The Influence of Residue Mixing on the Decomposition of Pepper Root Residues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Site and Collection of Residues
2.2. Experimental Design
2.2.1. Incubation Experiment Design
2.2.2. Greenhouse Experiment Design
2.2.3. Litterbags Sampling and Respiration Measurement
2.2.4. Residues DNA Extraction
2.2.5. Quantitative PCR Analysis
2.2.6. High-Throughput Amplicon Sequencing
2.2.7. Statistical Analysis
3. Results
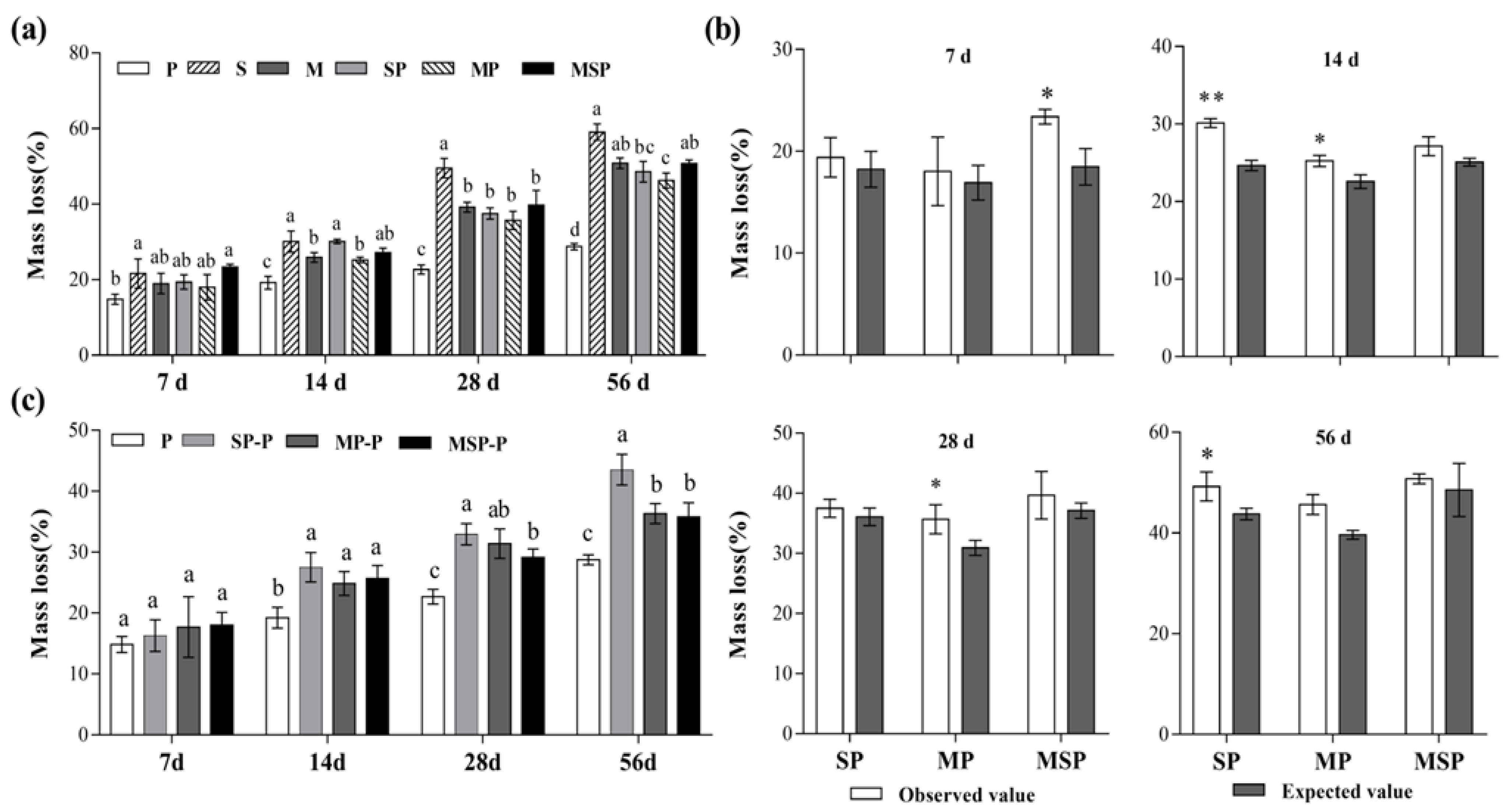
3.1. The Influence of Residue Mixing on the Decomposition Rate
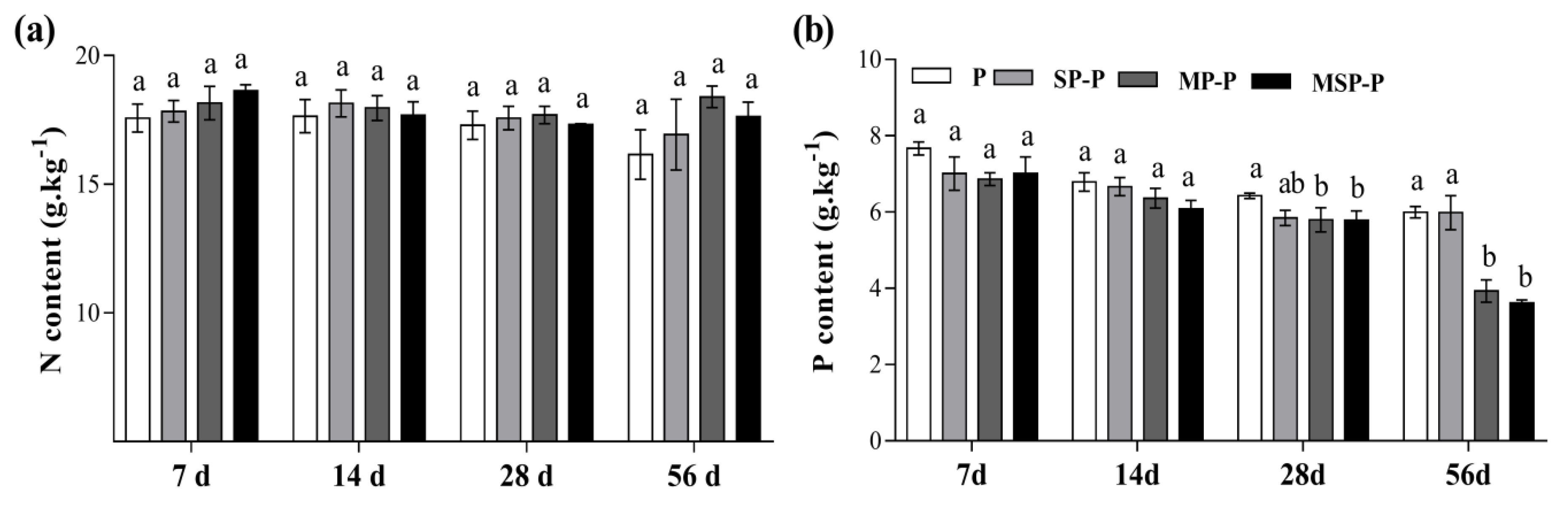
3.2. The Influence of Mixing Residues on Nitrogen and Phosphorus Contents in Pepper Root Residue
3.3. The Influence of Mixing Residues on Microbial Community in Pepper Root Residue
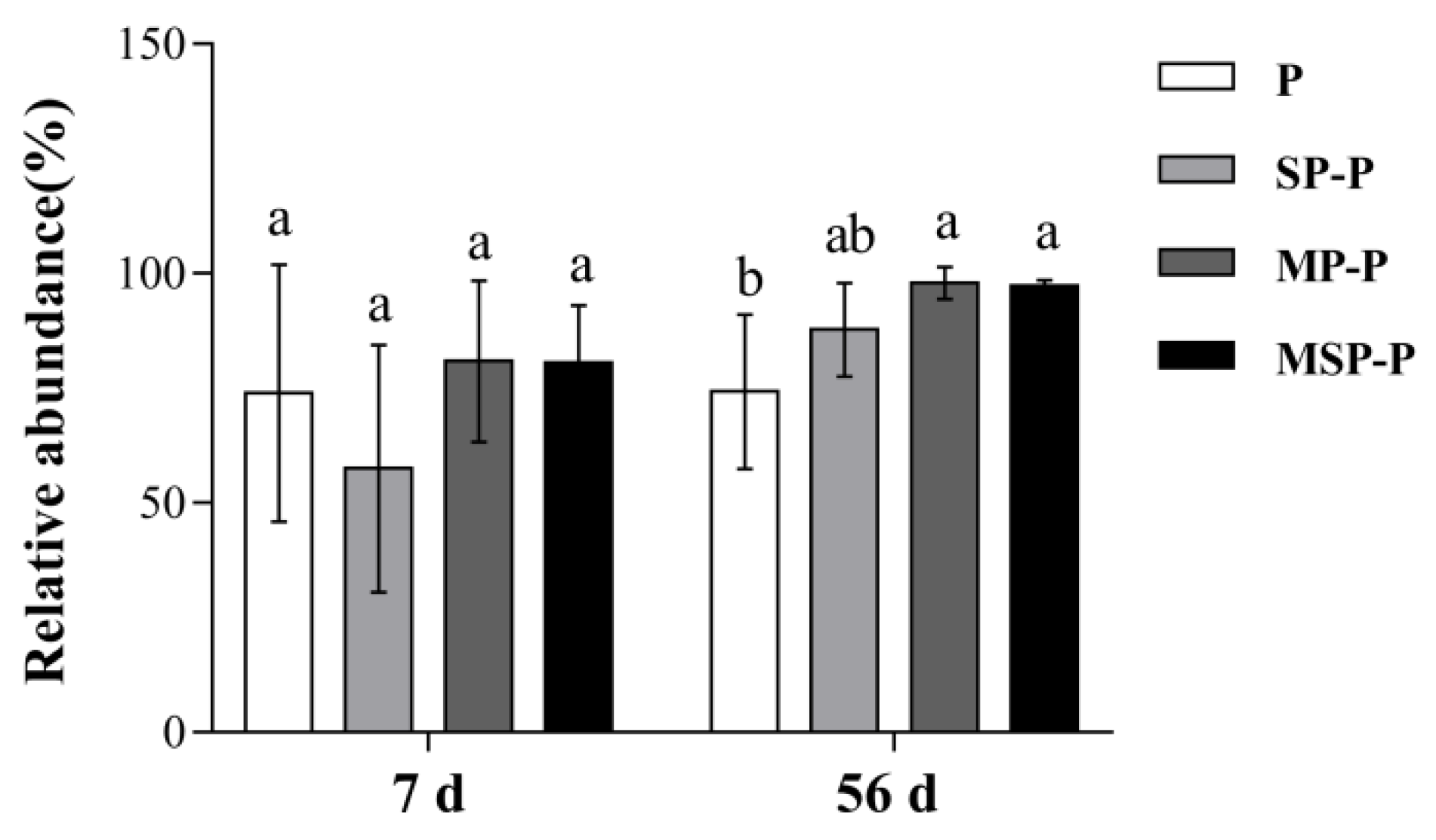
3.3.1. Effects of Residue Mixing on Bacterial and Fungal Community Abundance in Pepper Root Residue
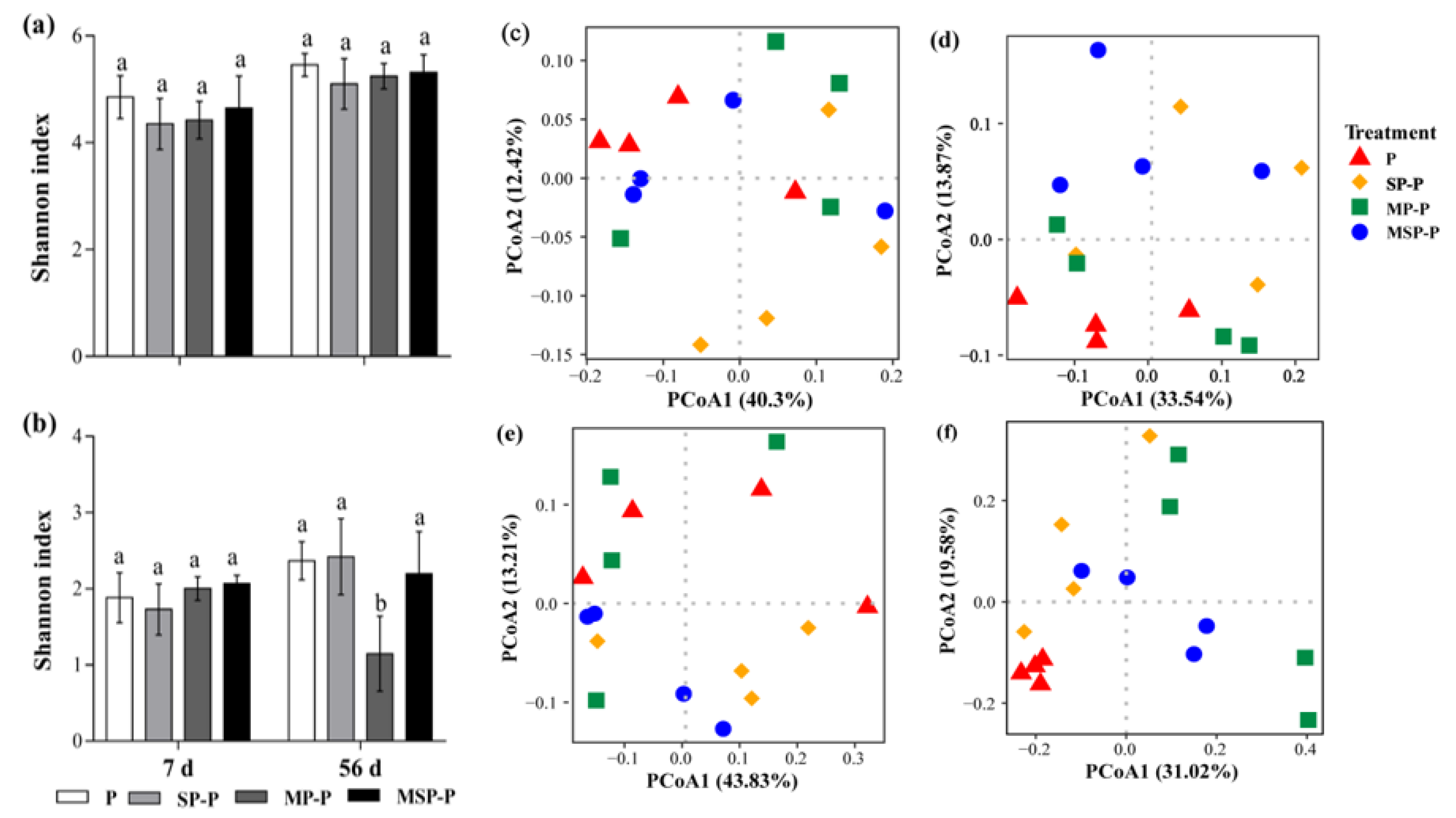
3.3.2. Effects of Residue Mixing on Bacterial and Fungal Community α- and β-Diversities of Pepper Root Residue
3.3.3. Effects of Residue Mixing on Relative Abundance of Bacteria and Fungi Community in Pepper Residue at the Level of Phylum
3.3.4. Effects of Residue Mixing on Relative Abundance of Bacteria and Fungi Community in Pepper Residue at the Genus Level
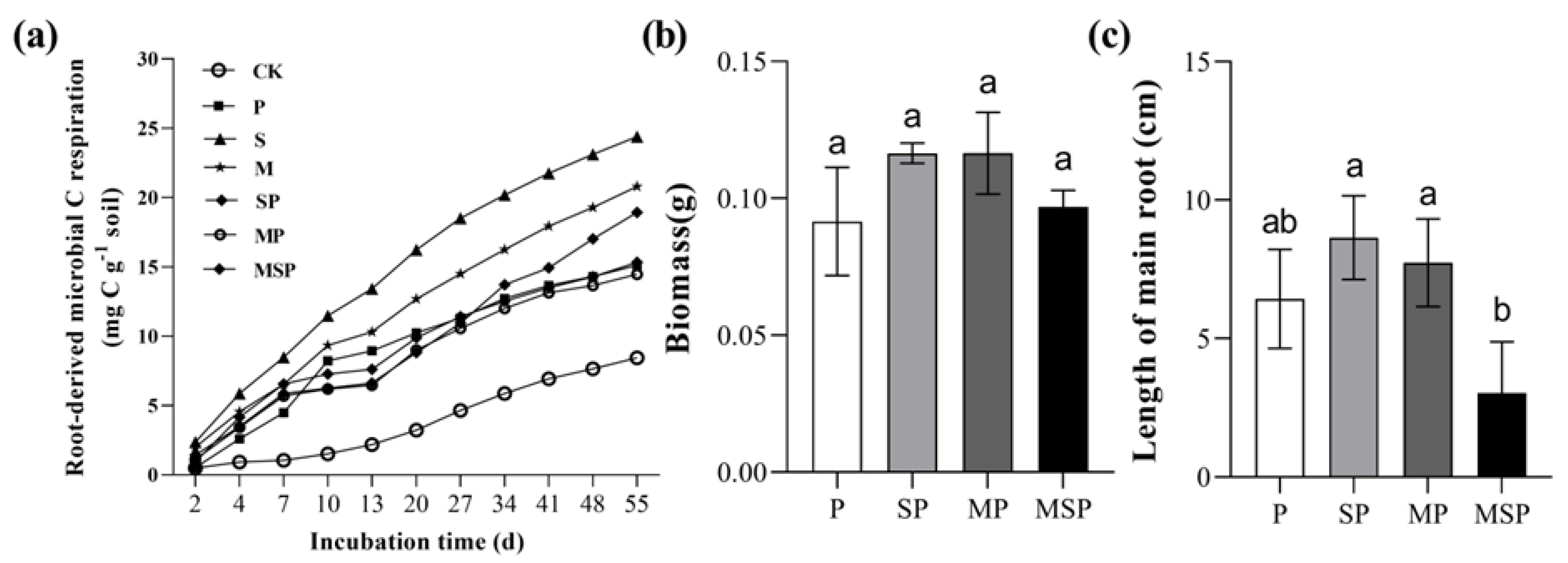
3.4. The Influence of Residues Mixing on Soil Respiration and Growth of Pepper Seedling
4. Discussion
4.1. Effect of Residue Mixing on Mass Loss of Pepper Root Residue and the Growth of Pepper Seedling
4.2. Effects of Residue Mixing on Nitrogen and Phosphorus Contents in Pepper Root Residue
4.3. Effects of Residue Mixing on Microbial Community in Pepper Residue
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zou, Z.; Zou, X. Geographical and ecological differences in pepper cultivation and consumption in China. Front. Nutr. 2021, 8, 718517. [Google Scholar] [CrossRef]
- Swan, C.M.; Gluth, M.A.; Horne, C.L. Leaf litter species evenness influences nonadditive breakdown in a headwater stream. Ecology 2009, 90, 1650–1658. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, J.; Pan, D.; Ge, X.; Jin, X.; Chen, S.; Wu, F. p-Coumaric can alter the composition of cucumber rhizosphere microbial communities and induce negative plant-microbial interactions. Biol. Fertil. Soils 2018, 54, 363–372. [Google Scholar] [CrossRef]
- Mao, R.; Zeng, D.-H. Non-additive effects vary with the number of component residues and their mixing proportions during residue mixture decomposition: A microcosm study. Geoderma 2012, 170, 112–117. [Google Scholar] [CrossRef]
- Abelho, M. Litter traits and decomposer complexity set the stage for a global decomposition model. Funct. Ecol. 2015, 30, 674–675. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Chen, H.Y.H. Global effects of plant litter alterations on soil CO2 to the atmosphere. Glob. Chang. Biol. 2018, 24, 3462–3471. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Liu, L.L.; Sayer, E.J. Variability of above-ground litter inputs alters soil physicochemical and biological processes: A meta-analysis of litterfall-manipulation experiments. Biogeosciences 2013, 10, 7423–7433. [Google Scholar] [CrossRef] [Green Version]
- Barzegar, A.; Yousefi, A.; Daryashenas, A. The effect of addition of different amounts and types of organic materials on soil physical properties and yield of wheat. Plant Soil 2002, 247, 295–301. [Google Scholar] [CrossRef]
- Schneider, T.; Keiblinger, K.M.; Schmid, E.; Sterflinger-Gleixner, K.; Ellersdorfer, G.; Roschitzki, B.; Richter, A.; Eberl, L.; Zechmeister-Boltenstern, S.; Riedel, K. Who is who in litter decomposition? Metaproteomics reveals major microbial players and their biogeochemical functions. ISME J. 2012, 6, 1749–1762. [Google Scholar] [CrossRef] [Green Version]
- Steinwandter, M.; Schlick-Steiner, B.; Steiner, F.M.; Seeber, J. One plus one is greater than two: Mixing litter types accelerates decomposition of low-quality alpine dwarf shrub litter. Plant Soil 2019, 438, 405–419. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Liu, X.; Song, Q.; Compson, Z.G.; LeRoy, C.J.; Luan, F.; Wang, H.; Hu, Y.; Yang, Q. Synergistic effects: A common theme in mixed-species litter decomposition. New Phytol. 2020, 227, 757–765. [Google Scholar] [CrossRef]
- Lidman, J.; Jonsson, M.; Burrows, R.; Bundschuh, M.; Sponseller, R.A. Composition of riparian litter input regulates organic matter decomposition: Implications for headwater stream functioning in a managed forest landscape. Ecol. Evol. 2017, 7, 1068–1077. [Google Scholar] [CrossRef]
- Mooshammer, M.; Wanek, W.; Schnecker, J.; Wild, B.; Leitner, S.; Hofhansl, F.; Blöchl, A.; Hämmerle, I.; Frank, A.; Fuchslueger, L.; et al. Stoichiometric controls of nitrogen and phosphorus cycling in decomposing beech leaf litter. Ecology 2012, 93, 770–782. [Google Scholar] [CrossRef]
- Vivanco, L.; Austin, A. The importance of macro- and micro-nutrients over climate for leaf litter decomposition and nutrient release in Patagonian temperate forests. For. Ecol. Manag. 2019, 441, 144–154. [Google Scholar] [CrossRef]
- Silver, W.L.; Miya, R.K. Global patterns in root decomposition: Comparisons of climate and litter quality effects. Oecologia 2001, 129, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Makkonen, M.; Berg, M.P.; Handa, I.T.; Hättenschwiler, S.; van Ruijven, J.; van Bodegom, P.M.; Aerts, R. Highly consistent effects of plant litter identity and functional traits on decomposition across a latitudinal gradient. Ecol. Lett. 2012, 15, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Cline, L.C.; Zak, D.R. Initial colonization, community assembly and ecosystem function: Fungal colonist traits and litter biochemistry mediate decay rate. Mol. Ecol. 2015, 24, 5045–5058. [Google Scholar] [CrossRef]
- Stemmer, M.; Watzinger, A.; Blochberger, K.; Haberhauer, G.; Gerzabek, M. Linking dynamics of soil microbial phospholipid fatty acids to carbon mineralization in a 13C natural abundance experiment: Impact of heavy metals and acid rain. Soil Biol. Biochem. 2007, 39, 3177–3186. [Google Scholar] [CrossRef]
- Banerjee, S.; Kirkby, C.A.; Schmutter, D.; Bissett, A.; Kirkegaard, J.; Richardson, A.E. Network analysis reveals functional redundancy and keystone taxa amongst bacterial and fungal communities during organic matter decomposition in an arable soil. Soil Biol. Biochem. 2016, 97, 188–198. [Google Scholar] [CrossRef]
- Orlova, O.V.; Kichko, A.A.; Pershina, E.V.; Pinaev, A.G.; Andronov, E.E. Succession of bacterial communities in the decomposition of oats straw in two soils with contrasting properties. Eurasian Soil Sci. 2020, 53, 1620–1628. [Google Scholar] [CrossRef]
- Purahong, W.; Kapturska, D.; Pecyna, M.J.; Schulz, E.; Schloter, M.; Buscot, F.; Hofrichter, M.; Krüger, D. Influence of different forest system management practices on leaf litter decomposition rates, nutrient dynamics and the activity of ligninolytic enzymes: A case study from central European forests. PLoS ONE 2014, 9, e93700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Fu, T.; Hu, C.; Shen, D.; Macchioni, N.; Sozzi, L.; Chen, Y.; Liu, J.; Tian, X.; Ge, Q.; et al. Microbial community analysis and biodeterioration of waterlogged archaeological wood from the Nanhai No. 1 shipwreck during storage. Sci. Rep. 2018, 8, 7170. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Li, Z.; Zhao, B.; Zhang, J. Relationship between the chemical structure of straw and composition of main microbial groups during the decomposition of wheat and maize straws as affected by soil texture. Biol. Fertil. Soils 2019, 56, 11–24. [Google Scholar] [CrossRef]
- Yue, K.; García-Palacios, P.; Parsons, S.A.; Yang, W.; Peng, Y.; Tan, B.; Huang, C.; Wu, F. Assessing the temporal dynamics of aquatic and terrestrial litter decomposition in an alpine forest. Funct. Ecol. 2018, 32, 2464–2475. [Google Scholar] [CrossRef]
- Derderian, M.D. Determination of calcium and magnesium in plant material with EDTA. Anal. Chem. 1961, 33, 1796–1798. [Google Scholar] [CrossRef]
- Li, X.; Lin, J.; Gao, Y.; Han, W.; Chen, D. Antioxidant activity and mechanism of Rhizoma Cimicifugae. Chem. Central J. 2012, 6, 140. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.; Wu, F.; Zhou, X. Different toxic effects of ferulic and p-hydroxybenzoic acids on cucumber seedling growth were related to their different influences on rhizosphere microbial composition. Biol. Fertil. Soils 2019, 56, 125–136. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, H.; Wang, Y.; Zhou, X. Effects of green manure of wild rocket (Diplotaxis tenuifolia L.) on cucumber rhizosphere fungal community. Allelopath. J. 2021, 53, 93–100. [Google Scholar] [CrossRef]
- Birouste, M.; Kazakou, E.; Blanchard, A.; Roumet, C. Plant traits and decomposition: Are the relationships for roots comparable to those for leaves? Ann. Bot. 2011, 109, 463–472. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Zhang, Y.; Rong, Y.; Bei, Y.; Wei, Y.; Liu, N. Temporal variation of Q 10 values in response to changes in soil physiochemical properties caused by fairy rings. Eur. J. Soil Biol. 2018, 86, 42–48. [Google Scholar] [CrossRef]
- Majumder, B.; Kuzyakov, Y. Effect of fertilization on decomposition of 14C labelled plant residues and their incorporation into soil aggregates. Soil Tillage Res. 2010, 109, 94–102. [Google Scholar] [CrossRef]
- Ran, L.; Li, J.; Xing, Y.; Zhang, J.; Zhou, X. Effects of p-coumaric acid on the structure and abundance of soil Pseudomonas spp. community. Allelopath. J. 2021, 53, 209–216. [Google Scholar] [CrossRef]
- Zhou, X.; Rahman, M.K.U.; Liu, J.; Wu, F. Soil acidification mediates changes in soil bacterial community assembly processes in response to agricultural intensification. Environ. Microbiol. 2021, 23, 4741–4755. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, J.; Wu, F. Soil microbial communities in cucumber monoculture and rotation systems and their feedback effects on cucumber seedling growth. Plant Soil 2017, 415, 507–520. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Z.; Jia, H.; Li, L.; Wu, F. Continuously Monocropped jerusalem artichoke changed soil bacterial community composition and ammonia-oxidizing and denitrifying bacteria abundances. Front. Microbiol. 2018, 9, 705. [Google Scholar] [CrossRef] [Green Version]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Stegarescu, G.; Escuer-Gatius, J.; Soosaar, K.; Kauer, K.; Tõnutare, T.; Astover, A.; Reintam, E. Effect of Crop Residue Decomposition on Soil Aggregate Stability. Agriculture 2020, 10, 527. [Google Scholar] [CrossRef]
- Hou, S.-L.; Lü, X.-T. Mixing effects of litter decomposition at plant organ and species levels in a temperate grassland. Plant Soil 2020, 459, 387–396. [Google Scholar] [CrossRef]
- Berg, B.; Johansson, M.-B.; Liu, C.; Faituri, M.; Sanborn, P.; Vesterdal, L.; Ni, X.; Hansen, K.; Ukonmaanaho, L. Calcium in decomposing foliar litter–A synthesis for boreal and temperate coniferous forests. For. Ecol. Manag. 2017, 403, 137–144. [Google Scholar] [CrossRef]
- Fornara, D.; Tilman, D. Plant functional composition influences rates of soil carbon and nitrogen accumulation. J. Ecol. 2008, 96, 314–322. [Google Scholar] [CrossRef]
- De Deyn, G.B.; Cornelissen, J.H.C.; Bardgett, R.D. Plant functional traits and soil carbon sequestration in contrasting biomes. Ecol. Lett. 2008, 11, 516–531. [Google Scholar] [CrossRef] [PubMed]
- Hishinuma, T.; Azuma, J.-I.; Osono, T.; Takeda, H. Litter quality control of decomposition of leaves, twigs, and sapwood by the white-rot fungus Trametes versicolor. Eur. J. Soil Biol. 2017, 80, 1–8. [Google Scholar] [CrossRef]
- Jarchow, M.E.; Liebman, M.; Dhungel, S.; Dietzel, R.; Sundberg, D.; Anex, R.P.; Thompson, M.L.; Chua, T. Trade-offs among agronomic, energetic, and environmental performance characteristics of corn and prairie bioenergy cropping systems. GCB Bioenergy 2014, 7, 57–71. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Tian, X.; He, X.; Song, F.; Ren, L.; Jiang, P. Effect of litter quality on its decomposition in broadleaf and coniferous forest. Eur. J. Soil Biol. 2008, 44, 392–399. [Google Scholar] [CrossRef]
- Liu, P.; Huang, J.; Han, X.; Sun, O.J. Litter decomposition in semiarid grassland of inner Mongolia, China. Rangel. Ecol. Manag. 2009, 62, 305–313. [Google Scholar] [CrossRef]
- Wardle, D.; Yeates, G.; Barker, G.; Bonner, K. The influence of plant litter diversity on decomposer abundance and diversity. Soil Biol. Biochem. 2006, 38, 1052–1062. [Google Scholar] [CrossRef]
- Chomel, M.; Guittonnylarcheveque, M.; DesRochers, A.; Baldy, V. Effect of mixing herbaceous litter with tree litters on decomposition and N release in boreal plantations. Plant Soil 2015, 398, 229–241. [Google Scholar] [CrossRef]
- Chen, B.-M.; Peng, S.-L.; D’Antonio, C.M.; Li, D.-J.; Ren, W.-T. Non-Additive effects on decomposition from mixing litter of the invasive mikania Micrantha H.B.K. with native plants. PLoS ONE 2013, 8, e66289. [Google Scholar] [CrossRef] [Green Version]
- Chapman, S.K.; Newman, G.S.; Hart, S.C.; Schweitzer, J.A.; Koch, G.W. Leaf litter mixtures alter microbial community development: Mechanisms for non-additive effects in litter decomposition. PLoS ONE 2013, 8, e62671. [Google Scholar] [CrossRef] [Green Version]
- Herzog, C.; Hartmann, M.; Frey, B.; Stierli, B.; Rumpel, C.; Buchmann, N.; Brunner, I. Microbial succession on decomposing root litter in a drought-prone Scots pine forest. ISME J. 2019, 13, 2346–2362. [Google Scholar] [CrossRef] [Green Version]
- Purahong, W.; Wubet, T.; Lentendu, G.; Schloter, M.; Pecyna, M.J.; Kapturska, D.; Hofrichter, M.; Krüger, D.; Buscot, F. Life in leaf litter: Novel insights into community dynamics of bacteria and fungi during litter decomposition. Mol. Ecol. 2016, 25, 4059–4074. [Google Scholar] [CrossRef] [PubMed]
- Wardle, D.A.; Nilsson, M.-C.; Zackrisson, O.; Gallet, C. Determinants of litter mixing effects in a Swedish boreal forest. Soil Biol. Biochem. 2003, 35, 827–835. [Google Scholar] [CrossRef]
- Tian, B.; Wang, C.; Lv, R.; Zhou, J.; Li, X.; Zheng, Y.; Jin, X.; Wang, M.; Ye, Y.; Huang, X.; et al. Community structure and succession regulation of fungal consortia in the lignocellulose-degrading process on natural biomass. Sci. World J. 2014, 2014, 845721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, B.-Y.; Huang, Q.-G.; Xu, Y.; Wang, C.-X.; Lv, R.-R.; Huang, J.-Z. Microbial community structure and diversity in a native forest wood-decomposed hollow-stump ecosystem. World J. Microbiol. Biotechnol. 2009, 26, 233–240. [Google Scholar] [CrossRef]

| Residues | N (g/kg) | P (g/kg) | Ca (g/kg) | Total Phenolics (mg/g) |
|---|---|---|---|---|
| Pepper | 26.97 ± 1.07a | 2.7 ± 0.11 a | 2.8 ± 0.00 a | 0.57 ± 0.06 a |
| Maize | 6.65 ± 0.6b | 2.89 ± 0.05 a | 1.4 ± 0.35 b | 0.36 ± 0.09 b |
| Soybean | 7.66 ± 0.13b | 1.57 ± 0.22 b | 2.8 ± 0.20 a | 0.19 ± 0.03 c |
| Taxonomy | P | SP-P | MP-P | MSP-P | |
|---|---|---|---|---|---|
| 7 d | Cyanobacteria | 53.67 ± 4.57 a | 39.61 ± 2.59 c | 46.73 ± 4.06 b | 50.38 ± 4.54 ab |
| WS6 | 24.69 ± 5.84 b | 39.22 ± 4.34 a | 32.40 ± 4.99 ab | 27.98 ± 7.29 b | |
| Nitrospirae | 2.38 ± 0.83 a | 1.75 ± 0.43 a | 1.86 ± 0.34 a | 2.33 ± 0.74 a | |
| Saccharibacteria | 1.11 ± 0.63 a | 0.79 ± 0.76 a | 0.80 ± 0.84 a | 0.79 ± 0.45 a | |
| Chloroflexi | 1.24 ± 025 a | 1.99 ± 1.38 a | 1.50 ± 0.87 a | 1.48 ± 0.17 a | |
| WWE3 | 0.77 ± 0.55 a | 0.40 ± 0.51 a | 0.37 ± 0.51 a | 0.32 ± 0.18 a | |
| BRC1 | 0.53 ± 0.36 a | 0.12 ± 0.07 a | 0.41 ± 0.53 a | 0.41 ± 0.23 a | |
| Latescibacteria | 0.41 ± 0.15 a | 0.29 ± 0.31 a | 0.24 ± 0.27 a | 0.37 ± 0.28 a | |
| Deinococcus-Thermus | 0.17 ± 0.12 a | 0.12 ± 0.03 a | 0.17 ± 0.18 a | 0.16 ± 0.07 a | |
| 56 d | Cyanobacteria | 50.92 ± 2.87 ab | 40.02 ± 4.52 c | 52.65 ± 2.29 a | 45.46 ± 6.49 bc |
| WS6 | 21.98 ± 4.24 b | 33.41 ± 6.83 a | 22.64 ± 3.89 b | 28.25 ± 6.95 ab | |
| Nitrospirae | 4.32 ± 1.59 a | 2.98 ± 1.68 a | 2.80 ± 0.36 a | 3.50 ± 1.41 a | |
| Saccharibacteria | 2.88 ± 0.41 a | 2.27 ± 1.07 a | 1.80 ± 0.46 a | 2.34 ± 0.99 a | |
| Chloroflexi | 2.14 ± 0.20 a | 1.73 ± 0.51 a | 2.04 ± 0.66 a | 1.89 ± 0.61 a | |
| WWE3 | 1.17 ± 0.54 a | 0.90 ± 0.72 a | 0.71 ± 0.43 a | 0.94 ± 0.74 a | |
| BRC1 | 0.85 ± 0.22 a | 0.56 ± 0.48 a | 0.63 ± 0.37 a | 0.66 ± 0.27 a | |
| Latescibacteria | 0.92 ± 0.36 a | 0.62 ± 0.51 a | 0.60 ± 0.30 a | 0.64 ± 0.30 a | |
| Deinococcus-Thermus | 1.22 ± 0.24 a | 0.62 ± 0.12 a | 0.62 ± 0.29 a | 0.71 ± 0.21 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Wang, Z.; Wu, F.; Zhou, X. The Influence of Residue Mixing on the Decomposition of Pepper Root Residues. Agriculture 2022, 12, 84. https://doi.org/10.3390/agriculture12010084
Zhang X, Wang Z, Wu F, Zhou X. The Influence of Residue Mixing on the Decomposition of Pepper Root Residues. Agriculture. 2022; 12(1):84. https://doi.org/10.3390/agriculture12010084
Chicago/Turabian StyleZhang, Xianhong, Zhilin Wang, Fengzhi Wu, and Xingang Zhou. 2022. "The Influence of Residue Mixing on the Decomposition of Pepper Root Residues" Agriculture 12, no. 1: 84. https://doi.org/10.3390/agriculture12010084
APA StyleZhang, X., Wang, Z., Wu, F., & Zhou, X. (2022). The Influence of Residue Mixing on the Decomposition of Pepper Root Residues. Agriculture, 12(1), 84. https://doi.org/10.3390/agriculture12010084






