Freshwater Cladophora glomerata Biomass as Promising Protein and Other Essential Nutrients Source for High Quality and More Sustainable Feed Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. C. glomerata Biomass Collection and Preparation
2.2. C. glomerata Biomass Chemical Analysis
2.3. C. glomerata Biomass Amino Acids Profile Analysis
2.4. C. glomerata Biomass Fatty Acids Profile Analysis
2.5. Statistical Analysis
3. Results
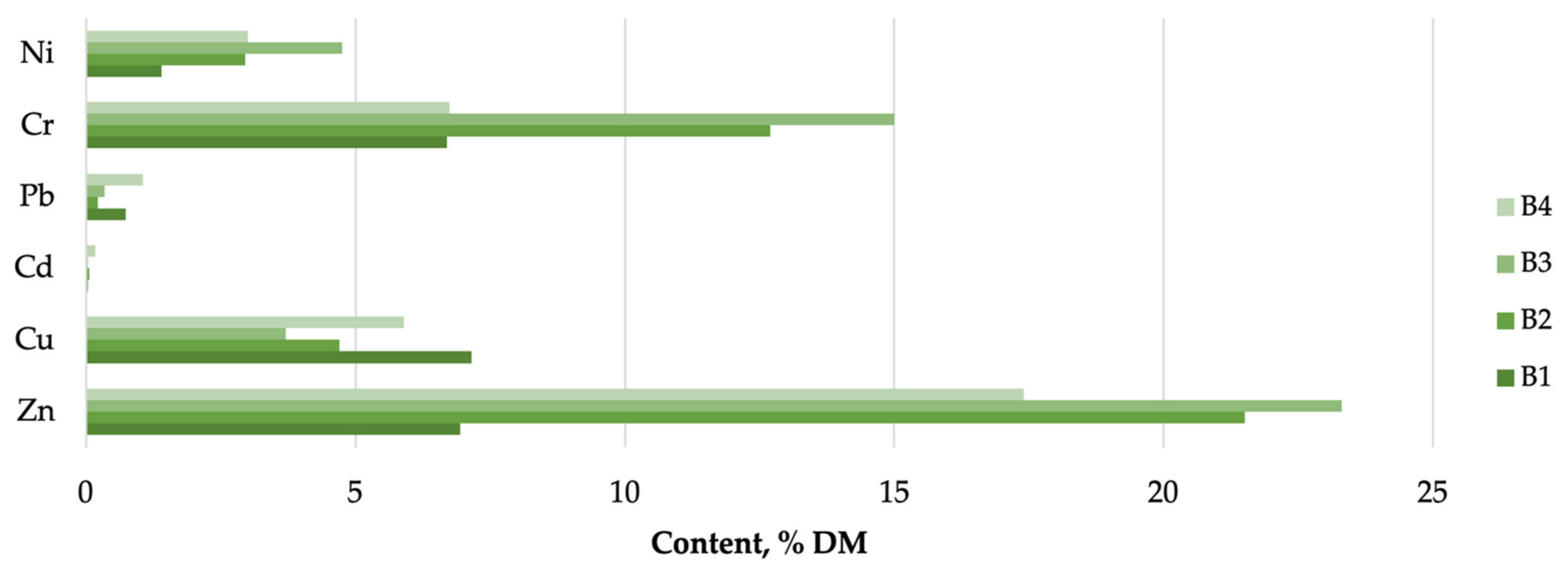
3.1. Chemical Composition of C. glomerata Biomass
3.2. Amino Acids Profile of C. glomerata Biomass
3.3. Fatty Acids Profile of C. glomerata Biomass
4. Discussion
4.1. Macro- and Micronutrients in Algal Biomass
4.2. Proteins and Amino Acids in Algal Biomass
4.3. Lipids and Fatty Acids in Algal Biomass
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Herrero, M.; Mason-D’Croz, D.; Godde, C.M.; Palmer, J.; Thornton, P.K.; Gill, M. Livestock, Land and the Environmental Limits of Animal Source-Food Consumption. In Proceedings of the Science Forum 2018, Stellenbosch, South Africa, 10–12 October 2018; CGIAR Independent Science & Partnership Council (ISPC): Stelle Nbosch, South Africa, 2018; p. 39. [Google Scholar]
- Wan, A.H.L.; Davies, S.J.; Soler-Vila, A.; Fitzgerald, R.; Johnson, M.P. Macroalgae as a sustainable aquafeed ingredient. Rev. Aquac. 2019, 11, 458–492. [Google Scholar] [CrossRef]
- Bruneel, C.; Lemahieu, C.; Fraeye, I.; Ryckebosch, E.; Muylaert, K.; Buyse, J.; Foubert, I. Impact of microalgal feed supplementation on omega-3 fatty acid enrichment of hen eggs. J. Funct. Foods 2013, 5, 897–904. [Google Scholar] [CrossRef]
- Konkol, D.; Górniak, W.; Świniarska, M.; Korczyński, M. Algae Biomass in Animal Production. In Algae Biomass: Characteristics and Applications; Springer International Publishing: Cham, Switzerland, 2018; pp. 123–130. [Google Scholar]
- Ayyildiz, M.; Erdal, G. The relationship between carbon dioxide emission and crop and livestock production indexes: A dynamic common correlated effects approach. Environ. Sci. Pollut. Res. Int. 2021, 28, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Messyasz, B. Concise review of Cladophora spp.: Macroalgae of commercial interest. J. Appl. Phycol. 2021, 33, 133–166. [Google Scholar] [CrossRef]
- Laungsuwon, R.; Chulalaksananukul, W. Chemical composition and antibacterial activity of extracts of freshwater green algae, Cladophora glomerata Kützing and Microspora floccosa (Vaucher) Thuret. J. Biosci. Biotechnol. 2014, 3, 211–218. [Google Scholar]
- Heiba, H.; Al-Easa, H.; Rizk, A. Fatty acid composition of twelve algae from the coastal zones of Qatar. Plant. Foods Hum. Nutr. 1997, 51, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Kovač, D.J.; Simeunović, J.B.; Babić, O.B.; Mišan, A.Č.; Milovanović, I.L. Algae in food and feed. Food Feed Res. 2013, 40, 21–31. [Google Scholar]
- Mihranyan, A. Cellulose from cladophorales green algae: From environmental problem to high-tech composite materials. J. Appl. Polym. Sci. 2011, 119, 2449–2460. [Google Scholar] [CrossRef]
- Zulkifly, S.B.; Graham, J.M.; Young, E.B.; Mayer, R.J.; Piotrowski, M.J.; Smith, I.; Graham, L.E. The Genus Cladophora Kützing (Ulvophyceae) as a Globally Distributed Ecological Engineer. J. Phycol. 2013, 49, 1–17. [Google Scholar] [CrossRef]
- Messyasz, B.; Leska, B.; Fabrowska, J.; Pikosz, M.; Roj, E.; Cieslak, A.; Schroeder, G. Biomass of freshwater Cladophora as a raw material for agriculture and the cosmetic industry. Open Chem. 2015, 13, 1108–1118. [Google Scholar] [CrossRef]
- Akköz, C.; Arslan, D.; Ünver, A.; Özcan, M.M.; Yilmaz, B. Chemical composition, total phenolic and mineral contents of Enteromorpha intestinalis (L.) Kütz. and Cladophora glomerata (L.) Kütz. seaweeds. J. Food Biochem. 2011, 35, 513–523. [Google Scholar] [CrossRef]
- Pikosz, M.; Messyasz, B.; Gąbka, M. Functional structure of algal mat (Cladophora glomerata) in a freshwater in western Poland. Ecol. Indic. 2017, 74, 1–9. [Google Scholar] [CrossRef]
- Kilkus, K.; Stonevičius, E. The Geography of Lithuanian Surface Waters; Vilnius University: Vilnius, Lithuania, 2012. [Google Scholar]
- Horwitz, W. Official Methods of Analysis of AOAC International, 18th ed.; Current through rev. 2, 2007 ed.; AOAC International: Gaithersburg, MD, USA; Rockville, MD, USA, 2005; pp. 24–36. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Christopherson, S.W.; Glass, R.L. Preparation of milk fat methylesters by alcoholysis in an essentially nonalcoholic solution. J. Dairy Sci. 1969, 52, 1289–1290. [Google Scholar] [CrossRef]
- Bojorge-García, M.; Carmona, J.; Beltrán, Y.; Cartajena, M. Temporal and spatial distribution of macroalgal communities of mountain streams in Valle de Bravo Basin, central Mexico. Hydrobiologia 2010, 641, 159–169. [Google Scholar] [CrossRef]
- Elenkov, I.; Stefanov, K.; Dimitrova-Konaklieva, S.; Popov, S. Effect of salinity on lipid composition of Cladophora vagabunda. Phytochemistry (Oxford) 1996, 42, 39–44. [Google Scholar] [CrossRef]
- Srimaroeng, C.; Ontawong, A.; Saowakon, N.; Vivithanaporn, P.; Pongchaidecha, A.; Amornlerdpison, D.; Soodvilai, S.; Chatsudthipong, V. Antidiabetic and Renoprotective Effects of Cladophora glomerata Kützing Extract in Experimental Type 2 Diabetic Rats: A Potential Nutraceutical Product for Diabetic Nephropathy. J. Diabetes Res. 2015, 2015, 320167. [Google Scholar] [CrossRef] [Green Version]
- Fabrowska, J.; Messyasz, B.; Szyling, J.; Walkowiak, J.; Łęska, B. Isolation of chlorophylls and carotenoids from freshwater algae using different extraction methods. Phycol. Res. 2018, 66, 52–57. [Google Scholar] [CrossRef]
- Holdt, S.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Nwosu, F.; Morris, J.; Lund, V.A.; Stewart, D.; Ross, H.A.; McDougall, G.J. Anti-proliferative and potential anti-diabetic effects of phenolic-rich extracts from edible marine algae. Food Chem. 2011, 126, 1006–1012. [Google Scholar] [CrossRef]
- Chojnacka, K. Biologically Active Compounds in Seaweed Extracts—The Prospects for the Application. Open Conf. Proc. J. 2012, 3, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Michalak, I.; Mironiuk, M.; Marycz, K. A comprehensive analysis of biosorption of metal ions by macroalgae using ICP-OES, SEM-EDX and FTIR techniques. PLoS ONE 2018, 13, e0205590. [Google Scholar] [CrossRef]
- Anh, N.; Hai, T.; Hien, T. Effects of partial replacement of fishmeal protein with green seaweed (Cladophora spp.) protein in practical diets for the black tiger shrimp (Penaeus monodon) postlarvae. J. Appl. Phycol. 2018, 30, 2649–2658. [Google Scholar] [CrossRef]
- De Blas, C.; Wiseman, J.; Carabano, R.; Abad-Guamán, R.; Allain, D.; Badiola, I.; Blas, E.; Cervera, C.; Zotte, A.D.; Carmona, J.F. Nutrition of the Rabbit, 3rd ed.; CAB International: Oxford, UK, 2020; pp. 243–254. [Google Scholar]
- Leonard, S.G.; Sweeney, T.; Pierce, K.M.; Bahar, B.; Lynch, B.P.; O’Doherty, J.V. The effects of supplementing the diet of the sow with seaweed extracts and fish oil on aspects of gastrointestinal health and performance of the weaned piglet. Livest. Sci. 2010, 134, 135–138. [Google Scholar] [CrossRef]
- Villamide, M.J.; Nicodemus, N.; Fraga, M.J.; Carabano, R. Protein digestion. In Nutrition of the Rabbit, 3rd ed.; De Blas, C., Wiseman, J., Eds.; CABI: Oxford, UK, 2020; pp. 41–58. [Google Scholar]
- Wu, G. Amino Acids: Biochemistry and Nutrition; CRC Press: Boca Raton, FL, USA, 2013; pp. 110–123. [Google Scholar]
- Sun, H.; Yang, W.R.; Yang, Z.B.; Wang, Y.; Jiang, S.Z.; Zhang, G.G. Effects of betaine supplementation to methionine deficient diet on growth performance and carcass characteris-tics of broilers. Am. J. Anim. Vet. Sci. 2008, 3, 78–84. [Google Scholar]
- Nutautaitė, M.; Alijošius, S.; Bliznikas, S.; Šašytė, V.; Vilienė, V.; Pockevičius, A.; Racevičiūtė-Stupelienė, A. Effect of betaine, a methyl group donor, on broiler chicken growth performance, breast muscle quality characteristics, oxidative status and amino acid content. Ital. J. Anim. Sci. 2020, 19, 621–629. [Google Scholar] [CrossRef]
- Alvarenga, R.R.; Rodrigues, P.B.; Cantarelli, V.d.S.; Zangeronimo, M.G.; Silva Júnior, J.W.d.; Silva, L.R.d.; Santos, L.M.d.; Pereira, L.J. Energy values and chemical composition of spirulina (Spirulina platensis) evaluated with broilers. Rev. Bras. Zootec. 2011, 40, 992–996. [Google Scholar] [CrossRef] [Green Version]
- Elango, R.; Ball, R.; Pencharz, P. Amino acid requirements in humans: With a special emphasis on the metabolic availability of amino acids. Amino Acids 2009, 37, 19–27. [Google Scholar] [CrossRef]
- Li, P.; Kim, S.W.; Li, X.; Datta, S.; Pond, W.G.; Wu, G. Dietary supplementation with cholesterol and docosahexaenoic acid affects concentrations of amino acids in tissues of young pigs. Amino Acids 2009, 37, 709–716. [Google Scholar] [CrossRef] [Green Version]
- National Research Council; Division on Earth and Life Studies; Board on Agriculture and Natural Resources; Committee on Nutrient Requirements of Swine. National Research Council Nutrient Requirements of Swine (11th Revised Edition); National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Blachier, F.; Boutry, C.; Bos, C.; Tomé, D. Metabolism and functions of L-glutamate in the epithelial cells of the small and large intestines. Am. J. Clin. Nutr. 2009, 90, 814–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabrera, R.A.; Usry, J.L.; Arrellano, C.; Nogueira, E.T.; Kutschenko, M.; Moeser, A.J.; Odle, J. Effects of creep feeding and supplemental glutamine or glutamine plus glutamate (Aminogut) on pre- and post-weaning growth performance and intestinal health of piglets. J. Anim. Sci. Biotechnol. 2013, 4, 29. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Dai, Z.; Zhang, Y.; Jia, H.; Chen, J.; Sun, S.; Wu, G.; Wu, Z. Maternal L-proline supplementation during gestation alters amino acid and polyamine metabolism in the first generation female offspring of C57BL/6J mice. Amino Acids 2019, 51, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Wu, G.; Bazer, F.; Bazer, F.; Burghardt, R.; Burghardt, R.; Johnson, G.; Johnson, G.; Kim, S.; Kim, S.; et al. Proline and hydroxyproline metabolism: Implications for animal and human nutrition. Amino Acids 2011, 40, 1053–1063. [Google Scholar] [CrossRef] [Green Version]
- Dai, Z.; Wu, G.; Zhu, W. Amino acid metabolism in intestinal bacteria: Links between gut ecology and host health. Front. Biosci. 2011, 16, 1768–1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, Z.; Li, X.; Xi, P.; Zhang, J.; Wu, G.; Zhu, W. Regulatory role for l -arginine in the utilization of amino acids by pig small-intestinal bacteria. Amino Acids 2012, 43, 233–244. [Google Scholar] [CrossRef]
- Dai, Z.; Dai, Z.; Li, X.; Li, X.; Xi, P.; Xi, P.; Zhang, J.; Zhang, J.; Wu, G.; Wu, G.; et al. L-glutamine regulates amino acid utilization by intestinal bacteria. Amino Acids 2013, 45, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Chojnacka, K. The application of macroalga Pithophora varia Wille enriched with microelements by biosorption as biological feed supplement for livestock. J. Sci. Food Agric. 2008, 88, 1178–1186. [Google Scholar] [CrossRef]
- Stabili, L.; Acquaviva, M.I.; Biandolino, F.; Cavallo, R.A.; De Pascali, S.A.; Fanizzi, F.P.; Narracci, M.; Cecere, E.; Petrocelli, A. Biotechnological potential of the seaweed Cladophora rupestris (Chlorophyta, Cladophorales) lipidic extract. New Biotechnol. 2014, 31, 436–444. [Google Scholar] [CrossRef]
- Karan, T.; Erenler, R. Fatty acid constituents and anticancer activity of Cladophora fracta (OF Müller ex Vahl) Kützing. Trop. J. Pharm. Res. 2019, 17, 1977. [Google Scholar] [CrossRef] [Green Version]
- Messyasz, B.; Michalak, I.; Łęska, B.; Schroeder, G.; Górka, B.; Korzeniowska, K.; Lipok, J.; Wieczorek, P.; Rój, E.; Wilk, R.; et al. Valuable natural products from marine and freshwater macroalgae obtained from supercritical fluid extracts. J. Appl. Phycol. 2018, 30, 591–603. [Google Scholar] [CrossRef]
- Çetingül, İ.; Yardımcı, M. The Importance of Fats in Farm Animal Nutrition. Kocatepe Vet. J. 2008, 1, 77–81. [Google Scholar]
- Huang, C.B.; Ebersole, J.L. A novel bioactivity of omega-3 polyunsaturated fatty acids and their ester derivatives. Mol. Oral Microbiol. 2010, 25, 75–80. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as sources of high added-value compounds—A brief review of recent work. Biotechnol. Prog. 2011, 27, 597–613. [Google Scholar] [CrossRef]
- Schmitz, G.; Ecker, J. The opposing effects of n−3 and n−6 fatty acids. Prog. Lipid Res. 2008, 47, 147–155. [Google Scholar] [CrossRef]
- Plaza, M.; Herrero, M.; Cifuentes, A.; Ibáñez, E. Innovative Natural Functional Ingredients from Microalgae. J. Agric. Food Chem. 2009, 57, 7159–7170. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; WU, J.H.Y. Omega-3 Fatty Acids and Cardiovascular Disease: Effects on Risk Factors, Molecular Pathways, and Clinical Events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, M.; Zuniga, E.; Ojima, I. Novel Taxoid-Based Tumor-Targeting Drug Conjugates. Chim. Oggi 2009, 27, 54–56. [Google Scholar] [PubMed]
- Pereira, H.; Barreira, L.; Figueiredo, F.; Custódio, L.; Vizetto-Duarte, C.; Polo, C.; Rešek, E.; Engelen, A.; Varela, J. Polyunsaturated Fatty Acids of Marine Macroalgae: Potential for Nutritional and Pharmaceutical Applications. Mar. Drugs 2012, 10, 1920–1935. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef]
- Farajzadeh Alan, D.; Naeli, M.H.; Naderi, M.; Jafari, S.M.; Tavakoli, H.R. Production of Trans-free fats by chemical interesterified blends of palm stearin and sunflower oil. Food Sci. Nutr. 2019, 7, 3722–3730. [Google Scholar] [CrossRef] [Green Version]
- Yates, C.M.; Calder, P.C.; Rainger, E.G. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol. Ther. 2014, 141, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, S.; Elwinger, K.; Pickova, J. Fatty acid and carotenoid composition of egg yolk as an effect of microalgae addition to feed formula for laying hens. Food Chem. 2006, 99, 530–537. [Google Scholar] [CrossRef]
- Marszalek, J.R.; Lodish, H.F. Docosahexaenoic acid, fatty acid-interacting proteins, and neuronal function: Breastmilk and fish are good for you. Annu. Rev. Cell Dev. Biol. 2005, 21, 633–657. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Machado, D.I.; López-Cervantes, J.; López-Hernández, J.; Paseiro-Losada, P. Fatty acids, total lipid, protein and ash contents of processed edible seaweeds. Food Chem. 2004, 85, 439–444. [Google Scholar] [CrossRef]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; Abd El-Hack, M.E.; Khafaga, A.F.; Taha, A.E.; Tiwari, R.; Yatoo, M.I.; Bhatt, P.; Khurana, S.K.; et al. Omega-3 and Omega-6 fatty acids in poultry nutrition: Effect on production performance and health. Animals 2019, 9, 573. [Google Scholar] [CrossRef] [Green Version]
- Al-Zuhairy, M.A. Effect of ND Vaccine, Multivitamins AD3E, and Omega-3 on Performance and Immune Response of Broilers. Mirror Res. Vet. Sci. Anim. 2014, 3, 43–52. [Google Scholar]
- Ibrahim, D.; El-Sayed, R.; Khater, S.I.; Said, E.N.; El-Mandrawy, S.A.M. Changing dietary n-6: N-3 ratio using different oil sources affects performance, behavior, cytokines mRNA expression and meat fatty acid profile of broiler chickens. Anim. Nutr. 2018, 4, 44–51. [Google Scholar] [CrossRef] [PubMed]
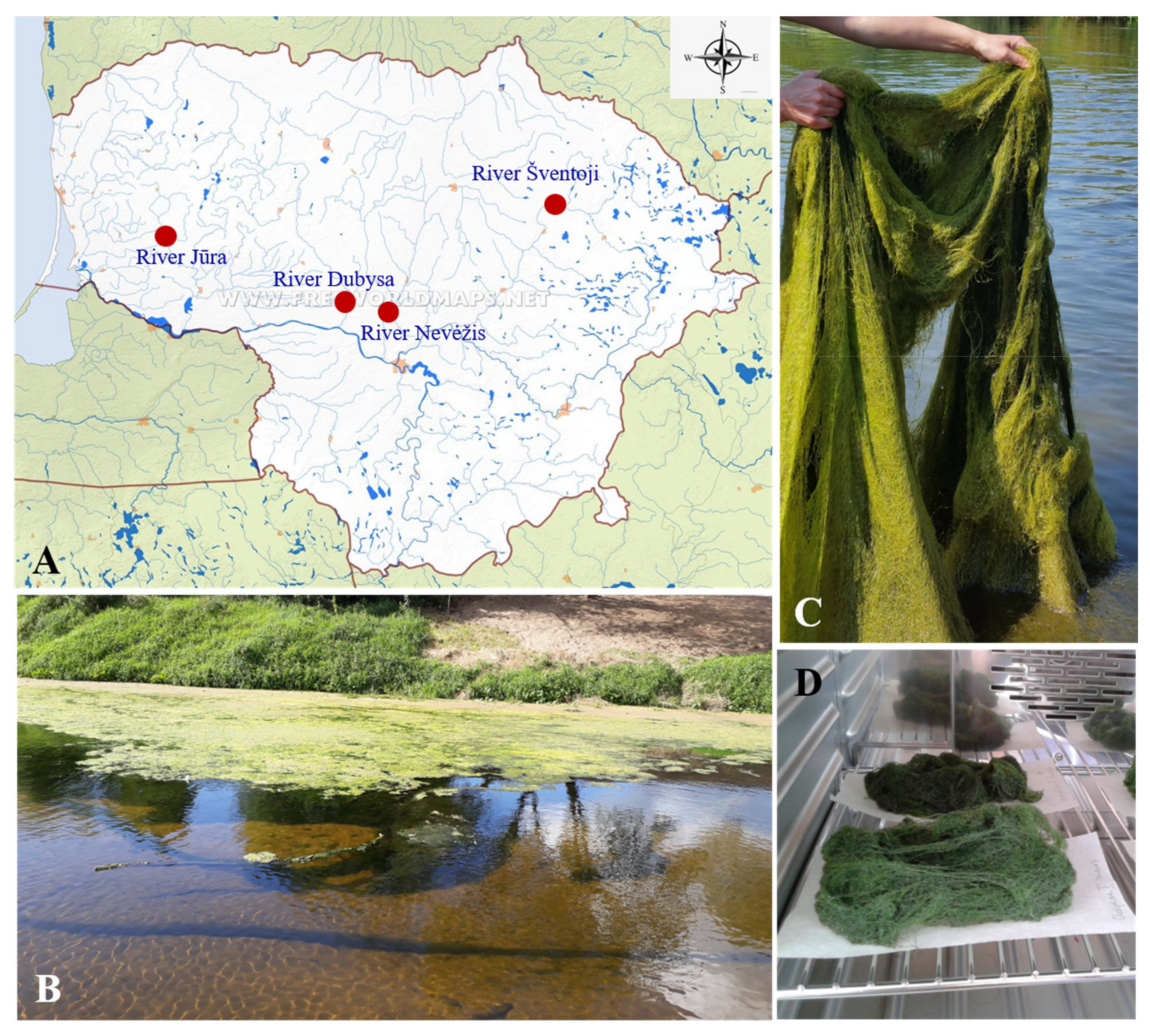

| River | * River length, km | * Catchment Area, km2 | * Average Annual Discharge at the Mouth, m3/s | Current Velocity, m/s | TP, mg/L | TN, mg/L | Water Temperature, °C | pH | Conductivity, µS/cm | Bottom Coverage by C. glomerata, % |
|---|---|---|---|---|---|---|---|---|---|---|
| Dubysa (B1) | 131 | 1972 | 14.2 | 0.11–0.57 | 0.012–0.035 | 0.18–0.40 | 16.4–20.1 | 8.41–8.80 | 535–581 | 40–90 |
| Šventoji (B2) | 246 | 6889 | 56.5 | 0.26–0.32 | 0.037–0.070 | 0.53–0.99 | 17.6–18.9 | 8.40–9.19 | 479–487 | 45–95 |
| Jūra (B3) | 172 | 3994 | 38 | 0.11–0.53 | 0.031–0.073 | 0.28–0.64 | 14.9–18.0 | 8.56–9.21 | 420–483 | 50–95 |
| Nevėžis (B4) | 209 | 6146 | 32.7 | 0.10–0.13 | 0.500–0.520 | 0.84–1.12 | 18.2–19.2 | 8.54–8.62 | 948–977 | 50–100 |
| Element | Method | Element | Method |
|---|---|---|---|
| N | The Commission Directive 72/199/EEC | Zn | LST EN ISO 15510:2017 |
| Cu | |||
| P | Directive 71/393/EEC | Cr | |
| K | Directive 71/250/EEC | Ni | LST EN ISO 15550:2017 |
| Ca | Cd | ||
| Mg | Directive 73/46/EEC | Pb |
| C. glomerata Biomass 2,3,4 | ||||||
|---|---|---|---|---|---|---|
| Item 1 | B1 | B2 | B3 | B4 | SEM 5 | p Value |
| DM (% of dried samples) | 94.95 a | 92.63 a | 95.19 a | 91.12 b | 0.35 | 0.000 |
| % DM | ||||||
| CP | 18.32 a | 15.98 a | 18.18 a | 21.52 b | 0.34 | 0.000 |
| CF | 0.35 a | 0.18 b | 0.19 b | 0.31 ab | 0.05 | 0.026 |
| CA | 48.45 a | 39.05 b | 49.83 c | 36.96 d | 0.23 | 0.000 |
| CFB | 13.97 a | 15.83 b | 10.88 c | 13.14 d | 0.19 | 0.000 |
| C. glomerata Biomass 1,2,3 | ||||||
|---|---|---|---|---|---|---|
| Item (g/kg DM) | B1 | B2 | B3 | B4 | SEM 4 | p Value |
| Aspartic acid | 10.90 a | 13.63 b | 14.18 c | 15.97 c | 0.63 | 0.001 |
| Threonine | 3.49 a | 6.52 b | 6.28 b | 6.77 b | 0.42 | 0.001 |
| Serine | 4.14 a | 6.21 b | 6.29 c | 7.25 c | 0.34 | 0.001 |
| Glutamic acid | 14.16 a | 16.90 b | 17.53 c | 19.50 c | 0.48 | 0.000 |
| Proline | 5.98 a | 5.92 a | 6.40 a | 7.22 b | 0.25 | 0.006 |
| Glycine | 8.06 a | 9.23 b | 9.54 c | 11.46 c | 0.32 | 0.000 |
| Alanine | 8.49 a | 8.12 a | 10.09 b | 10.73 b | 0.24 | 0.000 |
| Valine | 8.39 a | 8.48 a | 8.90 a | 10.42 b | 0.39 | 0.006 |
| Methionine | 1.94 b | 4.14 a | 2.24 b | 2.08 b | 0.21 | 0.000 |
| Isoleucine | 5.94 a | 6.17 a | 6.80 a | 7.69 b | 0.30 | 0.004 |
| Leucine | 9.71 a | 9.64 b | 10.81 c | 12.01 d | 0.31 | 0.002 |
| Tyrosine | 1.73 a | 1.71 a | 2.09 b | 2.35 b | 0.14 | 0.009 |
| Phenylalanine | 6.37 a | 6.93 a | 7.39 a | 8.50 b | 0.35 | 0.004 |
| Histidine | 3.04 | 3.01 | 3.33 | 3.68 | 0.28 | 0.077 |
| Lysine | 5.76 a | 5.85 a | 6.46 a | 7.88 b | 0.26 | 0.001 |
| Arginine | 5.25 a | 5.79 a | 6.31 a | 7.47 b | 0.20 | 0.000 |
| Total | 103.36 a | 118.25 b | 124.64 c | 140.99 d | 3.88 | 0.001 |
| C. glomerata Biomass 2,3,4,5 | ||||||
|---|---|---|---|---|---|---|
| Item 1 (% of the Total Fatty Acids Content) | B1 | B2 | B3 | B4 | SEM 6 | p Value |
| C10:0 | n/d | 0.37 a | n/d | 0.24 b | 0.02 | 0.000 |
| X | 0.75 a | 0.56 b | 0.88 c | 0.69 a | 0.02 | 0.000 |
| C12:0 | 0.25 a | 0.40 b | 0.80 c | 0.30 d | 0.01 | 0.000 |
| X | 0.64 ab | 0.50 a | n/d | 0.61 b | 0.03 | 0.000 |
| X | 5.03 a | 3.59 b | 5.58 a | 5.03 c | 0.04 | 0.000 |
| X | 0.19 b | 0.35 a | 0.22 b | n/d | 0.02 | 0.002 |
| C14:0 | 8.86 a | 12.48 b | 9.70 c | 11.21 d | 0.06 | 0.000 |
| X | 0.80 a | 0.76 a | 0.84 a | 0.62 b | 0.02 | 0.001 |
| C15:0 | 0.99 a | 0.18 b | 0.30 c | 0.82 d | 0.04 | 0.000 |
| iC15:0 | 5.95 a | 4.83 b | 7.65 c | 5.60 d | 0.07 | 0.000 |
| C16:0 | 33.54 a | 37.11 b | 32.65 c | 32.89 d | 0.08 | 0.000 |
| iC16:0 | 0.80 b | 0.50 a | 0.76 b | 0.40 c | 0.02 | 0.000 |
| trans-C16:1 n7 | 1.13 b | 0.81 a | 1.18 b | 1.03 b | 0.05 | 0.002 |
| C16:1 n9 | 1.71 a | 1.00 b | 1.88 c | 1.17 d | 0.06 | 0.000 |
| C16:1 n7 | 5.31 a | 6.89 b | 6.63 c | 7.88 d | 0.03 | 0.000 |
| C17:0 | n/d | 0.49 a | 0.50 a | 0.29 b | 0.03 | 0.000 |
| iC17:0 | 0.49 a | 0.18 b | 0.58 a | 0.23 c | 0.03 | 0.000 |
| C18:0 | 2.07 a | 2.52 b | 2.39 c | 3.25 d | 0.04 | 0.000 |
| C18:1 n9 | 12.72 a | 14.53 b | 11.16 c | 12.33 d | 0.05 | 0.000 |
| C18:1 n7 | 6.06 a | 5.16 b | 6.12 a | 5.16 c | 0.05 | 0.000 |
| trans-C18:2 n6 | 1.43 a | n/d | 1.11 b | n/d | 0.03 | 0.000 |
| cis-trans-C18:2 n6 | n/d | 0.74 a | n/d | 1.35 b | 0.02 | 0.000 |
| trans-cis-C18:2 n6 | 0.57 b | 0.31 a | 0.56 b | 0.45 c | 0.02 | 0.000 |
| C18:2 n6 | 4.15 a | 2.75 b | 3.83 c | 3.33 d | 0.04 | 0.000 |
| C18:3 n3 | 4.29 a | 1.91 b | 2.90 c | 2.96 c | 0.05 | 0.000 |
| C20:1 n9 | 0.59 a | n/d | 0.37 b | n/d | 0.01 | 0.000 |
| C20:4 n6 | 0.27 a | n/d | 0.30 a | 0.38 b | 0.01 | 0.000 |
| C20:5 n3 | 0.58 a | 0.77 a | 0.68 a | 1.13 b | 0.09 | 0.003 |
| C22:1 n9 | 0.22 | n/d | n/d | 0.24 | 0.02 | 0.264 |
| C22:2 n6 | 0.20 | n/d | 0.20 | n/d | 0.01 | 0.932 |
| C22:5 n3 | 0.22 | n/d | n/d | n/d | n/d | n/d |
| C24:0 | n/d | 0.30 | 0.24 | 0.21 | 0.04 | 0.067 |
| C24:1 | 0.20 | n/d | n/d | 0.21 | 0.01 | 0.393 |
| SFA | 52.94 a | 59.37 b | 55.57 c | 55.43 c | 0.11 | 0.000 |
| MUFA | 27.93 a | 28.39 b | 27.34 c | 28.03 a | 0.10 | 0.000 |
| PUFA | 11.71 a | 6.48 b | 9.58 c | 9.59 c | 0.11 | 0.000 |
| PUFA/SFA | 0.22 a | 0.11 b | 0.17 c | 0.17 c | 0.00 | 0.000 |
| Total n3 | 5.08 a | 2.68 b | 3.58 c | 4.09 d | 0.07 | 0.000 |
| Total n6 | 6.62 a | 3.80 b | 6.00 c | 5.51 d | 0.04 | 0.000 |
| n6/n3 | 1.30 a | 1.42 b | 1.68 c | 1.35 a | 0.02 | 0.000 |
| Total X | 7.42 a | 5.76 b | 7.51 c | 6.95 d | 0.02 | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nutautaitė, M.; Vilienė, V.; Racevičiūtė-Stupelienė, A.; Bliznikas, S.; Karosienė, J.; Koreivienė, J. Freshwater Cladophora glomerata Biomass as Promising Protein and Other Essential Nutrients Source for High Quality and More Sustainable Feed Production. Agriculture 2021, 11, 582. https://doi.org/10.3390/agriculture11070582
Nutautaitė M, Vilienė V, Racevičiūtė-Stupelienė A, Bliznikas S, Karosienė J, Koreivienė J. Freshwater Cladophora glomerata Biomass as Promising Protein and Other Essential Nutrients Source for High Quality and More Sustainable Feed Production. Agriculture. 2021; 11(7):582. https://doi.org/10.3390/agriculture11070582
Chicago/Turabian StyleNutautaitė, Monika, Vilma Vilienė, Asta Racevičiūtė-Stupelienė, Saulius Bliznikas, Jūratė Karosienė, and Judita Koreivienė. 2021. "Freshwater Cladophora glomerata Biomass as Promising Protein and Other Essential Nutrients Source for High Quality and More Sustainable Feed Production" Agriculture 11, no. 7: 582. https://doi.org/10.3390/agriculture11070582
APA StyleNutautaitė, M., Vilienė, V., Racevičiūtė-Stupelienė, A., Bliznikas, S., Karosienė, J., & Koreivienė, J. (2021). Freshwater Cladophora glomerata Biomass as Promising Protein and Other Essential Nutrients Source for High Quality and More Sustainable Feed Production. Agriculture, 11(7), 582. https://doi.org/10.3390/agriculture11070582









