1. Introduction
Cotton (
Gossypium hirsutum L.) is a natural fiber crop widely grown around the world [
1,
2]. However, cotton damping-off disease caused by
Rhizoctonia solani, is an important soil-borne and seedling disease [
3,
4] which affects cotton production and agricultural economy [
5]. Pathogenic isolates of this fungus can infect at least 188 kinds of higher plants, including vegetables, forest trees, cereals and ornamental plants [
6]. The species can be categorized into two groups: anastomosis groups (AG) and intraspecific groups (ISGs) of
R. solani species. Many AGs have been characterized [
7,
8] and the primary disease-causing
R. solani species of cotton belongs to the AG-4 group.
Lentinan (LNT) is a new type of natural functional polysaccharide isolated from
Lentinus edodes fruiting body that can reduce damage caused by oxygen free radicals to organelles and has a good inhibitory effect on plant diseases [
9]. Lentinan could promote the elongation of the roots but has no effect on dry weight since the concentration of lentinan in plant is low [
10]. LNT primary structure is a β-1,3-glucan (
Figure 1) [
11], which is a major component of cell walls of bacteria, fungi, seaweeds and plants. The biological activity of lentinan is closely related to its structure [
12]. These properties depend on the primary structure of the glucan, such as the degree of polymerization and branching [
13]. It has been reported that LNT exists as a right-handed triple-helical conformation in an aqueous solution [
14]. Trotel-Aziz et al. found that β-1,3-glucan has a good controlling effect on grape powdery mildew and grape downy mildew [
15]. Ménard et al. also found that β-1,3-glucan sulfate can induce tobacco disease resistance [
16]. In recent years, a substantial amount of evidence has shown that the plant’s inherent immunity plays a role in
R. solani infection [
17]. Moreover, many resistance proteins against
R. solani infection have been identified, such as enzymes in the glycolytic pathway, chitinase and glutathione peroxidase [
18].
Furthermore, fluopimomide, (
N-(3-chloro-5-trifluoromethyl-pyridin-2-ylmethyl-2,3,5,6-tetrafluoro-4-methoxy-benzamide)), a new fungicide released by Shandong Sino-Agri Union Biotechnology Co. Ltd., Jinan, China [
19], can effectively control diseases caused by oomycete pathogens [
20], such as
Meloidogyne incognita in tomato [
21] and cucumber [
22].
The use of chemical fungicides is still a major control measure against plant pathogens [
21], which can negatively impact human health and the environment [
23]. Alternatively, biopesticides obtained from diverse natural plants and microorganisms [
24], are less hazardous to the environment as compared with chemical pesticides [
25]. In recent years, there has been an increase in the adoption and application of biological controls, such as the application of biopesticides by farmers in controlling pest and plant diseases, thereby creating a hot spot for worldwide research into biopesticides [
26,
27].
In this research, we conducted in vivo toxicity experiments of seven fungicides against R. solani mycelial growth on cotton plants in the greenhouse and open field. This was carried out to assess the potential effect of LNT and fluopimomide on R. solani and the possible mechanisms of action. Together, this paper will provide a basis for the scientific and reasonable application of LNT and fluopimomide in the prevention and control of cotton diseases. It can also provide a theoretical and practical basis for the development, evaluation and utilization of new pesticides. Collectively, this study will significantly strengthen disease prevention and control techniques against cotton seedling diseases through the efficient and rational use of pesticides. This will enhance cotton production as well as quality of cotton.
2. Materials and Methods
2.1. Fungal Strains and Fungicides Preparation
Fungal strains AG-2 (standard strain, only used for in vivo toxicity test), AG-4 (standard strain, used in all tests in this study), and LQ-M (it belongs to AGs that were collected from the field, only used for in vivo toxicity test) were provided by the Institute of Plant Protection, Hebei Academy of Agricultural and Forestry Sciences. All chemicals and analytical reagents of the experiment were purchased from Nanjing Vazyme Biotech Co., Ltd. (Nanjing, China).
Hexaconazole (CAS: 79983-71-4, 96.7% purity), pyraclostrobin (CAS: 175013-18-0, 98.0% purity), fludioxonil (CAS: 131341-86-1, 98.0% purity), carbendazim (CAS: 10605-21-7, 98.0% purity), difenoconazole (CAS: 119446-68-3, 97% purity) and fluopimomide (CAS: 1309859-39-9, 98.0% purity) were provided from College of Plant Protection, Key Laboratory of Pesticide Toxicology and Application Technique, Shandong Agricultural University, Tai’an, Shandong, China. Extraction and purification of LNT were performed according to Zhang et al. [
9]. Carbendazim and other fungicides stock solutions (1 × 10
4 mg L
−1) were prepared with 3% hydrochloric acid and acetone, respectively, and stored at 4 °C.
2.2. Field Investigation of Main Cotton Seedling Diseases
According to the cotton planting situation in Shandong Province, China, the five-point sampling method was adopted in the six major cotton growing areas of Dezhou, Liaocheng, Dongying, Binzhou, Heze and Jining. At each point, 50 cotton seedlings were randomly selected to investigate the occurrence of diseases at the cotton seedling stage, and the incidence rate was calculated. The classification standards are as follows [
28]:
Grade 0: No spots at the base of the stem;
Grade 1: The diseased spots at the base of the stem occupy less than 1/3 of the entire stem circumference;
Grade 2: The diseased spots at the base of the stem account for 1/3–1/2 of the entire stem circumference;
Grade 3: The diseased spots at the base of the stem occupy 1/2–3/4 of the entire stem circumference;
Grade 4: The diseased spots at the base of the stem account for 3/4 or more of the entire stem circumference.
The disease index (DI) and control effect (CE) was calculated according to the following formula: DI = (Σ (diseased leaf number of every grade × corresponding grade number))/total (leaf number investigated × the highest grade number) × 100; CE (%) = ((DI of the control − DI of the treatment)/DI of the control) × 100.
2.3. In Vivo Toxicity Experiments
The purpose of the mycelium growth rate method was to determine pesticide sensitivity [
2]. Inoculate fungal cake (diameter = 7 mm) on PDA medium plates containing different concentrations of each pesticide. Meanwhile, sterile distilled water was used as a control, with three repetitions for each treatment, and culture in the dark at 25 °C. When the control mycelium grows above 70 mm, use the cross method to measure the colony diameter comparison of control containing medicaments. A regression equation, the inhibition concentration EC
50 and a correlation coefficient were used to evaluate the bacteriostatic effect according to the EC
50 size. The final active ingredient concentration of each agent was shown in
Table 1.
2.4. Seed Coating Formulation Screening and Coating Treatment
The preparation of seed coating agent was carried out according to Marín et al. [
29], by using the wet sand processing superfine grinding method. Pesticides with and without surfactants were prepared by dispersing the biopolymers (2%
w/v) in deionized water. The rotor-stator homogenizer makes the active ingredients of pesticides form a stable dispersion system. Then, other additives were added (including the plant growth regulators, film-forming auxiliaries, etc.) into the aqueous solution in a certain proportion, and stirring was continued at 25 °C for 4 to 5 h until completely dissolved. The CIPAC method was used to determine the stability, which fulfilled the requirements of pesticide preparation well.
Based on determining the toxicity of a single agent, we selected fungicides and plant antagonists for mixing and performed a combined toxicity determination of LNT and fluopimomide. The mixture was compounded according to 1:10, 1:20, 1:30, 1:40, 1:50 and the actual EC
50 value of the mixture of different proportions was determined. The test and calculation methods were the same as the single-agent indoor toxicity determination. The synergic ratio (SR) of the mixture was calculated to evaluate the synergistic effect of different proportions. The ratio with the highest synergistic coefficient was chosen: 50% fluopimomide WG, 2% LNT AS, and L311 and L811 film formers were used to prepare seed coatings. The seeds and seed coating agent were used according to the dosage, and dried in a cool place for later use.
EC
50 (ob) represents the actual EC
50, EC
50 (th) represents the theoretical EC
50, A and B are single agents, and a and b are the mass ratios of the two in the mixture. SR = 0.5–1.5 is additive, SR < 0.5 is antagonistic and SR > 1.5 is synergistic [
30].
2.5. Greenhouse Safety Assessment Experiment
The cultivar of cotton used in this study was Lumian 28, Lumian 37 and Lumian 338. Using 6% fluopimomide, 6.15% fluopimomide and LNT as a seed coating agent. The dosage of the active ingredients used in the seed coating experiment was 4 dosages, including 1, 1.5, 2, 2.5 times the recommended dosage in the field. The specific dosage of the seed coating agent was: 6% fluopimomide seed coating agent 9.6, 14.4, 19.2, 24.0 mg a.i./kg seed and 6.15% fluopimomide lentinan seed coating agent 9.6, 14.4, 19.2, 24.0 mg a.i./kg seed. The treatment without chemicals was used as the blank control. A set of 5 replicates was performed per treatment. The effect of plant height, stem thickness and germination rate were evaluated.
The cotton seeds were surface sterilized in 1% (
w/v) NaOCl and 70% (
v/v) ethanol for 3 min each and rinsed three times in sterile distilled water [
31]. Seed germination assay and seedling growth assay were carried out according to Zhang et al. [
32]. Germination assay: for each treatment, 4 replicates of 20 seeds were planted in sterilized sand (60–70 mesh, 20% moisture), and grown at 25 ± 1 °C with a 14 h: 10 h light:dark ratio (L:D) and 70 ± 5% relative humidity. When the main root length of cotton exceeds the seed length or the bud length exceeds 1/2 of the seed length, it could be regarded as seed germination. The hair-cutting bud rate was recorded and calculated at 3 days after germination. The germination rate was calculated according to the following formula:
Seedling growth assay: triplicates of 25 seeds each were seeded in pots with sterilized nutrient soil (60% moisture). Plants were cultivated under the same conditions described above. Plant heights were recorded for 20 days after emergence.
2.6. Greenhouse Control Effect Experiments
The germinated cotton seeds were transplanted to the greenhouse of Shandong Agricultural University. We measured the control effect of cotton damping-off disease caused by AG-4 at 7 days in the greenhouse experiment via the application of seed coating agent containing 6% fluopimomide, 6.15% fluopimomide and LNT, respectively. Pharmaceutical control azoxystrobin: clean water was used as a control. Each pot was then treated with 20 mL of the
R. solani AG-4 suspension. A set of 5 replicates was performed per treatment. The classification standard was as described in Huang et al. [
33].
2.7. Field Trials
Field tests were conducted in the cotton field of Wucheng village, Dezhou, Shandong, China (37.08° N, 115.93° E) and Cotton research center, Shandong academy of agricultural sciences, Liaocheng, Shandong, China (36.81° N, 115.70° E), two field trials were performed in 2020. The field is a serious damping-off region with years of continuous cotton cropping. The concentration of each treatment group was set as follows: 4.8, 7.2, 9.6 mg a.i./kg seed. Pharmaceutical control azoxystrobin: clean water was used as a control. The experimental field for each treatment covered 30 m2. A random block arrangement was adopted between each cell, with three replications. The seeding of cotton in the two test sites was carried out by manual seeding. The row spacing was kept at about 80 cm, and the ditching was used for direct seeding. The plant spacing was controlled at about 15 cm. 1 m protection rows were reserved at both ends of each plot to ensure the distance between the plots did not affect each other. We investigated the incidence of cotton damping-off and its control effect each year on 14 May and 22 May.
2.8. Spray and Irrigation Root Treatment
After the cotton seedling emerged, spray at 50, 100 and 200 mg·L
−1 LNT to irrigate the roots was carried out; water treatment was used as a control. Each treatment included 20 cotton seedlings. The incidence and index were recorded 7 days after the emergence. Calculate disease severity and control according to the description of Zhang et al. [
5]. The pesticide concentration with the most obvious growth-promoting effect was selected for the late enzyme activity and real-time quantitative PCR test.
2.9. Measurement of Defense Enzyme Activities
For crude enzyme extraction, refer to Mahunu et al. [
34]. Melatonin treatment groups of different concentrations were obtained, 0.5 g of seeds were germinated to the 2nd, 4th and 6th days, put in a mortar (mortar pre-cooled at −20 °C for 1 h), added 1:9 to the pre-cooled phosphate buffer (PBS Ph = 7.0, containing 1% polyvinylpyrrolidone and 0.1% mercaptoethanol) and ground in an ice bath; the homogenate was poured into a centrifuge tube, and refrigerated and centrifuged at 1000 r·min
−1 for 20 min, then stored at 4 °C until use.
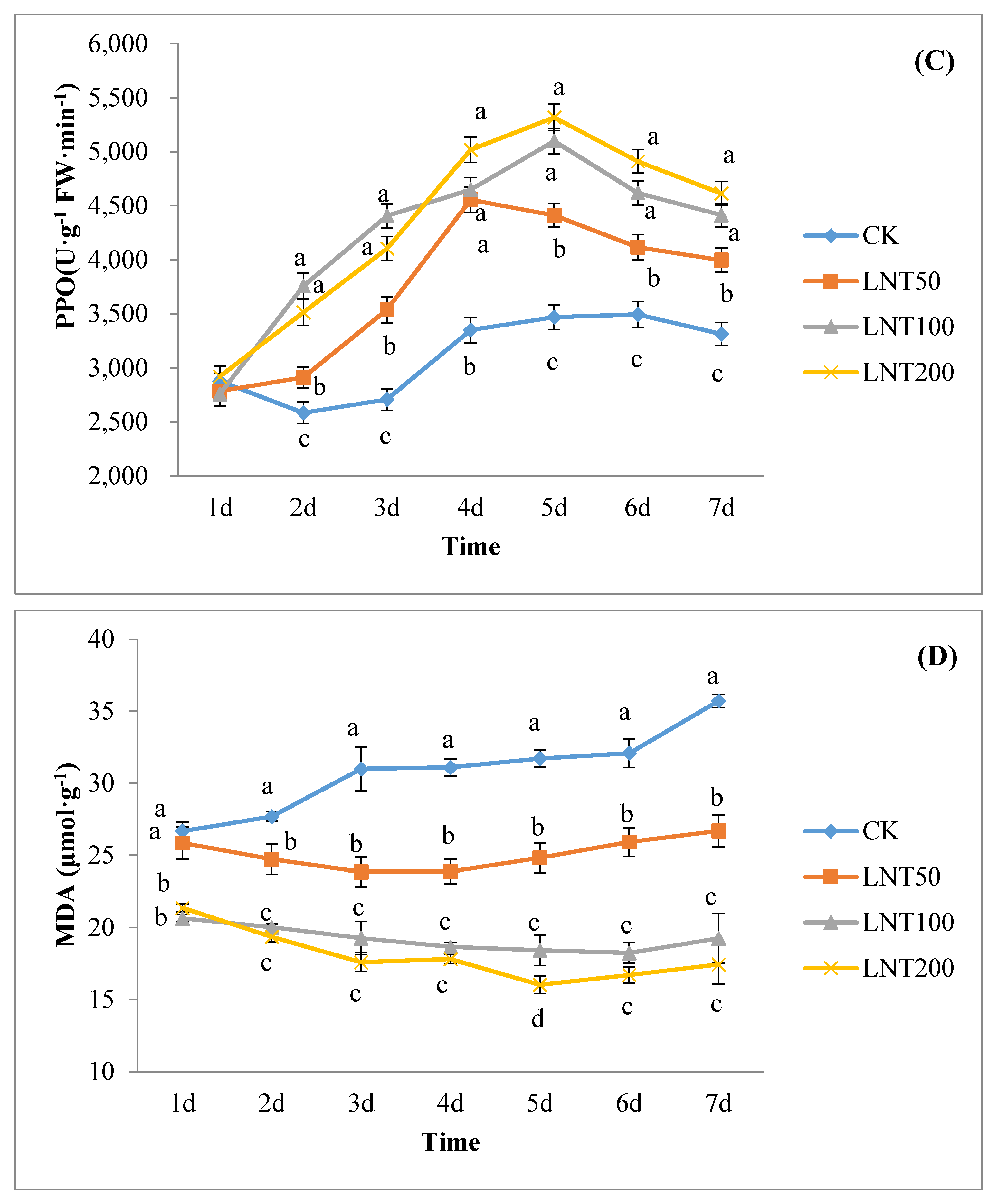
The lentinan with the best concentration for inducing effect was selected to treat cotton plants and inoculated with the spore suspension of the test species 2 days later. To study the physiological mechanism of lentinan-induced cotton wilt disease, the experimental design was as follows: (A) clear water (CK); (B) inoculation of R. solani (CK-inoculation) without lentinan treatment; (C) treatment with LNT but not R. solani; (D) treatment with LNT and inoculated with R. solani. 1–7 days after inoculation with R. solani, the 3rd to 4th unfolded leaves of cotton plants (without induced treatment) were used to determine the polyphenol oxidase SOD, PPO and POD activities.
2.10. Malondialdehyde (MDA) Assay
The content of malondialdehyde (μmol g
−1) was determined by the thiobarbituric acid method. Weigh 0.5 g of chopped cotton leaves in a mortar, added 1 mL mass fraction of 10% TCA and a small amount of quartz sand, and then added 4 mL TCA for further grinding. The homogenate was centrifuged at 4000 r min for 10 min, and the supernatant was the sample extract. A 2 mL pipette of centrifuged supernatant (control plus 2 mL of distilled water) was added to 2 mL of tbA solution with a mass fraction of 0.6%, mixed well, and the mixture was reacted in a boiling water bath for 15 min. After rapid cooling, it was centrifuged again. The supernatant was obtained and the extinction was measured at 450, 532 and 600 nm wavelength. CMDA (µmol L
−1) = 6.45 (A
532 − A
600) − 0.56 A
450, this yielded absolute MDA concentrations (µmol g
−1 R. solani) [
35].
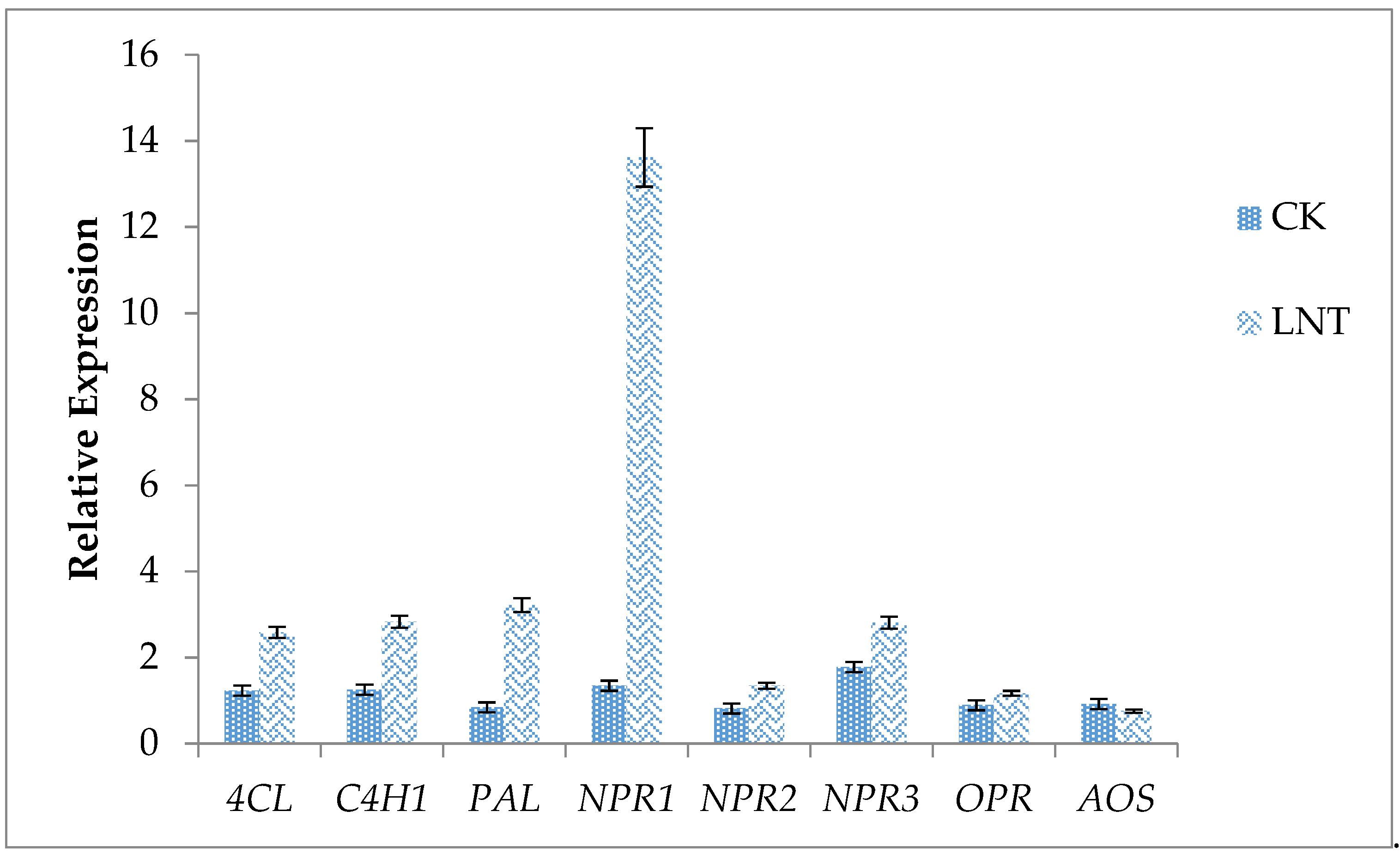
2.11. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
Cotton leaf tissue exposed to 100 mg L
−1 LNT was collected at 7 days after seedling emergence and stored at −80 °C. RNA extraction was performed as described by Zhang et al. [
9]. Draw a standard curve using a series of standards with known initial copy numbers to establish a linear relationship between the Ct value and the logarithm of the initial template amount. From the Ct value of the unknown sample, the initial copy number of the unknown sample can be calculated from the standard curve. This experiment uses the 2
−ΔΔCt method commonly used in relative quantification, where ΔΔCt = (Ct target gene-Ct internal reference) experimental group-(Ct target gene-Ct internal reference) control group. The detailed detection procedures were as follows: pre-denatured at 95 °C for 3 min, 95 °C denaturation for 10 s, 60 °C annealing extension 30 s, 40 cycles. The relative expression analysis was performed in triplicate. Specific primers were designed using the Primer Express software (Sandon Biotech, Shanghai, China) (
Table 2).
2.12. Statistical Analyses
Use SPSS software (v18, SPSS Inc., IBM Corp., Armonk, NY, USA) was used to calculate the inhibition rate/% = (control colony growth diameter-treated colony growth diameter)/control colony growth diameter × 100, EC50 value and 95% confidence interval. Different letters (abc) represent significant differences, p < 0.05; ABC mean significant level p < 0.01.
4. Discussion
Due to the continuous evolution of organisms, many have gradually evolved their own antioxidant defense systems [
36]. Plant immune elicitors are generally divided into protein peptides, oligosaccharides, organic acids and inorganic compounds. Examples include, chitosan oligosaccharide (COS) obtained by hydrolysis of chitosan, which can be used as an effective inducer of plant immunity [
37]; oligosaccharides can seriously reduce the severity of cucumber mosaic virus (CMV) [
38]; kelp polysaccharides can induce tobacco resistance to carrot soft rot Erwinia [
39], and also significantly increase expression levels of defense-related genes LOX and GST in grape leaf cells [
40]; LNT can regulate plant growth, induce the activation of the plant defense response, enhance the plant’s resistance against diseases, and thereby induce the plant’s resistance to plant viruses and fungal diseases [
41,
42]. In addition,
Ganoderma Lucidum polysaccharide can effectively control cotton fusarium wilt [
9] and LNT can effectively control sharp eyespot of wheat [
32].
For a long time, prolific plants have gradually formed a complete immune system [
43]. At present, the use of chemical pesticides is still the primary measure to prevent cotton damping-off disease in actual agricultural production but has been found to cause environmental pollution [
44] and increased resistance [
45]. Plant immune elicitors are usually a very conservative substance, mainly including oligosaccharides, peptides, proteins, lipids and other types [
46]. Researchers use plant immune inducers to control plant diseases and insect pests [
47] because they can reduce the pollution of chemical pesticides [
48], activate the plant’s own resistance system and enhance the plant’s resistance to pathogens. In this paper, LNT as a biological pesticide effectively controls cotton damping-off disease caused by
R. solani and reduces the frequent use of chemical pesticides to achieve the purpose of protecting the environment. The scientific and rational use of biological pesticides, such as LNT, can reduce the use of chemical pesticides, which will not cause harm to natural enemies. In the process of use, biological pesticides are difficult to produce drug resistance, and promote the healthy development of modern agriculture. Most biological pesticides are low-toxic or slightly toxic, they are easy to decompose, will not cause pollution to agricultural products and can effectively guarantee the quality and safety of agricultural products. Fluopimomide is more effective in controlling plant diseases compared to fuopicolide [
49,
50]. In vivo tests, the EC
50 values of fluopimomide against
R. solani were lower than fuopicolide. Meanwhile, as a new pesticide fluopimomide plays an important role in plant disease control and contribute to crop protection to some extent. Not only does it help with the scientific use of a new pesticide, but also controls plant diseases in actual crop protection by carrying out relevant research on fluopimomide.
Seed treatments will protect plant seedlings from pre-emergence damping off disease, and also ensure plants have a healthy root system among their growth and development period [
51]. Salicylic acid (SA) has physiological adjustment functions [
52], such as delaying senescence, inducing flowering, and heat production, and can also induce the synthesis of disease-related proteases (PRP) in plants, thereby increasing the disease resistance of plants.
Induced resistance is related to the enhancement of the activity of defense enzymes (such as POD, SOD and PPO) [
53]. Studies have shown that polysaccharides can induce plant POD, PPO and increase other defensive enzyme activities [
54]. POD is one of the key enzymes in the pathway of lignin synthesis. As a defense gene, POD can induce the biosynthetic pathway of SA, lignin and phytoalexin, activate SAR46, and promote cell-wall strengthening and pathogen suppression [
55]. In addition, POD can catalyze the decomposition of H
2O
2 and the polymerization of lignin monomers [
56]. Polyphenol oxidase (PPO) can promote the lignification of the plant cell walls to reduce the damage of pathogens to plants, changes in PPO activity being important physiological indicators of plant disease resistance [
57]. PPO is formed by catalyzing the formation of a protective barrier between lignin and quinone compounds, protecting cells from pathogens and directly exerting anti-disease effects by forming quinone compounds [
58]. Malondialdehyde (MDA) is one of the products of membrane lipid peroxidation, as an important indicator of membrane lipid peroxidation, reflecting the degree of cell membrane damage and the plant’s response to stress conditions [
59].
5. Conclusions
Plants are constantly faced with several pathogenic agents and controlling this disease-causing agent is crucial in the sustainable production of crops. The use of harmful synthetic chemical agents in controlling pathogens poses significant harm to human health and the environment. To overcome this, the use of organic control agents, such as lentinan (LNT), has considerable potential. We observed that LNT improved cotton seed germination rate and seedling growth, and also induced the expression of some defense genes in plants, hence is a good immune elicitor. Seeds treated with LNT increased in PPO, POD, and SOD activity and reduced MDA content. Lentinan is suitable for mixing with other chemical agents to improve the control effect, enhance plant resistance and ultimately increase yield. A large amount of pesticide use may cause problems, such as increased production costs, excessive residues on agricultural products, crop phytotoxicity, and environmental pollution. As a class of biological pesticides, plant immune inducers are changing the thinking of plant diseases and insect pest control. The use of plant immune elicitors, such as LNT, will improve this situation to a certain extent. There is a need for further in-depth understanding of plant immune elicitors in the future.