russelle, M.P. Forage legume roots and nodules and their role in nitrogen transfer. Agron. J. 1994, 86, 259–266. [Google Scholar] [CrossRef]
- Temperton, V.M.; Mwangi, P.N.; Scherer-Lorenzen, M.; Schmid, B.; Buchmann , N. Positive interactions between nitrogen-fixing legumes and four different neighbouring species in a biodiversity experiment. Oecologia 2007, 151, 190–205. [Google Scholar] [CrossRef]
- Guo, Z.Y.; Kong, C.H.; Wang, J.G.; Wang, Y.F. Rhizosphere isoflavones (daidzein and genistein) levels and their relation to the microbial community structure of mono-cropped soybean soil in field and controlled conditions. Soil Biol. Biochem. 2011, 43, 2257–2264. [Google Scholar] [CrossRef]
- Li, B.; Li, Y.Y.; Wu, H.M.; Zhang, F.F.; Li, C.J.; Li, X.X.; Lambers, H.; Li, L. Root exudates drive interspecific facilitation by enhancing nodulation and N2 fixation. Proc. Natl. Acad. Sci. USA 2016, 113, 6496–6501. [Google Scholar] [CrossRef] [PubMed]
- Moreau, D.; Bardgett, R.D.; Finlay, R.D.; Jones, D.L.; Philippot, L. A plant perspective on nitrogen cycling in the rhizosphere. Funct. Ecol. 2019, 33, 540–552. [Google Scholar] [CrossRef]
- Maj, D.; Wielbo, J.; Marek-Kozaczuk, M.; Skorupska, A. Response to flavonoids as a factor influencing competitiveness and symbiotic activity of Rhizobium leguminosarum. Microbiol. Res. 2010, 165, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Chen, J.; Xing, Y.; Wang, L.C. Influence of intercropping Chinese milk vetch on the soil microbial community in rhizosphere of rapeseed. Plant Soil 2019, 440, 85–96. [Google Scholar] [CrossRef]
- Bi, X.; Ren, L.; Gong, M.; He, Y.S.; Wang, L.; Ma, Z.D. Transfer of cadmium and lead from soil to mangoes in an uncontaminated area, Hainan Island, China. Geoderma 2010, 155, 115–120. [Google Scholar] [CrossRef]
- Fei, K.; Deng, L.Z.; Zhang, L.P.; Sun, T.Y.; Wu, Y.H. Lateral transport of soil total carbon with slope runoff and interflow: Effects of rainstorm characteristics under simulated rainfall. Catena 2019, 179, 39–48. [Google Scholar] [CrossRef]
- Wang, X.; Xiang, Z.; Li, C.; Zhu, J.; Wu, J.; Zhang, M.J. Optimization of the Method for Determination of Total Nitrogen in Soil by Automatic Kjeldahl Apparatus. J. Shandong Agric. Univ. (Nat. Sci. Ed.) 2020, 51, 438–440. [Google Scholar]
- Zheng, Y.K.; Fu, W.D.; Zhu, R.C.; Hu, Z.B.; Chen, G. Determination of total phosphorus in soil and sludge by an effective headspace gas chromatographic method. RSC Adv. 2019, 9, 40961–40965. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.L.; Yang, H.K.; Song, W.C.; Liu, C.Y.; Xu, J. Effect of N fertilization rate on soil alkali-hydrolyzable N, subtending leaf N concentration, fiber yield, and quality of cotton. Crop J. 2016, 4, 323–330. [Google Scholar] [CrossRef]
- Fu, M.M.; Huang, B.; Jia, M.M.; Hu, W.Y.; Sun, W.X. Effect of intensive greenhouse vegetable cultivation on selenium availability in soil. Pedosphere 2015, 25, 343–350. [Google Scholar] [CrossRef]
- Kwon, H.Y.; Hudson, R.; Mulvaney, R. Characterization of the organic nitrogen fraction determined by the Illinois soil nitrogen test. Soil Sci. Soc. Am. J. 2009, 73, 1033–1043. [Google Scholar] [CrossRef]
- Xu, N.; Tan, G.; Wang, H.; Gai, X.P. Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur. J. Soil Biol. 2016, 74, 1–8. [Google Scholar] [CrossRef]
- Nassab, A.D.; Amon, T.; Kaul, H.P. Competition and yield in intercrops of maize and sunflower for biogas. Ind. Crops Prod. 2011, 34, 1203–1211. [Google Scholar] [CrossRef]
- Yin, W.; Chen, G.P.; Feng, F.X.; Guo, Y.; Hu, F.L. Straw retention combined with plastic mulching improves compensation of intercropped maize in arid environment. Field Crops Res. 2017, 204, 42–51. [Google Scholar] [CrossRef]
- Tang, W.G.; Xiao, X.P.; Tang, H.M.; Yang, G.L. Effects of Different Planting Patterns on Water Use of Soil and Crops Annual Productivity in Southern Hilly Dryland. Sci. Agric. Sin. 2014, 47, 3606–3617. [Google Scholar]
- Zhao, B.Q.; Zhang, F.S.; Li, Z.J. Vertical distribution and its change of root quantity & activity of crops in the “winter wheat early spring maize/summer maize” cropping system: II. The vertical distribution and its changes of root quantity & activity of the early spring inter-plant. Acta Agron. Sin. 2001, 27, 974–980. [Google Scholar]
- Shili-Touzi, I.; Tourdonnet, S.D.; Launay, M.; Dore, T. Does intercropping winter wheat (Triticum aestivum) with red fescue (Festuca rubra) as a cover crop improve agronomic and environmental performance? A modeling approach. Field Crops Res. 2010, 116, 218–229. [Google Scholar] [CrossRef]
- Ouyang, Y.; Reeve, J.R.; Norton, J.M. Soil enzyme activities and abundance of microbial functional genes involved in nitrogen transformations in an organic farming system. Biol. Fertil. Soils 2018, 54, 437–450. [Google Scholar] [CrossRef]
- Giller, K.E.; Ormesher, J.; Awah, F.M. Nitrogen transfer from phaseolus bean to intercropped maize measured using 15N-enrichment and 15N-isotope dilution methods. Soil Biol. Biochem. 1991, 23, 339–346. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, L.; Zhang, F.S. Effect of root contact on interspecifific competition and N transfer between wheat and fababean using direct and indirect 15N techniques. Plant Soil 2004, 262, 45–54. [Google Scholar] [CrossRef]
- Hauggaard-Nielsen, H.; Ambus, P.; Jensen, E.S. The comparison of nitrogen use and leaching in sole cropped versus intercropped pea and barley. Nutr. Cycl. Agroecosyst. 2003, 65, 289–300. [Google Scholar] [CrossRef]
- Wang, Y.J.; Wang, T.Q.; Hou, Z.J.; Wang, T.Q.; Zhou, Q. Responses of root exudates to intercropping of Chinese milk vetch with rapeeed. Chin. J. Appl. Ecol. 2021, 32, 1783–1790. [Google Scholar] [CrossRef]
- Chen, C.P.; Cheng, C.H.; Huang, Y.H.; Chen, C.T.; Lai, C.M. Converting leguminous green manure into biochar: Changes in chemical composition and C and N mineralization. Geoderma 2014, 232, 581–588. [Google Scholar] [CrossRef]
- Hassan, S.; Mathesius, U. The role of flavonoids in root-rhizosphere signalling: Opportunities and challenges for improving plant–microbe interactions. J. Exp. Bot. 2012, 63, 3429–3444. [Google Scholar] [CrossRef]
- Begum, A.A.; Leibovitch, S.; Migner, P.; Zhang, F. Specific flavonoids induced nod gene expression and pre-activated nod genes of Rhizobium leguminosarum increased pea (Pisum sativum L.) and lentil (Lens culinaris L.) nodulation in controlled growth chamber environments. J. Exp. Bot. 2001, 52, 1537–1543. [Google Scholar] [CrossRef]
- Shu, X.Y.; He, J.; Zhou, Z.H. Organic amendments enhance soil microbial diversity, microbial functionality and crop yields: A meta-analysis. Sci. Total Environ. 2022, 829, 154627. [Google Scholar] [CrossRef]
- Bargaza, A.; Noyceb, G.L.; Fulthorpe, R.; Carlssona, G.; Furze, J.R. Species interactions enhance root allocation, microbial diversity and P acquisition in intercropped wheat and soybean under P deficiency. Appl. Soil Ecol. 2017, 120, 179–188. [Google Scholar] [CrossRef]
- Morshedi, A.; Akbari, F.; Dahmardeh, M.; Ghanbari, A.; Khoramdel, S. The Influences of Tillage System and Plant Residue on Nitrogen Uptake and Use Efficiency in Corn and Bean Intercropping Systems. J. Crops Improv. 2018, 20, 785–799. (In Persian) [Google Scholar] [CrossRef]
- Hua, C.P.; Wang, Y.J.; Xie, Z.K.; Guo, Z.H.; Zhang, Y.B.; Qiu, Y.; Wang, L. Effects of intercropping on rhizosphere soil microorganisms and root exudates of Lanzhou lily (Lilium davidii var. unicolor). Sci. Cold Arid. Reg. 2018, 10, 0159–0168. [Google Scholar] [CrossRef]
- Corey, D.; Broeckling, A.; Broz, M.K.; Manter, D.K.; Vivanco, J.M. Root Exudates Regulate Soil Fungal Community Composition and Diversity. Appl. Environ. Microb. 2008, 74, 738–744. [Google Scholar]
- Neal, A.L.; Ahmad, S.; Gordon-Weeks, R.; Ton, J. Benzoxazinoids in root exudates of maize attract Pseudomonas putida to the rhizosphere. PLoS ONE 2012, 7, e35498. [Google Scholar] [CrossRef]
- Abdel-Lateif, K.; Bogusz, D.; Hocher, V. The role of flavonoids in the establishment of plant roots endosymbioses with arbuscular mycorrhiza fungi, Rhizobia, and Frankia bacteria. Plant Signal. Behav. 2012, 7, 636–641. [Google Scholar] [CrossRef]
- Fontainea, S.; Mariottib, A.; Abbadie, L. The priming effect of organic matter: A question of microbial competition? Soil Biol. Biochem. 2003, 35, 837–843. [Google Scholar] [CrossRef]
- Cong, W.F.; Hoffland, E.; Li, L.; Janssen, B.H.; Werf, W. Intercropping affects the rate of decomposition of soil organic matter and root litter. Plant Soil 2015, 391, 399–411. [Google Scholar] [CrossRef]
- Duchene, O.; Vian, J.F.; Celette, F. Intercropping with legume for agroecological cropping systems: Complementarity and facilitation processes and the importance of soil microorganisms. A review. Agric. Ecosyst. Environ. 2017, 240, 148–161. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Howarth, R.W. Nitrogen limitation on land and in the sea: How can it occur? Biogeochemistry 1991, 13, 87–115. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Li, S.; Liu, N.; Huang, G.; Zhou, Q. Soil Microbial Community Driven by Soil Moisture and Nitrogen in Milk Vetch (Astragalus sinicus L.)–Rapeseed (Brassica napus L.) Intercropping. Agriculture 2022, 12, 1538. https://doi.org/10.3390/agriculture12101538
Liu Z, Li S, Liu N, Huang G, Zhou Q. Soil Microbial Community Driven by Soil Moisture and Nitrogen in Milk Vetch (Astragalus sinicus L.)–Rapeseed (Brassica napus L.) Intercropping. Agriculture. 2022; 12(10):1538. https://doi.org/10.3390/agriculture12101538
Chicago/Turabian StyleLiu, Zeqin, Shujuan Li, Ning Liu, Guoqin Huang, and Quan Zhou. 2022. "Soil Microbial Community Driven by Soil Moisture and Nitrogen in Milk Vetch (Astragalus sinicus L.)–Rapeseed (Brassica napus L.) Intercropping" Agriculture 12, no. 10: 1538. https://doi.org/10.3390/agriculture12101538
APA StyleLiu, Z., Li, S., Liu, N., Huang, G., & Zhou, Q. (2022). Soil Microbial Community Driven by Soil Moisture and Nitrogen in Milk Vetch (Astragalus sinicus L.)–Rapeseed (Brassica napus L.) Intercropping. Agriculture, 12(10), 1538. https://doi.org/10.3390/agriculture12101538






