Abstract
The hair follicle is a complex mini-organ in the skin that undergoes organ induction, morphogenesis, and regeneration. However, the accurate molecular mechanism of skin and hair diversity regulation is still elusive. The sheep is an animal model that can be used to further explore the mechanisms of skin and hair diversity. In this study, we carried out a transcriptomic analysis of the mRNA expression in the skin of Xinji fine-wool sheep at different growth stages (3 and 12 months old) and 12-month-old Tan sheep and explored the transcripts’ relationship with hair follicle growth. A total of 1327 mRNAs and 67 transcription factors were identified to be differentially expressed in the different breeds and during different periods of skin development. The differentially expressed genes were enriched in GO terms represented by system development, multicellular organism development, animal organ development, and skin development, and three KEGG pathways typified those governing differences in skin structure. Combining protein–protein interaction networks of skin development (GO:0043588) and functional annotation, nine important candidate genes, namely, LAMA5, OVOL1, SRF, DHCR24, NGFR, SMO, CDSN, HOXC13, and KDF1, and many core genes with minor effects were confirmed to be associated with hair follicle development. Furthermore, members of the zf-C2H2 and homeobox transcription factor families, which were identified to play a crucial role in producing finer and denser wool, were mainly upregulated in 12-month-old Xinji fine-wool sheep when compared with expression in 12-month-old Tan sheep and 3-month-old Xinji fine-wool sheep. This study revealed the major–minor gene interactions in the developmental pathway and provided ideas for an in-depth understanding of the genetic structure and gene regulation in the skin/hair growth process.
1. Introduction
The hair follicle is a unique mini-system organ revealing the mechanisms underlying growth and regeneration [1]. The quality and output of the hair of mammals, which is collectively known as fur or wool in sheep breeds, are decided by the structure and location of the hair follicles [2,3]. Each hair comprises a hair follicle and hair shaft, and the hair shaft consists of differentiated keratinocytes which are produced by the hair follicle [4]. Hair follicles are mainly divided into primary hair follicles (PFs) and secondary hair follicles (SFs). SFs are smaller than PFs, do not have a sweat gland or arrector pili muscle, and tend to grow finer wool fibers that contribute the majority of fibers to the adult fleece [5]. Compared with SFs, PFs have a hair bulb with a larger diameter and complete sebaceous glands. Hair follicles can define the molecular and cellular changes that guide wool diversity ranging from coarse to fine and short to long hair.
Wool is one of the most important animal products and income sources from sheep farming; in particular, the irreplaceable textile and economic value of fine wool place it in short supply in international markets. Tan sheep, a breed of Mongolian sheep, are a rare local sheep breed in China that grows fur. They are famous for their one-month lamb fur, which enjoys a high reputation in the fur market at home and abroad [6]. The Xinji fine-wool sheep (XFWS) is a typical representative of the fine-wool sheep breed which originated in Northeast China, has the advantages of stable breeding performance, a high wool yield rate, and strong adaptability that is compatible with breeding in arid zones. The wool has excellent textile performance, with a fineness less than 23 μm and a hair length greater than 6 cm [7]. In fine-wool sheep, SFs determine the yield and density of fine wool. Wool fineness, length, and bending along with other indicators are important standards to evaluate the comprehensive quality of wool in sheep breeding. Research on the hair follicle development network has become a hotspot aimed at accelerating wool quality and yield [5,8]. In the last century, the molecular genetics of hair follicle development and wool production has been reported and many of the signaling pathways are well known at the cellular level [8,9,10,11].
RNA sequencing (RNA-Seq) has become a broadly used and essential method for studying gene expression and metabolism. In fact, the transcription data obtained by RNA-Seq provides an opportunity to determine multiple mechanisms of gene regulation, such as differential gene expression analysis, new transcript prediction, untranslated region (UTR) analysis, RNA editing, and the presence of regulatory RNAs [12,13]. Recent advances, including sheep reproduction [14], sheep muscle growth [15], and liver function [16], on the basis of the application of RNA-Seq have been reported. These results provide the additional benefit of obtaining sequence information of all mRNAs within a specific tissue of the species.
In recent years, researchers have thoroughly investigated the regulatory mechanism of hair follicles which involved different animal models that are partially conserved with those of sheep. In the mouse and chicken models, the PFs first emerge from focal epidermal thickening and then grow downward into the dermis to form lamellar structures with dense cells and weak dermal aggregates [17,18,19]. Several molecules and signaling pathways have been implicated as key factors in the induction/initiation phase of PF morphogenesis, including ectodysplasin A receptor (EDAR), fibroblast growth factor (FGF), bone morphogenetic proteins (BMPs), and wingless-related integration site (WNT) [20,21,22]. These factors not only affect the formation of PFs but also play a vital role in regulating the size, quantity, and quality of hair follicles during development phases.
Many candidate mRNAs, miRNAs, and lncRNAs functioning in the development of hair follicles have been confirmed in prenatal sheep skin, including in fine-wool sheep (Merino) [23], miRNAs in Chinese Merino sheep [24], early regulators in coarse and fine-wool sheep [24], and lncRNA-miRNA-mRNA network interactions in Aohan fine-wool sheep skin [25]. However, the regulatory factors of skin and hair diversity in the postnatal stages remain largely unknown in different sheep breeds and growth stages. The study of the molecular mechanism of hair follicle morphogenesis in different breeds and postnatal growth stages and how regulatory factors and functional genes regulate hair follicle growth and development can supply a scientific basis for improving the comprehensive quality of wool by applying to molecular breeding technology.
In this study, transcriptome sequencing technology was used to examine the mRNA expression profile of sheep shoulder skin tissue in two different breeds and growth stages. We also conducted Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses, constructed protein–protein interaction networks of skin development, and annotated transcription factor responses to skin and wool diversity, displaying the potential regulatory mechanisms for further study of hair follicle development.
2. Materials and Methods
2.1. Skin Tissue Sample Information
Xinji fine-wool sheep and Tan sheep were in farms in Yanchi County of Ningxia and Songyuan City of Jilin Province, respectively, and fed based on the farm’s feeding plan. Skin tissue samples from the shoulder (diameter 2 cm) were collected from 3-month-old Xinji fine-wool sheep (3-m-XFWS, n = 4; S1, S2, S3, S4), 12-month-old Xinji fine-wool sheep (12-m-XFWS, n = 3; X1, X2, X3), and 12-month-old Tan sheep (12-m-TS, n = 6; C1, C2, C3, C4, C5, C6). These skin samples were stored in RNAlater (Invitrogen, Carlsbad, CA, USA), taken back to the laboratory at 4 °C, and immediately placed in liquid nitrogen for RNA-Seq and qPCR analysis.
2.2. RNA Extraction, Library Construction, and RNA Sequencing
Total RNA was extracted from shoulder skin tissue by TRIzol according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA). Detection of RNA concentration, integrity, and quantity was evaluated by a NanoDrop 2000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA) and an Agilent 2100 (Agilent, Technologies, Santa Clara, CA, USA).
According to the manufacturer’s instruction, 13 libraries (12-m-XFWS, n = 3; 3-m-XFWS, n = 4; 12-m-TS, n = 6) were constructed with 1 μg of total RNA per sample using TruSeq RNA Sample Preparation kit V2 (Illumina, San Diego, CA, USA). In accordance with the TruSeq PE Cluster Kit v3-cBot-HS kit instruction, cBot Cluster Generation system was used to cluster samples with index coding. After cluster generation, the library preparations were sequenced on an Illumina HiSeq platform (Illumina, San Diego, CA, USA). The sequencing strategy was double-terminal sequencing with a reading length of 150 bp.
2.3. Sequencing Data Mapping and Transcriptome Assembly
Raw data (raw reads) of FASTQ format were firstly processed through primary quality control. In this step, clean reads were obtained by removing those raw reads containing more than three poly-N sequences, adapter sequences, or where the proportion of bases with a quality value below 5 was more than 20%. All the downstream analyses were based on the clean data with high quality. TopHat version 2.0.11 software (http://ccb.jhu.edu/software/tophat/index.shtml, accessed on 10 March 2021) was used to align clean reads from each sample to the sheep reference genome Oarv4.0. (GCA_000298735.2, https://www.ncbi.nlm.nih.gov/assembly/GCA_000298735.2,accessed on 10 September 2021) [26]. HTSeq version 0.6.1 (https://pypi.org/project/HTSeq/, accessed on 3 April 2021) software was used for transcriptome assembly, annotation, and expression calculations.
2.4. Analysis of Differentially Expressed Genes
The transcripts per kilobase of exon model per million reads mapped (TPM) value was used to estimate the expression levels of mRNAs [27]. For experiments with three biological replicates, the differentially expressed genes (DEGs) were identified by using the R package DEseq2 behind a negative binomial distribution [28]. The p.adjust (p.adj) was set as 0.01, and the log2 Fold Change absolute value was set to be greater than 1. We only identified coding genes.
2.5. Clustering and Correlation Analysis
The principal component analysis (PCA), hierarchical clustering, and correlation analysis used TPM values of all annotated transcripts in transcriptomes of different periods and breeds which were implemented by gmodels in R (version 3.1.3, https://cran.r-project.org/, accessed on 20 September 2021).
2.6. Functional Enrichment and Protein–Protein Interaction Analyses of DEGs
GO and KEGG enrichment analyses were executed with the DEGs by g:Profiler [29,30,31]. Significantly enriched GO terms or KEGG pathways were identified by Fisher’s exact test, and p.adj < 0.05 was used as the threshold. The results were visualized by R language package. Protein–protein interaction analysis of DEGs was based upon the universally performed String database and Cytoscape software to show the interaction network [32].
2.7. Relative Gene Expression Analysis by Quantitative Real-Time PCR (qPCR)
We used qPCR to identify the gene expression level. Each RNA sample of about 0.1 μg was reverse-transcribed into cDNA by RT reagent. The qPCR was carried out at 95 °C for 10 min, followed by 95 °C for 15 s, 60 °C for 60 s for 45 cycles, and 72 °C for 30 s. qPCR was carried out on ABI MicroAmp Optical 96-well reaction plate by using SYBR Premix Ex Taq (Takara, Kyoto city, Japan) in ABI 7500 real-time PCR instrument. GAPDH was used as an internal reference to standardize goal gene expression. All primers utilized in the qPCR are displayed in Supplementary Table S1. Each qPCR experiment was completed in triplicate, and the relative RNA expression values were computed by way of the 2−ΔΔCt method [33].
2.8. qPCR Statistical Analysis
Statistical analyses of the qPCR results and graphs were executed in GraphPad Prism 7 (GraphPad Software Inc, San Diego, CA, USA). Statistical significance of the data was examined by performing T-tests. The p values were defined by Student’s t test. Error bars show standard error of the mean, *** p < 0.001, ** p < 0.01, * p < 0.05.
3. Results
3.1. Transcriptome Sequencing and Alignment
A total of 13 high-quality RNA libraries were constructed. After removing the low-quality sequences, a total of 181027689, 133459123, and 230146178 clean reads with more than 91.25% Q30 were acquired in 3-m-XFWS, 12-m-XFWS, and 12-m-TS, respectively. The mapping rate ranged from 72.60% to 83.76% of the reads that were successfully aligned with the Ovis aries reference genome (Supplementary Table S2). TPM analysis revealed that 28.75–37.67% of the genes had low expression (TPM ≤ 1.0), 20.13–30.02% had moderate expression (1.0 < TPM < 10.0) and 32.32–50.33% had high expression (10.0 ≥ TPM) (Figure 1A, Supplementary Table S3). To evaluate the reliability of the samples, PCA and Pearson’s correlation coefficient r were utilized as assessment indices for the correlation of biological repetitions in the 13 libraries. The PCA results show that 12-m-XFWS samples clustered together distinct from the other samples indicating a different mRNA expression pattern from the 3-m-XFWS and 12-m-TS. The 3-m-XFWS and 12-m-TS mixed together sample clusters overlapped showing that the mRNA expression patterns were similar (Figure 1B). Pearson’s correlation coefficient r showed that the repeated values between the samples were all over 70% (Figure 1C, Supplementary Table S4). The similarity value of 12-m-XFWS and 12-m-TS was less than that of 3-m-XFWS and 12-m-TS. Sequencing results indicate that the original data were good for subsequent analyses.
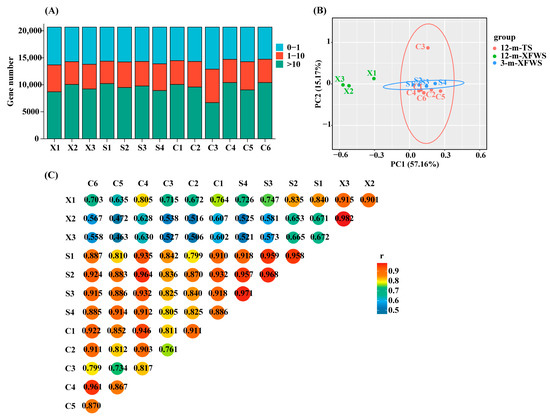
Figure 1.
Sequencing quality analysis. (A) Statistical analysis of TPM from the 13 RNA sequencing libraries (TPM, the transcripts per kilobase of exon model per million reads mapped value). (B) PCA plot of the 13 RNA-Seq libraries (PCA, the principal component analysis; 12-m-TS, 12-month-old Tan sheep, n = 6; 12-m-XFWS, 12-month-old Xinji fine-wool sheep, n = 3; 3-m-XFWS, 3-month-old Xinji fine-wool sheep, n = 4). (C) Pearson correlation of the 13 RNA sequencing samples.
3.2. Analysis of the Differentially Expressed Genes
HTSeq version 0.6.1 software was applied to measure the level of expression in each sample, and TPM was performed as an index to quantify the expression level of each sample sequence (Supplementary Table S5). Using the R package DEseq2, we set log fold change |FC| > 1 and p.adj < 0.01 as the criteria for selecting DEGs. The expression of the differentially expressed genes is shown in the Supplementary information (Supplementary Tables S6 and S7). In total, 2079 DEGs were determined in the 12-m-XFWS vs. 12-m-TS (upregulated: 1035, downregulated: 1044) and 4111 in the 12-m-XFWS vs. 3-m-XFWS (upregulated: 2364, downregulated: 1747) (Figure 2A, Supplementary Table S8). The distribution of DEGs in 12-m-XFWS vs. 12-m-TS and 12-m-XFWS vs. 3-m-XFWS was described by a volcano map (Figure 2B,C, Supplementary Tables S9 and S10). We also showed a Venn map (Figure 2E) and clustering map (Figure 2D) to visually display the expression level of the overlapping DEGs in different breeds (12-m-XFWS vs. 12-m-TS) and periods (12-m-XFWS vs. 3-m-XFWS). In this study, a total of 1327 overlapping candidate genes played important roles in regulating breed and time–space specificity (Supplementary Table S11).
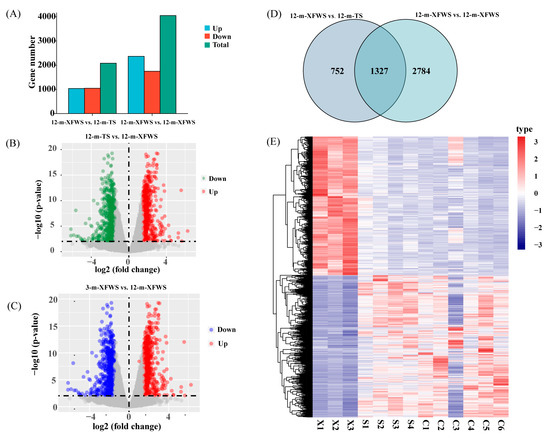
Figure 2.
Gene expression characterization in the skin of Xinji fine-wool sheep at 3 and 12 months of age and Tan sheep at 12 months of age. (A) The number statistics of upregulated and downregulated DEGs (DEGs, differentially expressed genes). (B,C) A volcano plot of DEGs in different breeds and ages. (D,E) Venn diagram and heat map of overlapped DEGs at different breeds and growth stages.
3.3. GO Enrichment and KEGG Pathway Analysis
To further study the functions of the DEGs, GO and KEGG enrichment analyses were performed for the DEGs by g:Profiler. The results are shown in Supplementary Table S12. A total of 1327 DEGs were used in the GO enrichment analysis (p.adj ≤ 0.05), and 14 cellular component terms, 9 molecular function terms, and 39 biological process terms were significantly enriched. The top 20 GO terms (Figure 3A) identified included the growth of skin/hair terms, such as system development (GO:0048731), multicellular organism development (GO:0007275), animal organ development (GO:0048513), skin development (GO:0043588), mesenchymal cell development (GO:0014031), and intermediate filament cytoskeleton (GO:0045111). Three KEGG (p ≤ 0.05) signaling pathways were significantly enriched with 1327 DEGs (Figure 3B), namely, the arrhythmogenic right ventricular cardiomyocyte pathway, vascular smooth muscle contraction, and cGMP-PKG signaling pathway, which were associated with regulating skin and hair follicle development.
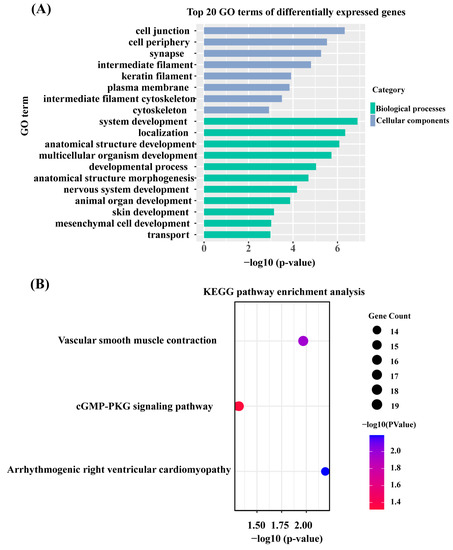
Figure 3.
The most importantly enriched GO terms of the KEGG pathway of DEGs. (A) Top 20 GO terms of DEGs. (B) KEGG pathway of DEGs (GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes).
3.4. Identification of Candidate Genes for Hair Follicle Development
To enhance our knowledge of the molecular regulation mechanism in skin development and hair follicle growth, we mainly focused on biological processes associated with skin development (GO:0043588). We detected genes (referred to as core genes) affecting skin development. Protein–protein interaction analysis revealed that eight hair-follicle-related genes were in the protein–protein interaction clustering network (Supplementary Figure S1), including LAMA5, OVOL1, SRF, DHCR24, NGFR, SMO, CDSN, and KDF1. Protein–protein interaction network analysis showed that the hub genes NGFR, DHCR24, and SRF interact with other genes in skin development. Moreover, a literature survey identified another candidate gene among the core genes, HOXC13, that potentially regulates hair follicle development [34]. All of them, including LAMA5, OVOL1, SRF, DHCR24, NGFR, SMO, CDSN, KDF1, and HOXC13, were upregulated in 12-m-XFWS compared with 12-m-TS and 3-m-XFWS. The expression of nine candidate genes related to the regulation of hair follicle development was visualized by a heat map (Figure 4A). To assess the reliability and reproducibility of the RNA-Seq data of the transcriptome, we performed the same samples for qPCR of nine candidate genes. The relative tendency of the expression pattern of the qPCR results was consistent with that of the RNA-Seq data, showing significant differences in different breeds and periods (Figure 4B,C, Supplementary Table S13). In conclusion, we identified nine candidate genes that make up the most significant major gene regulatory relationships and, together with other core genes, collectively are associated with hair follicle development.
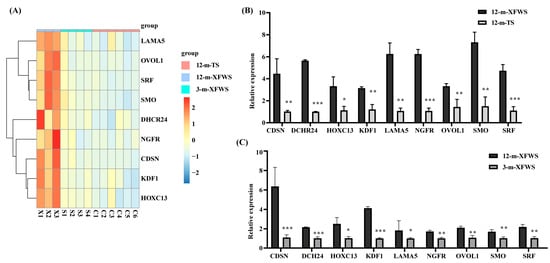
Figure 4.
Major genes affecting hair follicle development. (A) A heat map TPM value of nine candidate genes affecting hair follicle development (orange–red represents high expression; light green means low expression). (B,C) Validation of the nine candidate genes by qPCR in different breeds and during different periods. (qPCR, quantitative real-time PCR; data are expressed as the mean ± SEM, * p < 0.05, ** p < 0.01, *** p <0.001).
3.5. Analysis and Annotation of Transcription Factors
Previously researchers have reported that transcription factors (TFs) play a significant role in regulating the skin and wool diversity of coarse- and fine-wool sheep [35,36]. We downloaded sheep transcription factor annotation files from Animal TFDB 3.0 (http://bioinfo.life.hust.edu.cn/AnimalTFDB/, accessed on 13 July 2021) as background files and then extracted transcription factors from the identified differential genes. A total of 108 differentially expressed TFs were identified in the 12-m-XFWS vs. 12-m-TS (upregulated: 69, downregulated: 39) (Supplementary Table S14) and 197 in the 12-m-XFWS compared to the 3-m-XFWS (upregulated: 107, downregulated: 100) (Supplementary Table S15). In our study, 67 TFs were differentially expressed in both different breeds and ages (Figure 5A). Of these, fifteen were zf-C2H2, eleven homeobox, five were ZBTB, five were MYB-like, five were TF_bZIP, three were bHLH, three were Fork_head, and three were HMG (Figure 5B). In the 12-m-XFWS, the expression of zf-C2H2 and homeobox TFs was higher, which was different from the TFs in the 12-m-TS and 3-m-XFWS (Figure 5C, Supplementary Table S16). At the same time, the expression of zf-C2H2 and homeobox was increased significantly, showing that zf-C2H2 and homeobox may be expressed specifically in the 12-m-XFWS to regulate the growth of skin/hair. Three TFs were differentially expressed in the regulation of hair follicle development: OVOL1, SRF, and HOXC13. In the 12-m-XFWS, compared with the 12-m-TS and 3-m-XFWS, a total of 15 TFs were determined in zf-C2H2 (upregulated: 9, downregulated: 6) and 11 in homeobox (upregulated: 9, downregulated: 3), indicating that the expression of zf-C2H2 and homeobox increased in the 12-m-XFWS, which may positively regulate the expression of other genes and produce finer and denser wool.
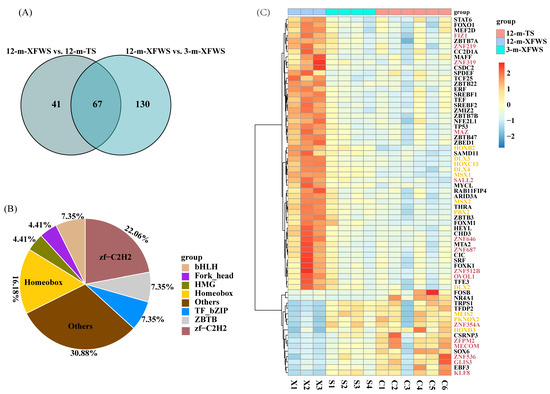
Figure 5.
Annotation of transcription factors of specific DEGs. (A) A Venn diagram of overlapped TFs in different breeds and during different periods (TFs, transcription factors). (B) The family classification of TFs. (C) TPM value of the clustering of overlapped TF expression level in skin tissue. (Genes marked deep pink represent members of the zf-C2H2 TF family; genes marked yellow represent members of the homeobox TF family).
4. Discussion
4.1. RNA-Seq Analysis and Shoulder-Skin-Related DEGs in Different Breeds and Ages
The molecular mechanism behind the development of hair follicles broadens our understanding of hair growth biology and the genetic basis of wool. This knowledge could be used to underpin the development of gene markers for use in wool production or use other molecular approaches to assist animal production such as gene editing. In this study, we systematically investigated the complexity of sheep skin and hair follicle development by comparing transcriptome data from the skin of Xinji fine-wool sheep and Tan sheep. The most prominent differences of the wool fibers between the coarse- and fine-wool sheep are produced by the primary hair follicles with long, thick, and medullated filaments in coarse-wool sheep and with fine, thin, and unmedullated filaments in fine-wool sheep, respectively. In the study of fine-wool sheep’s hair follicles, along with aging, the PFs decreased and the SFs increased [37]. Our results indicate the asynchronous development of sheep hair follicles in different breeds and ages.
In this study, we took the DEGs that regulate breed and time–space specificity into account. Our analysis showed that multiple GO terms and signaling pathways form complex mechanisms that regulate skin and hair development. We also identified three KEGG signaling pathways, namely the arrhythmogenic right ventricular cardiomyocyte pathway, vascular smooth muscle contraction, and cGMP-PKG signaling pathway, that participate in the morphogenesis and development of hair follicles and regulate the development of fibroblasts and epithelium during skin and hair/hair follicle induction. The regulation of skin cell proliferation and differentiation is directly involved in the development of hair follicles. Some important genes were identified in three KEGG signaling pathways. For example, a few recent studies illustrated the significance of LMNB1 in regulating cell proliferation and senescence in cultured human cells, whose depletion and overexpression restrain proliferation, but senescence was only induced by LMNB1 overexpression [38,39]. Overexpression of PLA2G10 resulted in hair loss in transgenic mice, accompanied by hair follicle distortion and decreased expression of genes related to hair development during the postnatal hair cycle [40]. SRF plays an important role in epidermal development and homeostasis. Mice lacking SRF in the epidermis displayed morphogenetic defects, including an eye-open-at-birth phenotype and a lack of whiskers [41]. In the analysis of the expression profile of lncRNAs in Hu sheep lambskin, it was found that TCONS_00279168 may target ATP1B4 and FGF12 to regulate MAPK, PI3K-Akt, and Ras signaling pathways involved in the sheep hair follicle development process [42,43].
4.2. DEGs Correlated with Hair Follicle Development
The protein–protein interaction networks of skin development (GO:0043588) provide clues for understanding their interactions and relationships with sheep hair follicle development. Protein–protein interaction results show that some hub genes interact with several different core genes, whereas one core gene can also target numerous hub genes. This phenomenon reveals that the core gene owns a central position affecting skin development and hair follicle growth. Numerous lines of evidence (such as gene expression, functional annotation, protein–protein interactions, and a literature survey) showed that we identified nine important candidate genes, LAMA5, OVOL1, SRF, DHCR24, NGFR, SMO, CDSN, KDF1, and HOXC13, implicated in regulating hair follicle development, and all of them were upregulated in 12-m-XFWS compared with 12-m-TS and 3-m-XFWS. The genes identified have been reported to be involved in skin and hair follicle development, representing regulation of epidermal cell differentiation and migration (LAMA5, SRF, CDSN) [38,44] and regulation of hair follicle development (OVOL1) [45], stem cell proliferation and differentiation (DHCR24, NGFR, SMO) [46,47], and regulation of hair cycle (KDF1, HOXC13) [48]. Previously, researchers have reported that there are some genes responsible for the production of the “FGF” protein in the root of the hair [49]. We found that FGF10 was downregulated in 12-m-XFWS compared with 12-m-TS and 3-m-XFWS. Therefore, we deduced that these important candidate genes in 12-month-old Xinji fine-wool sheep are significantly advantageous for secondary hair follicle development.
Previous studies have illustrated that members of the zf-C2H2 TF family are involved in the development response in vertebrates and arthropods, such as embryonic development, skeletal muscle development, and the nervous system [50,51,52]. However, there are no reports about their roles in the regulation of skin and development in sheep. Our transcriptome data suggested that zf-C2H2 TFs function in the diversity of sheep skin and wool, which is consistent with the conserved genes OVOL1 and OVOL2 encoding zf-C2H2 transcription factors being commonly found in mammals [53] that participate in epithelial cell proliferation and differentiation. In addition, homeobox TF genes were also enriched. For example, MSX2 contributes to epidermal competency during wound-induced hair follicle neogenesis [54]. DLX is also known to be associated with the morphogenesis of branchial arches, forebrain, and sensory organs [55]. The transcription factor HOXC13 has a stage-specific involvement in the morphogenesis of Merino sheep hair follicles [56]. Therefore, we speculate that high expression levels of zf-C2H2 and homeobox TFs in 12-m-XFWS enhanced finer and denser wool.
Xinji fine-wool sheep and Tan sheep were in the farms in Yanchi County of Ningxia and Songyuan City of Jilin Province, respectively, and fed based on the farm’s feeding plan; thus the impact of environment and nutrition was not considered. Overall, we identified nine hair-follicle-related genes and established multiple core genes potentially having minor effects on skin development and hair growth. So far, few studies have been shown to investigate the TFs contributing to skin morphogenesis and development between different sheep breeds and ages [57]. Our report first revealed that members of the zf-C2H2 and homeobox TF families were involved in the induction of hair follicle development between Xinji fine-wool sheep and Tan sheep with enormous phenotypic differences in their wool fibers. These results demonstrate the typical major–minimal gene interactions that jointly affect skin/hair growth and help to better understand the molecular events that regulate the diversity of wool fibers in different sheep lines.
5. Conclusions
In conclusion, this study provides a genome-wide view of mRNA expression profiles in the shoulder skin of sheep from two different breeds, and for one of those breeds, two different ages were also profiled. Moreover, GO annotation and KEGG pathway analysis were performed to identify the candidate DEGs affecting skin and wool diversity under different breeds and periods. We also constructed protein–protein interaction networks of skin development (GO:0043588) to confirm the interaction relationships in this network to reveal hub genes that regulate hair follicle growth. As a result of the analysis and characterization of TFs, the expression of zf-C2H2 and homeobox TFs was mainly upregulated in the 12-m-XFWS compared with the 12-m-TS and 3-m-XFWS and therefore associated with the production of finer and denser wool. These conclusions contribute to the knowledge of skin/hair growth, have value for improving hair follicle development, and provide a new perspective for the regulation of wool growth.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture12010015/s1, Figure S1: Protein–protein interaction analysis of skin development (GO:0043588). Table S1: Details of primers used for qPCR analysis. Table S2: Summary of raw reads after quality control and mapping to the reference genome. Table S3: Statistical analysis of TPM value. Table S4: Statistical analysis of Pearson’s correlation coefficient value. Table S5: The expression level of each sample sequence is measured by TPM value. Table S6: DEGs between 12-m-XFWS and 12-m-TS. Table S7: DEGs between 12-m-XFWS and 3-m-XFWS. Table S8: Statistics of the number of upregulated and downregulated DEGs. Table S9: Statistics of the DEGs in 12-m-XFWS vs. 12-m-TS. Table S10: Statistics of the DEGs in 12-m-XFWS vs. 3-m-XFWS. Table S11: 1327 overlapped candidate genes regulating breed and time–space specificity. Table S12: GO annotation and KEGG pathway analysis of 1327 DEGs. Table S13: The qPCR nine candidate genes for hair follicle development. Table S14: Differentially expressed TFs between 12-m-XFWS and 12-m-TS. Table S15: Differentially expressed TFs between 12-m-XFWS and 3-m-XFWS. Table S16: The expression level of 67 overlapped TFs is measured by TPM value.
Author Contributions
Formal analysis, T.B. and B.L.; Funding acquisition, Y.M.; Investigation, Y.P.; Methodology, J.H.; Project administration, Y.M.; Resources, C.W.; Software, Y.Z.; Validation, L.J.; Writing—original draft, T.B.; Writing—review and editing, L.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 31961143021), the earmarked fund for Modern Agro-industry Technology Research System (CARS-39-01), and the Agricultural Science and Technology Innovation Program of China (ASTIP-IAS01).
Institutional Review Board Statement
All experiments were performed following the relevant guidelines and regulations set by the Ministry of Agriculture of the People’s Republic of China. Ethical approval on animal survival was given by the animal ethics committee of CAAS-IAS (IAS2019-57).
Informed Consent Statement
Not applicable.
Data Availability Statement
All raw data generated in this study were submitted to the National Center for Biotechnology Information Sequence Read Archive (NCBI SRA) database under BioProject No. PRJNA765722 Available online: https://dataview.ncbi.nlm.nih.gov/object/PRJNA765722?reviewer=fv3n54gddeqqmbvm37n1aqst2c (accessed on 3 October 2021).
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial conflict of interest.
References
- Schneider, M.R.; Schmidt-Ullrich, R.; Paus, R. The Hair Follicle as a Dynamic Miniorgan. Curr. Biol. 2009, 19, R132–R142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mann, S.J. The Tylotrich (Hair) follicle of the american opossum. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 1968, 160, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Nixon, A.J.; Broad, L.; Saywell, D.P.; Pearson, A.J. Transforming growth factor-alpha immunoreactivity during induced hair follicle growth cycles in sheep and ferrets. J. Histochem. Cytochem. 1996, 44, 377–387. [Google Scholar] [CrossRef] [Green Version]
- Grymowicz, M.; Rudnicka, E.; Podfigurna, A.; Napierala, P.; Smolarczyk, R.; Smolarczyk, K.; Meczekalski, B. Hormonal Effects on Hair Follicles. Int. J. Mol. Sci. 2020, 21, 5342. [Google Scholar] [CrossRef]
- Rogers, G.E. Biology of the wool follicle: An excursion into a unique tissue interaction system waiting to be re-discovered. Exp. Dermatol. 2006, 15, 931–949. [Google Scholar] [CrossRef]
- Wang, X.; Ma, Q.; Zhao, Z. Research progress of regulator genes of Tan sheep furcolor. Biot. Resour. 2019, 41, 143–148. [Google Scholar]
- Wu, C.; Wang, C. Polymorphism analysis of five reproductive hormone receptor genes in Xinji fine wool sheep. Sci. Technol. 2019, 55, 63–69. [Google Scholar]
- Moore, G.; Jackson, N.; Isaacs, K.; Brown, G. Pattern and Morphogenesis in Skin. J. Theor. Biol. 1998, 191, 87–94. [Google Scholar] [CrossRef]
- Bandmann, H.J.; Bosse, K. Histology and anatomy of the hair follicle in the course of the hair cycle. Arch. Klin. Exp. Dermatol. 1966, 227, 390–409. [Google Scholar] [CrossRef]
- Hollis, D.E.; Chapman, R.E.; Panaretto, B.A.; Moore, G. Morphological Changes in the Skin and Wool Fibres of Merino Sheep Infused with Mouse Epidermal Growth Factor. Aust. J. Biol. Sci. 1983, 36, 419–434. [Google Scholar] [CrossRef] [Green Version]
- Gillespie, J.M.; Frenkel, M.J.; Reis, P.J. Changes in the matrix proteins of wool and mouse hair following the administra-tion of depilatory compounds. Aust. J. Biol. Sci. 1980, 33, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Tisserant, E.; Da Silva, C.; Kohler, A.; Morin, E.; Wincker, P.; Martin, F. Deep RNA sequencing improved the structural anno-tation of the Tuber melanosporum transcriptome. New Phytol. 2011, 189, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, G.C.; Arnaud, M.B.; Inglis, D.O.; Skrzypek, M.S.; Binkley, G.; Simison, M.; Miyasato, S.R.; Binkley, J.; Orvis, J.; Shah, P.; et al. The Aspergillus Genome Database: Multispecies curation and incorporation of RNA-Seq data to improve structural gene annotations. Nucleic Acids Res. 2014, 42, D705–D710. [Google Scholar] [CrossRef] [Green Version]
- La, Y.; He, X.; Zhang, L.; Di, R.; Wang, X.; Gan, S.; Zhang, X.; Zhang, J.; Hu, W.; Chu, M. Comprehensive Analysis of Differentially Expressed Profiles of mRNA, lncRNA, and circRNA in the Uterus of Seasonal Reproduction Sheep. Genes 2020, 11, 301. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; You, S.; Yao, Y.; Liu, Z.-J.; Hazi, W.; Li, C.-Y.; Zhang, X.-Y.; Hou, X.-X.; Wei, J.-C.; Li, X.-Y.; et al. Expression profiles of circular RNAs in sheep skeletal muscle. Asian-Australas. J. Anim. Sci. 2018, 31, 1550–1557. [Google Scholar] [CrossRef]
- Li, Y.; Kong, L.; Deng, M.; Lian, Z.; Han, Y.; Sun, B.; Guo, Y.; Liu, G.; Liu, D. Heat Stress-Responsive Transcriptome Analysis in the Liver Tissue of Hu Sheep. Genes 2019, 10, 395. [Google Scholar] [CrossRef] [Green Version]
- Mou, C.; Thomason, H.A.; Willan, P.M.; Clowes, C.; Harris, W.E.; Drew, C.F.; Dixon, J.; Dixon, M.J.; Headon, D.J. Enhanced ectodys-plasin-A receptor (EDAR) signaling alters multiple fiber characteristics to produce the East Asian hair form. Hum. Mutat. 2008, 29, 1405–1411. [Google Scholar] [CrossRef] [Green Version]
- Kamberov, Y.G.; Wang, S.; Tan, J.; Gerbault, P.; Wark, A.; Tan, L.; Yang, Y.; Li, S.; Tang, K.; Chen, H.; et al. Modeling Recent Human Evolution in Mice by Expression of a Selected EDAR Variant. Cell 2013, 152, 691–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, Y.; Li, S.; Zheng, X.; Chen, W.; Li, X.; Liu, Z.; Hu, Y.; Qiao, H.; Qi, Q.; Pei, Q.; et al. Transcriptome Reveals Long Non-coding RNAs and mRNAs Involved in Primary Wool Follicle Induction in Carpet Sheep Fetal Skin. Front. Physiol. 2018, 9, 446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, W.K.W.; Freem, L.; Zhao, D.; Painter, K.J.; Woolley, T.E.; Gaffney, E.A.; McGrew, M.J.; Tzika, A.; Milinkovitch, M.C.; Schneider, P.; et al. Feather arrays are patterned by interacting signalling and cell density waves. PLoS Biol. 2019, 17, e3000132. [Google Scholar] [CrossRef] [Green Version]
- Drew, C.F.; Lin, C.M.; Jiang, T.X.; Blunt, G.; Mou, C.; Chuong, C.M.; Headon, D.J. The Edar subfamily in feather placode formation. Dev. Biol. 2007, 305, 232–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mou, C.; Pitel, F.; Gourichon, D.; Vignoles, F.; Tzika, A.; Tato, P.; Yu, L.; Burt, D.W.; Bed’Hom, B.; Tixier-Boichard, M.; et al. Cryptic Patterning of Avian Skin Confers a Developmental Facility for Loss of Neck Feathering. PLoS Biol. 2011, 9, e1001028. [Google Scholar] [CrossRef] [Green Version]
- Yue, Y.; Guo, T.; Yuan, C.; Liu, J.; Guo, J.; Feng, R.; Niu, C.; Sun, X.; Yang, B. Integrated Analysis of the Roles of Long Noncoding RNA and Coding RNA Expression in Sheep (Ovis aries) Skin during Initiation of Secondary Hair Follicle. PLoS ONE 2016, 11, e0156890. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.Y.; Yang, H.; Shi, G.Q.; Shen, M.; Yang, J.Q.; Yang, Y.L.; Liu, X.J. Expression profile analysis of microRNAs during hair folli-cle development in the sheep foetus. Biosci. Biotechnol. Biochem. 2019, 83, 1045–1061. [Google Scholar] [CrossRef]
- Zhao, R.; Li, J.; Liu, N.; Li, H.; Liu, L.; Yang, F.; Li, L.; Wang, Y.; He, J. Transcriptomic Analysis Reveals the Involvement of lncRNA-miRNA-mRNA Networks in Hair Follicle Induction in Aohan Fine Wool Sheep Skin. Front. Genet. 2020, 11, 590. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript as-sembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differen-tiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [Green Version]
- Bray, N.L.; Pimentel, H.; Melsted, P.; Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016, 34, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive func-tional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Ishiguro-Watanabe, M.; Tanabe, M. KEGG: Integrating viruses and cellular organisms. Nucleic Acids Res. 2021, 49, D545–D551. [Google Scholar] [CrossRef]
- Reimand, J.; Arak, T.; Adler, P.; Kolberg, L.; Reisberg, S.; Peterson, H.; Vilo, J. g: Profiler-a web server for functional interpreta-tion of gene lists (2016 update). Nucleic Acids Res. 2016, 44, W83–W89. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Potter, C.S.; Pruett, N.D.; Kern, M.J.; Baybo, M.A.; Godwin, A.R.; Potter, K.A.; Peterson, R.L.; Sundberg, J.P.; Awgulewitsch, A. The Nude Mutant Gene Foxn1 Is a HOXC13 Regulatory Target during Hair Follicle and Nail Differentiation. J. Investig. Dermatol. 2011, 131, 828–837. [Google Scholar] [CrossRef] [Green Version]
- Ma, G.-W.; Chu, Y.-K.; Yang, H.; Yan, X.-H.; Rong, E.-G.; Li, H.; Wang, N. Functional Analysis of Sheep POU2F3 Isoforms. Biochem. Genet. 2019, 58, 335–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wu, K.; Wang, L.; Wang, Z.; Han, W.; Chen, D.; Wei, Y.; Su, R.; Wang, R.; Liu, Z.; et al. Comparative study on seasonal hair follicle cycling by analysis of the transcriptomes from cashmere and milk goats. Genome 2020, 112, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dong, H. Study on the development law of hair follicle of Gansu alpine fine wool sheep and its hybrid sheep. Chin. Herbiv. Anim. Sci. 2019, 39, 67–69. [Google Scholar]
- Dreesen, O.; Chojnowski, A.; Ong, P.F.; Zhao, T.Y.; Common, J.E.; Lunny, D.; Lane, E.B.; Lee, S.J.; Vardy, L.A.; Stewart, C.L.; et al. Lamin B1 fluctuations have differential effects on cellular proliferation and senescence. J. Cell Biol. 2013, 200, 605–617. [Google Scholar] [CrossRef] [Green Version]
- Taimen, P.; Pfleghaar, K.; Shimi, T.; Möller, D.; Ben-Harush, K.; Erdos, M.R.; Adam, S.A.; Herrmann, H.; Medalia, O.; Collins, F.S.; et al. A progeria mutation reveals functions for lamin A in nuclear assembly, architecture, and chromosome organization. Proc. Natl. Acad. Sci. USA 2009, 106, 20788–20793. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, K.; Taketomi, Y.; Isogai, Y.; Miki, Y.; Sato, H.; Masuda, S.; Nishito, Y.; Morioka, K.; Ishimoto, Y.; Suzuki, N.; et al. Hair fol-licular expression and function of group X secreted phospholipase A2 in mouse skin. J. Biol. Chem. 2011, 286, 11616–11631. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.; Hindes, A.; Burns, C.J.; Koppel, A.C.; Kiss, A.; Yin, Y.; Ma, L.; Blumenberg, M.; Khnykin, D.; Jahnsen, F.L.; et al. Serum Response Factor Controls Transcriptional Network Regulating Epidermal Function and Hair Follicle Morphogenesis. J. Investig. Dermatol. 2013, 133, 608–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, X.; Chen, W.; Sun, W.; Hussain, Z.; Wang, S.; Wang, J. Analysis of lncRNAs Expression Profiles in Hair Follicle of Hu Sheep Lambskin. Animal 2020, 10, 1035. [Google Scholar] [CrossRef]
- Li, S.; Chen, W.; Zheng, X.; Liu, Z.; Yang, G.; Hu, X.; Mou, C. Comparative investigation of coarse and fine wool sheep skin indicates the early regulators for skin and wool diversity. Gene 2020, 758, 144968. [Google Scholar] [CrossRef]
- Leclerc, E.A.; Huchenq, A.; Mattiuzzo, N.R.; Metzger, D.; Chambon, P.; Ghyselinck, N.B.; Serre, G.; Jonca, N.; Guerrin, M. Cor-neodesmosin gene ablation induces lethal skin-barrier disruption and hair-follicle degeneration related to desmo-some dysfunction. J. Cell Sci. 2009, 122, 2699–2709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, T.; Tsuji, G.; Ohno, F.; Uchi, H.; Nakahara, T.; Hashimoto-Hachiya, A.; Yoshida, Y.; Yamamoto, O.; Oda, Y.; Furue, M. Activation of the OVOL1-OVOL2 Axis in the Hair Bulb and in Pilomatricoma. Am. J. Pathol. 2016, 186, 1036–1043. [Google Scholar] [CrossRef] [Green Version]
- Gilanchi, S.; Esmaeilzade, B.; Eidi, A.; Barati, M.; Mehrabi, S.; Ghoroghi, F.M.; Nobakht, M. Neuronal differentiation of rat hair follicle stem cells: The involvement of the neuroprotective factor Seladin-1 (DHCR24). Iran. Biomed. J. 2014, 18, 136–142. [Google Scholar] [PubMed]
- Veraitch, O.; Mabuchi, Y.; Matsuzaki, Y.; Sasaki, T.; Okuno, H.; Tsukashima, A.; Amagai, M.; Okano, H.; Ohyama, M. Induction of hair follicle dermal papilla cell properties in human induced pluripotent stem cell-derived multipotent LNGFR(+)THY-1(+) mesenchymal cells. Sci. Rep. 2017, 7, 42777. [Google Scholar] [CrossRef]
- Yang, X.; Cui, Y.; Yue, J.; He, H.; Yu, C.; Liu, P.; Liu, J.; Ren, X.; Meng, Y. The histological characteristics, age-related thickness change of skin, and expression of the HSPs in the skin during hair cycle in yak (Bos grunniens). PLoS ONE 2017, 12, e0176451. [Google Scholar] [CrossRef]
- Qi, Y.; Fu, S.; He, X.; Wang, B.; Da, L.; Te, R.; Yuejun, M.; Suzhen, S.; Zhang, W.; Liu, Y. Preliminary comparison of skin transcriptome from sheep with different wool fibre diameters. Anim. Prod. Sci. 2021, 61, 708. [Google Scholar] [CrossRef]
- Zhuang, Z.H.; Zhong, Y.; Chen, Y.H.; Zhang, Z.W. Research progress on the roles of Krüppel-like factors in muscle tissues. Hereditas 2018, 40, 733–748. [Google Scholar]
- Vanhoutteghem, A.; Bouche, C.; Maciejewski-Duval, A.; Hervé, F.; Djian, P. Basonuclins and disco: Orthologous zinc finger proteins essential for development in vertebrates and arthropods. Biochimie 2011, 93, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Runko, A.P.; Sagerström, C.G. A novel subfamily of zinc finger genes involved in embryonic development. J. Cell Biochem. 2004, 93, 887–895. [Google Scholar] [CrossRef]
- Tsuji, G.; Ito, T.; Chiba, T.; Mitoma, C.; Nakahara, T.; Uchi, H.; Furue, M. The role of the OVOL1-OVOL2 axis in normal and dis-eased human skin. J. Dermatol. Sci. 2018, 90, 227–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, M.W.; Jiang, T.-X.; Plikus, M.V.; Guerrero-Juarez, C.F.; Lin, C.-H.; Schafer, C.; Maxson, R.; Widelitz, R.B.; Chuong, C.-M.; Lin, C.-H. Msx2 Supports Epidermal Competency during Wound-Induced Hair Follicle Neogenesis. J. Investig. Dermatol. 2018, 138, 2041–2050. [Google Scholar] [CrossRef] [Green Version]
- Depew, M.J.; Liu, J.K.; Long, J.E.; Presley, R.; Meneses, J.J.; Pedersen, R.A.; Rubenstein, J. Dlx5 regulates regional development of the branchial arches and sensory capsules. Development 1999, 126, 3831–3846. [Google Scholar] [CrossRef]
- Zhao, B.; Luo, H.; He, J.; Huang, X.; Chen, S.; Fu, X.; Zeng, W.; Tian, Y.; Liu, S.; Li, C.J.; et al. Comprehensive transcriptome and methylome analysis delineates the biological basis of hair follicle development and wool-related traits in Me-rino sheep. BMC Biol. 2021, 19, 197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, F.; Jin, H.; Dalrymple, B.P.; Cao, Y.; Wei, T.; Vuocolo, T.; Zhang, M.; Piao, Q.; Ingham, A.B. A comparison of transcriptomic patterns measured in the skin of Chinese fine and coarse wool sheep breeds. Sci. Rep. 2017, 7, 14301. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).