Abstract
Sorghum is a major staple food crop for the people in semi-arid areas of Africa and Asia. Post-flowering drought is a global constraint of sorghum production. The study aimed to improve stay-green (STG) characteristics of farmer-preferred sorghum varieties in Tanzania using marker-assisted backcrossing. A total of 752 individuals representing five BC2F1 populations and their parents were genotyped using previously reported KASP markers linked with STG 3A and STG 3B quantitative trait loci (QTL). In the BC2F1 populations, the maximum number of individuals with heterozygous alleles were observed in S35*Pato background (37) whereas only seven individuals derived from the B35*Wahi parents’ background contained heterozygous alleles. Of the 30 single nucleotide polymorphism (SNP) markers, favourable alleles were observed at 18 loci in BC2F1 populations. In the BC2F1 generation, the highest (0.127 kg/panicle) grain yield was observed in the B35*NACO Mtama 1 background population. The genotypic analysis revealed the presence of favourable alleles in homozygous conditions at markers loci associated with STG 3A and STG 3B QTLs in BC2F3 populations, suggesting successful introgression of STG QTLs from the donor parents to the recurrent parents. Across water irrigation regimes, the highest (0.068 kg/panicle) mean grain weight was observed in the genotype NA316C. Therefore, our study demonstrated the utility of marker-assisted backcrossing for drought tolerance improvement of locally adapted sorghum varieties in Africa.
1. Introduction
Undoubtedly, drought is a major environmental threat to agriculture sustainability in the world which affects physiological plant growth and performance [1,2]. Nonetheless, both pre-and post-flowering drought stress limits crop yield, though post-flowering drought stress is a major concern for sorghum farmers in Tanzania where it occurs when plants are at the grain filling stage and critical for the determination of yield performance [3,4]. Drought affects water and nutrients uptake from soil and their distribution to plant parts leading to leaf rolling, leaf senescence and lower vegetative plant growth [4]. Consequently, it affects plant stand, grain size, grain quality and quantity. It is indicated that retention of greenness (referred as ‘non-senescence’) in leaves during limited water supply accelerates carbon fixation for the starch production [5]. The greenness of leaves allows the photosynthesis process to produce food for the plant and minimises the premature death of flowers, ultimately maintaining yield under drought stress conditions [6]. Generally, farmers escape long dry spells by delaying the planting of crop at the later stage where post-flowering does not fall within the dry spell period [7]; however, recent climate change events make it difficult for farmers and breeders to predict the best time to do it. The common traits indicative of drought tolerance include leaf rolling, leaf senescence, chlorophyll content, days to flowering as well as maturity and root biomass [8]. Moreover, traits such as panicle length, panicle weight, number of seeds per panicle, number of tillers, grain size, dry matter and inflorescence exertion indicate genetic prepotency for drought tolerance [9]. In the past, drought stress in sorghum has been extensively addressed using conventional breeding approaches, but application of modern breeding tools such as genome-wide association, marker-assisted selection (MAS), as well as marker-assisted backcrossing (MABC), is still limited [10]. Nevertheless, drought stress is a highly complex trait, where environmental and crop management practices significantly contribute to the observed variability [10,11] and individual performance. Therefore, the identification of plants that associate correctly with the traits of interest is quite challenging. The exploitation of drought tolerance traits in sorghum had been achieved by mapping STG quantitative trait loci (QTLs) associated with the expression of phenotypic traits such as delay of leaf senescence and maintaining of green leaves at maturity [12]. The efficiency of green leaf area retention depends on the genetic makeup of sorghum genotypes under limited water conditions [5] and governed by traits such as transpiration efficiency, water use efficiency (including water extraction and water use pattern) and leaf canopy development [13]. It is difficult to select plants by phenotyping only because variations may not be due to genetic differences but may be due to environmental effects that may mislead selections [14]. Consequently, researchers have dissected the traits encoding STG [13] and developed molecular markers that are tightly linked to STG QTLs associated with drought tolerance. Based on studies conducted to map STG QTLs in sorghum genotypes SC56, B35, KS19 and E36-1 using different molecular markers, target trait-linked markers were identified [15]. For instance, genotypes B35 and EF36-1 contain novel STG loci such as STG1 and STG2 located on chromosome 3 (SB1-03), STG3 on SBI-02 and, STG4, on SBI-05 which contribute to the expression of the STG phenotypes in sorghum [16]. The genotype B35 sorghum line is characterised by high chlorophyll content, STG and stable root system traits that enable plants to grow and survive better under limited moisture conditions [17]. Moreover, genotypes with STG have high efficiency in utilising nitrogen nutrients from the soil resulting in lower leaf senescence under limited moisture conditions [18,19]. Among the diverse DNA-based markers, SNPs markers clearly detect the existing polymorphism among genotypes, including in sorghum [20,21]. Compared with electrophoresis system-based traditional SNP markers, Kompetitive allele-specific PCRs (KASP) are useful because of low genotyping error rate and amenability to automation that may complement direct phenotyping under field conditions resulting in short breeding cycles in complement with conventional breeding method [2,22]. Additionally, codominant nature of KASP markers facilitates identification of favourable alleles in heterozygous conditions for the introgression of traits through MABC which are either governed by dominant or recessive gene action [23,24,25]. Previously, two QTLs (STG 3A and STG 3B) expressing leaf greenness under post-drought stress in sorghum have been mapped in 56 to 72 Mbp region of chromosome (SBI-02) for efficiency transpiration and vapour pressure deficit response [26,27]. Therefore, we aimed to introgress both STG 3A and STG 3B QTLs previously described [17,26] from donor parents B35 and S35 to the most important Tanzanian sorghum varieties NACO Mtama 1, Seguifa, Tegemeo, Macia, Pato, Wagita, Wahi, Hakika and Kenya through MABC for diversifying drought tolerance of sorghum varieties.
2. Materials and Methods
2.1. Experimental Materials and Location
The seeds of nine Tanzanian farmers’ preferred sorghum varieties, namely Pato, Macia, Seguifa, NACO Mtama 1, Tegemeo, Wagita, Wahi, Hakika and Kenya, were obtained from the Tanzania Agricultural Research Institute (TARI)—Ilonga and Hombolo Centres. The Pato variety originated in Ethiopia but was released in Tanzania after multi-location screening. Pato is a white grain variety that is used for food, and it is characterised by fast recovery from drought, having a maturity period between 95–100 days, highly palatable with hard grain endosperm and no testa. Macia and Seguifa varieties are also white grain and used for food while NACO Mtama1 is white grain used for food and brewing. Tegemeo has white endosperm, cooking quality and cream white grain colour; the grain is used for food and brewing. Wahi and Hakika were released as varieties for tolerance to drought and the Striga species in 2002. Wagita and Kenya are local varieties with red-coloured grains, used for food and local alcohol. Wagita and Kenya are late maturing varieties commonly grown in lake zone of Tanzania. Seeds of donor lines B35 and EF36-1 for STG were provided by ICRISAT.
The present study was conducted at TARI-Makutupora Centre located 23 km North of Dodoma city in the Dodoma region of Tanzania (Longitude: 35°, 46.093′ E and Latitude: 05°, 58.669′ S; Altitude: 1070 m) where rainfalls occur between December and April with a dry spell in February. The site is classified as semi-arid and is characterised by a monomodal rainfall pattern. The annual rainfall at TARI-Makutupora ranges from 300–500 mm with poor distribution and over 500 mm in few years, and the temperature varies from 15–35.1 °C. The crossing and backcrossing were performed in the breeding nursery at TARI-Makutupora from 2017 to 2019 using drip irrigation systems. The field condition for evaluation of BC2F3 population performance involved two blocks, the first with well-watered and the second with water stressed at post-flowering. Water stress was imposed from the post-flowering of plants to physiological maturity where no water was applied to plants. Drip irrigation was allowed once a day in well water environment until plant maturity.
2.2. Markers Used for Drought Tolerance
A combined approach of using morphological and molecular markers that track drought tolerance and related traits of agronomic importance was used in this study. This integrated approach made the selection of genotypes of interest effective. The morphological markers used in this study were leaf rolling, leaf senescence, plant height, days to 50% flowering, days to 50% plant maturity, the total number of green leaves at plant maturity, grain colour and grain size, grain weight, root biomass, stem biomass and chlorophyll content were utilised in each generation during field evaluation while molecular markers were integrated at later generations (BC2F1 and BC2F3 generations) for tracing the favourable alleles at STG 3A as well as STG 3B QTLs, together with some additional markers associated with drought-contributing traits [28,29]. Initially, a total of 30 SNP Kompetitive allele-specific PCR (KASP) markers were used to screen materials [30]. Eight out of these markers were associated with STG 3A and STG 3B, while the remaining twenty-two SNP markers were linked with traits contributing to STG (Table 1). SNP Ids snpSB0035, snpSB0037, snpSB0038, snpSB0072 and snpSB0091 had favourable allele for alternate allele, snpSB0075 and snpSB0080 for recurrent parent allele and the rest had favourale allele for the donor parent allele.

Table 1.
List of SNPs markers associated with STG QTLs used for genotyping sorghum.
Of the KASP SNP markers used for genotyping, about 73% and 53% were observed to be polymorphic for the donor and recurrent parents, respectively.
2.3. Introgression of STG QTLs from Donor Parents to the Recurrent Parents and Genotyping
The MABC method was employed to introgress STG QTLs from the donor parents B35 and S35 into the farmers’ preferred sorghum varieties (NACO-Mtama 1, Macia, Seguifa, Hakika, Wahi, Pato, Tegemeo, Kenya and Wagita) in Tanzania. The donor parent B35 is drought tolerant and contains STG 1, STG 2, STG 3 and STG 4 QTLs. Parental line S35 is an introgression line derived from B35 as STG QTL donor (STG A). In order to strengthen the stability of drought tolerance in farmers’ preferred varieties (recurrent parents), the donor STG alleles were introgressed into recurrent parents by developing backcrossed generations of F1, BC1F1 BC2F1, BC2F2 and BC2F3. Besides field evaluation, molecular markers were also applied at BC2F1 and BC2F3 generations for screening STG 3A and STG 3B QTLs.
2.4. The Development of F1, BC1F1 and BC2F1 Populations
A total of 12 crossing plots with 5 rows each were planted with spacing of 0.75 m by 0.30 m (row to row and plant to plant) in two crossing blocks. For crossing, selected plants in recurrent parents were emasculated using the plastic bag method [31]. Pollen was collected in paper bags from the donor parent plants and dusted on the stigma of panicles of the recurrent parents to obtain the F1 seeds. Pollination bags were removed at the soft dough stage (10–15 days after pollination) and the seed sets on bagged heads were assessed visually using a scale of 0 to 100%, where 0% represented a complete sterile head without seed set and 100% represented complete seed set. Bird scaring was conducted using tailored small pieces of bags from white mosquito nets which were covered per panicle to prevent birds from eating the seed set after removing the selfing bags.
To generate BC1F1 populations, each of the F1 generations together with their recurrent parents were planted in single row plot of 5.4 m long and were backcrossed to their respective recurrent parents. Similarly, BC1F1 seeds were used to generate subsequent BC2F1 populations.
2.5. Development of BC2F2 and BC2F3
For generation of BC2F2 population, a total of five genotypes comprising of three genotypes with heterozygous alleles (W82, NA241 and NA307) and two genotypes with homozygous alleles (NA316 and SE438) from the BC2F1 population were selected following genotyping analysis and agronomic performance under field conditions. Subsequently, genotypes NA241A, NA241B, NA316A, NA316B, NA316C, NA307, SE408 and SE438 were screened from the population BC2F2 as the best-performing under field conditions to generate the BC2F3 population.
2.6. Genotyping of BC2F1 and BC2F3 Populations
For introgression of STG QTLs, genotyping of BC2F1 populations was performed with a KASP marker (LGC, Middlesex, UK) in an agreement with Intertek-AgriTech (https://www.intertek.com/agriculture/agritech/: accessed on 20 July 2021) as an external service provider [32]. A total of 752 samples representing 150 individual leaf samples per BC2F1 population, together with donor and recurrent parents, were collected in the 96-well plates. Three-leaf discs of approximately 4–5 cm per plant sample were collected using single-hole punching methodology. Following genotyping of the BC2F1 population, the presence of favourable alleles at STG loci in the BC2F3 population was also confirmed with ten KASP markers tightly linked to STG 3A and STG 3B QTLs (SnpSB0042, SnpSB0049, SnpSB0053, SnpSB0054, SnpSB0072, SnpSB0089, SnpSB0098, SnpSB0101, SnpSB0102 and SnpSB0103). Seven BC2F3 populations (NA241A, NA241B, NA316A, NA316B, NA316C, SE408 and SE438) were chosen and at least 100 samples per populations together with parents, making a total of 752 samples, were genotyped. The genotype NA307 was evaluated under field conditions only following the limitation of samples needed for genotyping. The SNP markers used for genotyping at BC2F1 and BC2F3 populations are indicated in Table 1.
2.7. Molecular Data Analysis
Marker data analysis was performed using SNPviewer from Biosearch Technologies (https://www.biosearchtech.com/support/tools/genotyping-software/snpviewer, LGC, Middlesex, UK: accessed on 25 December 2020) for insurance of marker quality. Subsequently, genotyped lines were compared in flapjack v. 1.21.02.04 (https://ics.hutton.ac.uk/flapjack/: accessed on 25 December 2020).
3. Results
3.1. Phenotypic Data
Five percent of the best-performing genotypes of the BC2F1 populations for the traits plant height, STG, total number of green leaves at maturity, panicle length, panicle width and panicle weight that were screened under field conditions showed significance differences of performance in all traits evaluated across replication. The highest mean plant height (146.4 cm) was recorded in the population S35*Pato (Table 2), whereas donor parent B35 exhibited the lowest (88 cm) plant height. The selected 5 percent BC2F1 population performed better than their parents. The population B35*NACO Mtama 1 showed the highest grain yield (0.127 kg/panicle), while the donor parent B35 produced the lowest (0.013 kg/panicle). The best-performing BC2F1 genotypes under field conditions and those identified to be desirable at a genotypic level were screened for further improvements.

Table 2.
Five per cent of the best performance BC2F1 populations and their parents.
3.2. BC2F1 Genotyping
Of the 728 BC2F1 samples collected for genotyping, about 10% samples (71 plants) were observed to be heterozygous as representative SNP markers, detailed in Table 3. Of these, three (snpSB00075, snpSB00102 and snpSB00103) and two (snpSB00102 and snpSB00103) SNPs markers were scored as heterozygous in a total of seven and eight samples with BC2F1 of the B35*Wahi and BS35*Hakika backgrounds, respectively. Similarly, heterozygous alleles at three (snpSB00072, snpSB00077 and snpSB00103) and four markers (snpSB00049, snpSB00077, snpSB00102 and snpSB00103) loci were also identified in 37 samples of S35*Pato and 10 samples of BC2F1 with the B35*Seguifa background. The markers snpSB00037, snpSB00041, snpSB00098, snpSB00102 and snpSB00103 showed heterozygous alleles in 10 samples with B35*Macia backgrounds, respectively. The markers snpSB00042, snpSB00098, snpSB00102 and snpSB00103 expressed heterozygous alleles in a total of 18 samples of the B35*NACO Mtama 1 background (Table 3). The rest (19) of the SNP markers showed homozygous alleles of the BC2F1 samples used for genotyping. The complete details of markers are shown in the Supplementary Table S1 in the main text connecting Table 3 data.

Table 3.
BC2F1 sorghum populations scored with heterozygous alleles using SNP markers.
Eighteen SNP markers showed favourable alleles among 728 of BC2F1 samples genotyped (Table 4). The markers that indicated favourable alleles that were most important for STG included: snpSB00049 and snpSB00054 for STG 3A, and snpSB00102 and snpSB00103 for STG 3B.

Table 4.
Favourable alleles and STG QTLs identified after genotyping BC2F1 populations of sorghum.
In order to generate BC2F3 populations, three samples (W82, NA241 and NA307) showing heterozygous alleles and two samples (NA316 and SE438) with homozygous favourable alleles for STG QTL were chosen. The favourable alleles at markers associated with STG 3B QTL were found in plant W82 (snpSB00102), NA241 (snpSB00102 and snpSB00103) and NA307 (snpSB00101 and snpSB00102). Moreover, plant sample NA316 had favourable alleles in homozygous conditions for SNP markers associated with STG 3A (snpSB00049, snpSB00053 and snpSB00054) and STG 3B (snpSB00102). Similarly, plant SE438 also had homozygous alleles of SNP marker snpSB00054 and snpSB00102 associated with STG 3A and STG 3B, respectively. Plants which expressed heterozygous alleles after genotyping were categorised as the priority because of the availability of traits from both parents for successful STG introgression from either of the donor parents B35 or S35 to the recurrent parents. Prior to selection, phenotypic data were recorded for traits such as days to 50% flowering, plant height, days to 50% plant maturity, panicle weight, grain weight per plant, seed vigour, stability of plants, leaf senescence, susceptible to pests and diseases. Plants with good performance based on phenotypic data, such as days to 50% flowering, plant height, days to 50% plant maturity, panicle weight, grain weight per plant, seed vigour, stability of plants, leaf senescence and susceptibility to pest and diseases, were selected and compared with genotyped data. Finally, best-performing plants under field conditions with favourable alleles for STG 3A and STG 3B were selected.
3.3. Phenotypic Data of BC2F3 Populations’ Results
The mean grain weight per panicle evaluated across water irrigation and water stress condition was the highest (0.068 kg) in the genotype NA316C followed by NA307 with 0.064kg (Figure 1). The lowest (0.039 kg) mean grain yield was recorded in the donor parent B35. The mean grain weight of the rest of the genotypes ranged from 0.049–0.061 kg.
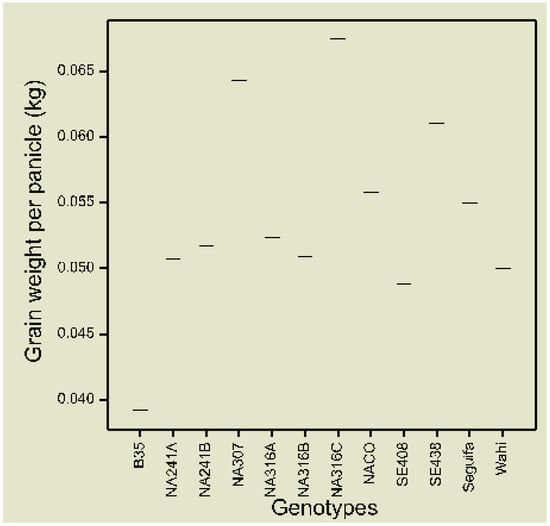
Figure 1.
Boxplot of mean grain weight per panicle of BC2F3 genotypes and parents across well-watered and water-stressed environments.
The mean STG per genotype screened across water regimes was scored the highest (4.45) in the donor parent B35 next by the control Wahi with 4.7 score (Figure 2). The lowest (6.23) score was recorded by the genotype NA307. However, of the BC2F3 populations, the genotype NA316A showed the highest STG retention in sorghum leaves. No significance differences were recorded in the rest of the genotypes.
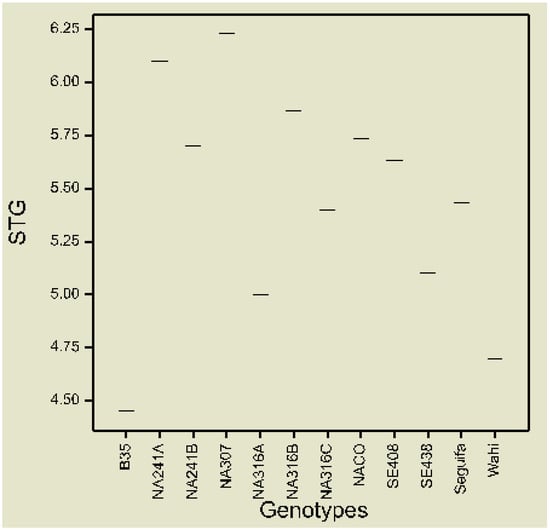
Figure 2.
Boxplot of mean STG of BC2F3 genotypes and parents across well-watered and water-stressed environments (Scale: 1–9 where, 1—all leaves in plant are green, 9—all leaves in plant are dried).
3.4. BC2F3 Genotyping Results
Eight out of ten SNP markers (snpSB0042, snpSB0049, snpSB0053, snpSB0054, snpSB0072, snpSB0089, snpSB0101 and snpSB0102) used for genotyping of BC2F3 population identified favourable homozygous alleles for STGs in sorghum as in the representative SNP marker details in Table 5. The SNP markers snpSB0042, snpSB0049, snpSB0053, snpSB0054, snpSB0072 and snpSB00089 identified favourable homozygous alleles for STG 3A QTL. The snpSB0101 and snpSB0102 markers linked favourable alleles for STG 3B QTL (Table 5). The SNP markers, snpSB00042 identified the lowest (2) number of favourable alleles in the donor parent B35. The snpSB00098 and snpSB0103 markers did not link favourable alleles in the population genotyped. The SNP markers, snpSB00054, snpSB00089 and snpSB00101, recorded the highest number (124) of samples with favourable alleles in almost all of the genotypes analysed. SNP markers which failed to score the favourable alleles and STG 3A and STG 3B QTLs were not included in Table 5. The complete details are shown in the Supplementary Table S2 in the main body text connecting Table 5 data.

Table 5.
Genotypes of BC2F3 populations scored by favourable alleles using SNP markers.
The SNP marker snpSB00089 indicated the highest (729) total number of samples with favourable homozygous alleles for STG 3B followed by snpSB00101 (728; Table 6). The SNP markers which failed to show favourable alleles linked to STG QTLs such as snpSB00098 and snpSB00103 were not included in Table 6. The SNP markers snpSB0042, snpSB0049, snpSB0053, snpSB0054, snpSB0072 and snpSB00089 were associated with STG 3A QTL. The snpSB0101 and snpSB0102 markers were linked with STG 3B QTL in the BC2F3 populations.

Table 6.
Favourable alleles and STG QTLs identified in BC2F3 populations of sorghum.
4. Discussion
Findings in this study revealed the importance of molecular markers to shorten breeding cycles in sorghum. The genotyping information is crucial for exploiting traits from the parents to improve new lines, as STG is a complex trait associated with several genes. The introgression of STG QTLs by MABC enhances post-flowering drought tolerance in sorghum [4]. The transfer of the STG trait from the donor parents B35 and S35 into the farmers’ preferred varieties using SNP markers is highly desirable for post-flowering drought tolerance. This study identified favourable alleles for donor and recurrent parents on STG 3A and STG 3B in the samples of the genotype BC2F1 and BC2F3 populations, indicating successful introgression. The incorporation of STG QTLs improves plants’ delayed leaf senescence from post-flowering to physiological plant maturity [12]. Sukumaran et al. [33] reported 8–24% phenotypic variations contributed by STG QTLs with the flanking SNP markers. Other studies which used SSRs markers for improvement of STG 1, STG 2, STG 3 and STG 4 in sorghum reported successful introgression [34,35]. The SNP markers indicate the arrangement of bialleles in the whole genome. The markers identify alleles with a high rate of recovery in the genome, which make their precision higher than other markers [30]. Findings in the current study show that some of the new genotypes generated by the backcrossing method performed well in grain yield compared with control and recurrent parents planted on the same date under similar environments. The outperformance of new genotypes over farmers’ cultivation varieties shows the advantages of SNP markers for high efficiency STG QTLs in sorghum improvement. STG 1 QTL mapped by SSR markers adapts post-flowering drought stress and it increases yield grain more than other QTLs, thus it is named as the best trait of the SSR markers [1,36]. However, QTLs STG 3A and STG 3B introgression to farmers’ drought susceptible cultivated sorghum varieties have reported significant improved efficiency of green area retention at post-flowering drought stress [37]. The QTLs have improved grain yield and water use efficiency at physiological plant maturity, making SNP the best markers for mapping tightly linked QTLs for STG compared with SSRs in the post-drought tolerance improvement in sorghum [26,37]. Currently, SNPs markers are the most commonly used markers for identification of genetic polymorphism which are used in the study of genetic variation among populations [38,39]. Da Silva et al. [30] used two backcrosses to introgress the bmr6 allele for biomass from the donor parent CMSXS170 line to elite lines CMSXS652 and IS23 of sweet sorghum using SNP markers. Results revealed a successful transfer of the bmr6 allele to the recurrent parent for biomass improvement. For successful backcrosses, the promising genotypes selected for traits of interest should contain favourable alleles from either one of the parents and must be expressed phenotypically [12]. Backcrosses must be done at least three to four times for sufficient transfer of the QTLs from the donor to the recurrent parents, followed by successive selfing until the backcross line is close to the original recurrent parent [30]. SNPs markers narrow the specific location of QTLs which is closely linked to the gene expressing the STG trait. The SNP markers identify favourable alleles for STG expression, thus hastening screening of drought tolerance sorghum genotypes. The current study identified favourable alleles for STG that were screened for further studies of sorghum improvement. It was noted that some of the plants performed well in the field environments by expressing STG, low leaf rolling, high yield and high vigour of sorghum grains, but were not tightly linked by SNP markers used for genotyping. This indicates that there is a need to map a large number of SNP markers to widen the chance of identifying the right STG QTLs. If SNP markers fail to locate the position of the QTL expressing STG in the chromosomes, SSR and DArT markers can alternatively be used [32]. Moreover, findings have reported that there are some QTLs which are detected by molecular markers in the backcrosses but fail to express under field conditions; such genotypes do not qualify for selection of crop improvement [40]. Differential transcriptome analysis encompasses bioprocess to map genes controlling the expression of drought tolerance in sorghum [41]. The STG expression on drought tolerant sorghum varieties is due to the reaction of genes which increase the production of antioxidant capacity, regulatory factors and the repressors of early senescence [41]. The application of modern technology supplements conventional breeding techniques to address drought stress in sorghum. With modern technology, for instance, molecular markers map traits of interest which saves time of breeding cycles. In this study, SNP markers identified favourable alleles for STG QTLs from the genotypes of BC2F1 and BC2F3 populations which simplified the comparison with field data to screen the best plants with the target traits. It is important to use markers that are powerful and reliable to identify the favourable alleles for STG, QTLs and chromosome position of genotypes for inclusion in drought tolerance sorghum improvement.
5. Conclusions and Recommendations
In the present study, marker-assisted selection was performed in BC2F1 and BC2F3 populations, and QTLs (STG 3A and STG 3B) associated with stay-green (STG) characteristics from the donor parent B35 were successfully introgressed into two farmers’ preferred recurrent varieties, NACO Mtama 1 and Seguifa. Nonetheless, populations generated from Pato, Macia, Wagita, Kenya, Tegemeo, Hakika and Wahi backgrounds did not continue due to the poor performance of either STG expression or associated traits such as leaf rolling, grain yield and its related traits. Besides better drought tolerance, improved lines also exhibited higher grain weight. The improved lines generated in the present study will further diversify the base of farmers’ preferred sorghum varieties in Tanzania that may help to cope with scarcity of rainfall, especially in semi-arid areas of Sub-Saharan Africa. The introgression lines of sorghum obtained in our study may be used as the bases for climate resilience seed to cope with climate change in Sub-Saharan Africa. Finally, our results suggest that the marker-assisted backcrossing together with field evaluations is sufficient to introgress the target traits from the donor source and recover desirable lines from recurrent parents.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/agriculture11090883/s1. Table S1: BC2F1 sorghum populations scored with heterozygous alleles using SNP markers, Table S2: Genotypes of BC2F3 populations scored by favourable alleles using SNP markers.
Author Contributions
A.M. designed the research study, data collection, data cleaning, data analysis and the development of first draft of manuscript. T.F. reviewed the first draft of the manuscript. J.S.Y.E., K.O. and P.T. participated in the experimental design and model of data analysis; they reviewed the second draft of the manuscript. S.D. provided breeding materials from ICRISAT India to Tanzania and part of the data analysis. A.L.G.-O., M.K. and R.B. participated in the guidance of sampling and genotyping activities. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the German Academic Exchange Service (DAAD) (Reference ID: 57377190), Shared Industrial-scale Low-density SNP Genotyping for CGIAR and Partner Breeding Programs Serving SSA and SA (High Through-put Genotyping—HTPG) BMGF funded project (OPP1130244) for genotyping support and The Africa Higher Education Centers of Excellence (ACE) Project II.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in supplementary material.
Acknowledgments
The authors acknowledged the Tanzania Agricultural Research Institute (TARI) for providing technical support when conducting research.
Conflicts of Interest
Authors declare no conflict of interest in this research manuscript.
References
- Barron, J.; Rockström, J.; Gichuki, F.; Hatibu, N. Dry spell analysis and maize yields for two semi-arid locations in East Africa. Agric. Meteorol. 2003, 117, 23–37. [Google Scholar] [CrossRef]
- Chander, S.; Garcia-Oliveira, A.L.; Gedil, M.; Shah, T.; Otusanya, G.O.; Asiedu, R.; Chigeza, G. Genetic diversity and population structure of soybean lines adapted to sub-Saharan Africa using single nucleotide polymorphism (SNP) markers. Agronomy 2021, 11, 604. [Google Scholar] [CrossRef]
- Premachandra, G.S.; Hahn, D.T.; Joly, R.J. Leaf water relations and gas exchange in two grain sorghum genotypes differing in their pre- and post-flowering drought tolerance. J. Plant Physiol. 1994, 143, 96–101. [Google Scholar] [CrossRef]
- Kamal, S.M.; Gorafi, Y.S.A.; Tsujimoto, H.; Ghanim, A.M.A. Stay-Green QTLs response in adaptation to post flowering drought depends on the drought deverity. BioMed Res. Int. 2018, 2018, 1–15. [Google Scholar] [CrossRef]
- Borrell, A.K.; van Oosterom, E.J.; Mullet, J.E.; George-Jaeggli, B.; Jordan, D.R.; Klein, P.E.; Hammer, G.L. Stay-green alleles individually enhance grain yield in sorghum under drought by modifying canopy development and water uptake patterns. New Phytol. 2014, 203, 817–830. [Google Scholar] [CrossRef]
- Rosenow, D.T.; Clark, L.E. Drought tolerance in sorghum. In Proceedings of the 36th Annual Corn and Sorghum Industry Research Conference; Loden, H.D., Wilkinson, D., Eds.; American Seed trade Association: Washington, DC, USA, 1981; pp. 18–31. [Google Scholar]
- Slegers, M.F.W. Exploring Farmers’ Perceptions of Drought in Tanzania and Ethiopia. Ph.D Thesis, Wageningen University, Wageningen, The Netherlands, 2008. [Google Scholar]
- Velazco, J.G.; Jordan, D.R.; Mace, E.S.; Hunt, C.H.; Malosetti, M.; van Eeuwijk, F.A. Genomic Prediction of Grain Yield and Drought-Adaptation Capacity in Sorghum is Enhanced by Multi-Trait Analysis. Front. Plant Sci. 2003, 10, 997. [Google Scholar] [CrossRef]
- Sory, S. Marker Assisted Selection for Post-Flowering Drought Tolerance in Sorghum bicolor [L.] Moench. Ph.D. Thesis, University of Ghana, Accra, Ghana, December 2015. [Google Scholar]
- Serraj, R.; Hash, T.C.; Buhariwalla, H.K.; Bidinger, F.R.; Folkertsma, R.T.; Chandra, S.; Gaur, P.; Kashiwagi, J.; Nigam, S.N.; Rupakula, A.; et al. Marker-assisted breeding for crop drought tolerance at ICRISAT: Achievements and prospects. In Proceedings of the International Congress; In the Wake of the Double Helix: From the Green Revolution to the Gene Revolution; Tuberosa, R., Phillips, R.L., Gale, M., Eds.; Avenue Media: Bologna, Italy, 2003; pp. 217–238. [Google Scholar]
- Boyles, R.E.; Brenton, Z.W.; Kresovich, S. Genetic and genomic resources of sorghum to connect genotype with phenotype in contrasting environments. Plant J. 2019, 97, 19–39. [Google Scholar] [CrossRef]
- Kamal, N.M.; Gorafi, Y.S.A.; Abdeltwab, H.; Abdalla, I.; Tsujimoto, H.; Ghanim, A.M.A. A New Breeding Strategy towards Introgression and Characterization of Stay-Green QTL for Drought Tolerance in Sorghum. Agriculture 2021, 11, 598. [Google Scholar] [CrossRef]
- Vadez, V.; Deshpande, S.P.; Kholova, J.; Hammer, G.L.; Borrell, A.K.; Talwar, H.S.; Hash, C.T. Stay-green quantitative trait loci’s effects on water extraction, transpiration efficiency and seed yield depend on recipient parent background. Funct. Plant Biol. 2011, 38, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Ribaut, J.M.; Betran, J. Single large-scale marker assisted selection (SLS-MAS). Mol. Breed. 1999, 5, 531–541. [Google Scholar] [CrossRef]
- Xu, W.; Subudhi, P.K.; Crasta, O.R.; Rosenow, D.T.; Mullet, J.E.; Nguyen, H.T. Molecular mapping of QTLs conferring staygreen in grain sorghum (Sorghum bicolor L. Moench). Genome 2000, 43, 461–469. [Google Scholar] [CrossRef]
- Kebede, H.; Subudhi, P.K.; Rosenow, D.T.; Nguyen, H.T. Quantitative trait loci influencing drought tolerance in grain sorghum (Sorghum bicolor L. Moench). Theor. Appl. Genet. 2001, 103, 266–276. [Google Scholar] [CrossRef]
- Ayalew, K.; Adugna, A.; Fetene, M.; Sintayehu, S. Evaluation of bio-physiological and yield responses of stay-green QTL introgression sorghum lines to post-flowering drought stress. Afr. Crop Sci. J. 2018, 26, 447–462. [Google Scholar] [CrossRef][Green Version]
- Abdelrahman, M.; El-Sayed, M.; Jogaiah, S.; Burritt, D.J.; Tran, L.P. The “STAY-GREEN” trait and phytohormone signaling networks in plants under heat stress. Plant Cell Rep. 2017, 36, 1009–1025. [Google Scholar] [CrossRef] [PubMed]
- Ngugi, K.; Kimani, W.; Kiambi, D.; Mutitu, E.W. Improving Drought Tolerance in Sorghum bicolor L. Moench: Mar ker-Assisted transfer of the Stay-Green Quantitative Trait Loci (Q T L) from a Characterized Donor Source into a Local Farmer Variety. Int. J. Sci. 2013, 1, 154–162. [Google Scholar] [CrossRef][Green Version]
- Gimode, D.; Odeny, D.A.; de Villiers, E.P.; Wanyonyi, S.; Dida, M.M.; Mneney, E.E.; Muchugi, A.; Machuka, J.; de Villiers, S.M. Identification of SNP and SSR Markers in Finger Millet Using Next Generation Sequencing Technologies. PLoS ONE 2016, 11, e0159437. [Google Scholar] [CrossRef]
- Mammadov, J.; Aggarwal, R.; Buyyarapu, R.; Kumpatla, S. SNP markers and their impact on plant breeding. Int. J. Plant Genom. 2012, 2012, 728398. [Google Scholar] [CrossRef] [PubMed]
- Semagn, K.; Babu, R.; Hearne, S.; Olsen, M. Single nucleotide polymorphism genotyping using Kompetitive Allele Specific PCR (KASP): Overview of the technology and its application in crop improvement. Mol. Breed. 2013, 33, 1–14. [Google Scholar] [CrossRef]
- Thomson, M.J. High-throughput SNP genotyping to accelerate crop improvement. Plant Breed Biotech. 2014, 2, 195–212. [Google Scholar] [CrossRef]
- Meade, F.; Byrne, S.; Griffin, D.; Kennedy, C.; Mesiti, F.; Dan Milbourne, D. Rapid Development of KASP Markers for Disease Resistance Genes Using Pooled Whole-Genome Re-sequencing. Potato Res. 2020, 63, 57–73. [Google Scholar] [CrossRef]
- Kiranmayee, K.N.S.U.; Hash, C.T.; Sivasubramani, S.; Ramu, P.; Amindala, B.P.; Rathore, A.; Kishor, P.B.K.; Gupta, R.; Deshpande, S.P. Fine-Mapping of Sorghum Stay-Green QTL on Chromosome10 Revealed Genes Associated with Delayed Senescence. Genes 2020, 11, 1026. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, S.; Rakshit, S.; Manasa, K.G.; Pandey, S.; Gupta, R. Genomic Approaches for Abiotic Stress Tolerance in Sorghum. In the Sorghum Genome; Springer: Cham, Switzerland, 2016; pp. 169–187. [Google Scholar]
- Meru, G.M. Genotyping BC3F2 Populations of Four Ethiopian Sorghum Varieties for Stay Green QTLs Introgression through Marker Assisted Selection with SSRs. Master’s Thesis, Kenyata University, Nairobi, Kenya, 2010. [Google Scholar]
- International Board for Plant Genetic Resources (IBPGR); International Crops Research Institute for the Semi-Arid Tropics (ICRISAT). Descriptors for Sorghum [Sorghum bicolor (L.) Moench]; IBPGR: Rome, Italy; ICRISAT: Patancheru, India, 1993. [Google Scholar]
- Burow, G.; Chopra, R.; Sattler, S.; Burke, J.; Acosta-Martinez, V.; Xin, Z. Deployment of SNP (CAPS and KASP) markers for allelic discrimination and easy access to functional variants for brown midrib genes bmr6 and bmr12 in Sorghum bicolor. Mol. Breed. 2019, 39, 115. [Google Scholar] [CrossRef]
- Da Silva, M.J.; Ribeiro, P.C.O.; Silva, R.A.; Silva, K.J.; Schaffert, R.E.; Parrella, R.A.C. Multi-trait selection of sweet sorghum (Sorghum bicolor (L.) Moench) genotypes for bioenergy production. J. Bioenergy Food Sci. 2020, 7, e2952020. [Google Scholar] [CrossRef]
- Schertz, K.F.; Clark, L.E. Controlling dehiscence with plastic bags for hand crosses in Sorghum 1. Crop Sci. 1967, 7, 540–542. [Google Scholar] [CrossRef]
- Sakuraba, Y.; Park, S.; Kim, Y.; Wang, S.; Yoo, S.; Hörtensteiner, S.; Paek, N. Arabidopsis STAY-GREEN2 Is a Negative Regulator of Chlorophyll Degradation during Leaf Senescence. Mol. Plant 2014, 7, 1288–1302. [Google Scholar] [CrossRef]
- Sukumaran, S.; Li, X.; Xianran Li, X.; Zhu, C.; Bai, G.; Perumal, R.; Tuinstra, M.R.; Prasad, P.V.; Mitchell, S.E.; Tesso, T.T.; et al. QTL Mapping for Grain Yield, Flowering Time, and Stay-Green Traits in Sorghum with Genotyping-by-Sequencing Markers. Crop Sci. 2016, 56, 1429–1442. [Google Scholar] [CrossRef]
- Kimani, W.; Ngugi, K.; Kiambi, D.; MutituI, E.W.; De Villiers, S. Marker-assisted introgression of stay-green trait in a Kenyan sorghum variety. E. Afr. Agric. For. J. 2012, 78, 109–115. [Google Scholar]
- Reddy, N.R.; Ragimasalawada, M.; Sabbavarapu, M.M.; Nadoor, S.; Patil, J.V. Detection and va Perrierlidation of stay-green QTL in post-rainy sorghum involving widely adapted cultivar, M35-1 and a popular stay-green genotype B35. BMC Genom. 2014, 15, 909. [Google Scholar] [CrossRef]
- Kamal, N.M.; Gorafi, Y.S.A.; Ghanim, A.M.A. Performance of Sorghum stay-green introgression lines under post-flowering drought. Int. J. Plant Res. 2017, 7, 65–74. [Google Scholar]
- Kholova, J.; Deshpande, S.; Madhusudhana, R.; Blummel, M.; Borrell, A.; Hammer, G. Improving Post-Rainy Sorghum Varieties to Meet the Growing Grain and Fodder Demand in India—Phase 2; Australian Centre for International Agricultural Research: Canberra, Australia, 2019; p. 29.
- Adu-Boakyewaa, G.A.; Badu-Apraku, B.; Akromah, R.; Garcia-Oliveira, A.L.; Awuku, F.J.; Gedil, M. Genetic diversity and population structure of early-maturing tropical maize inbred lines using SNP markers. PLoS ONE 2019, 14, e021481. [Google Scholar]
- Nelimor, C.; Badu-Apraku, B.; Garcia-Oliveira, A.L.; Tetteh, A.; Paterne, A.; N’guetta, A.S.; Gedil, M. Genomic Analysis of Selected Maize Landraces from Sahel and Coastal West Africa Reveals Their Variability and Potential for Genetic Enhancement. Genes 2020, 11, 1054. [Google Scholar] [CrossRef] [PubMed]
- Platten, J.D.; Cobb, J.N.; Zantua, R.E. Criteria for evaluating molecular markers: Comprehensive quality metrics to improve marker-assisted selection. PLoS ONE 2019, 14, e0210529. [Google Scholar] [CrossRef]
- Azzouz-Olden, F.; Hunt, A.G.; Dinkins, R. Transcriptome analysis of drought-tolerant sorghum genotype SC56 in response to water stress reveals an oxidative stress defense strategy. Mol. Biol. Rep. 2020, 47, 3291–3303. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).