Effect of Vegetable Oils Feed Additives on Endoparasites Associated with Dewormed Racing Horses
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Samples Collection
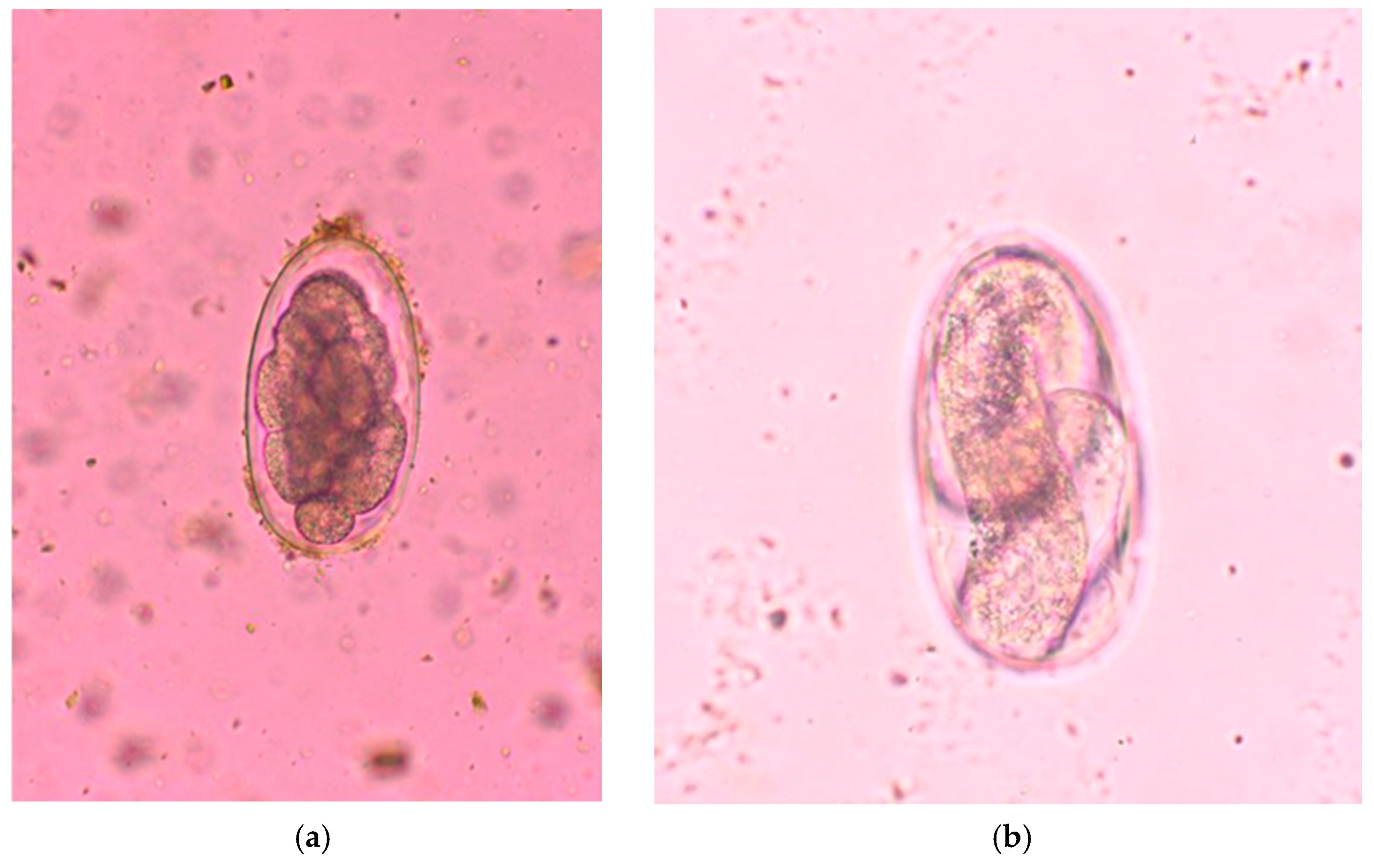
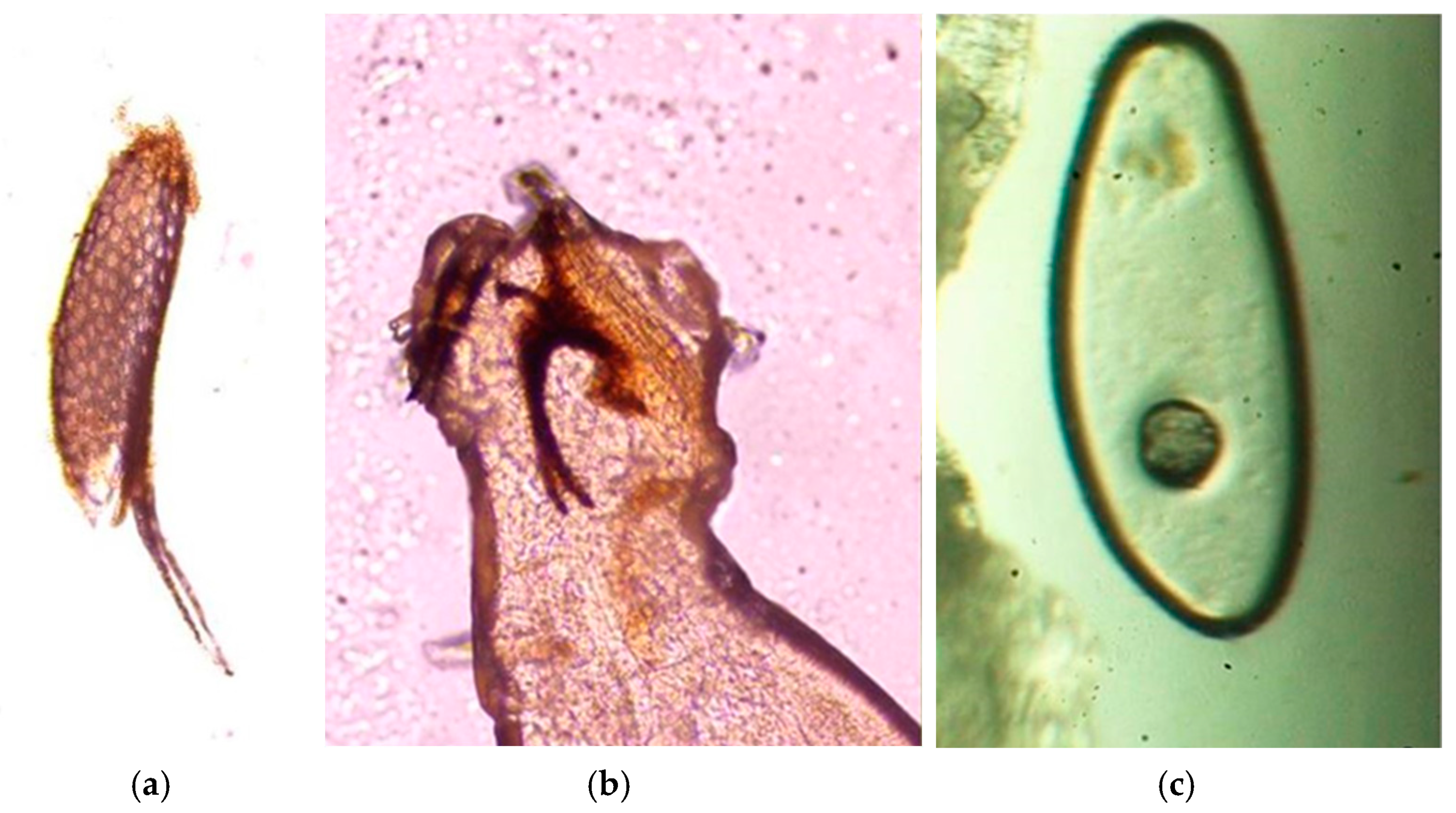
2.3. Parasitological Analysis
2.4. Data Analysis
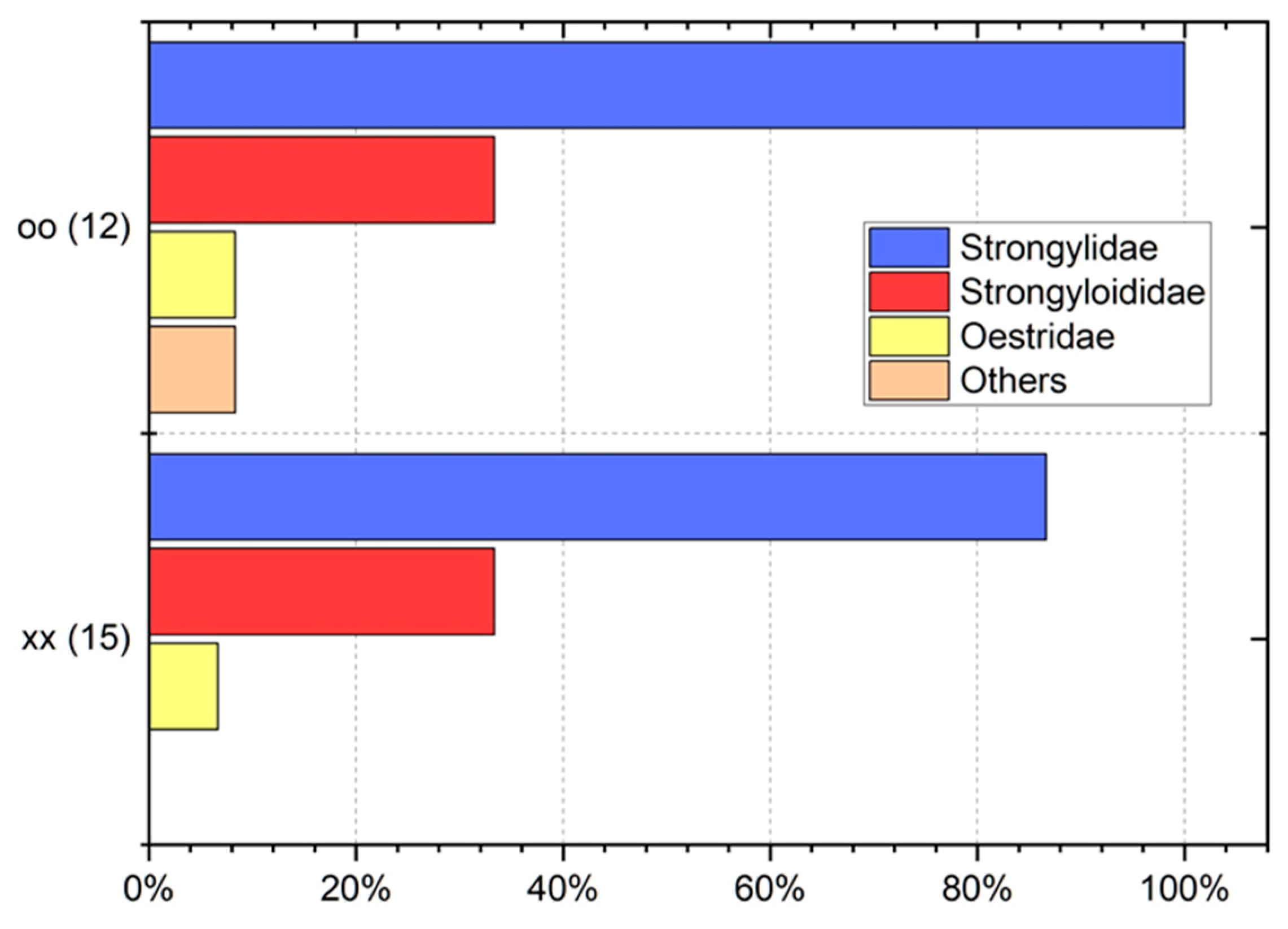
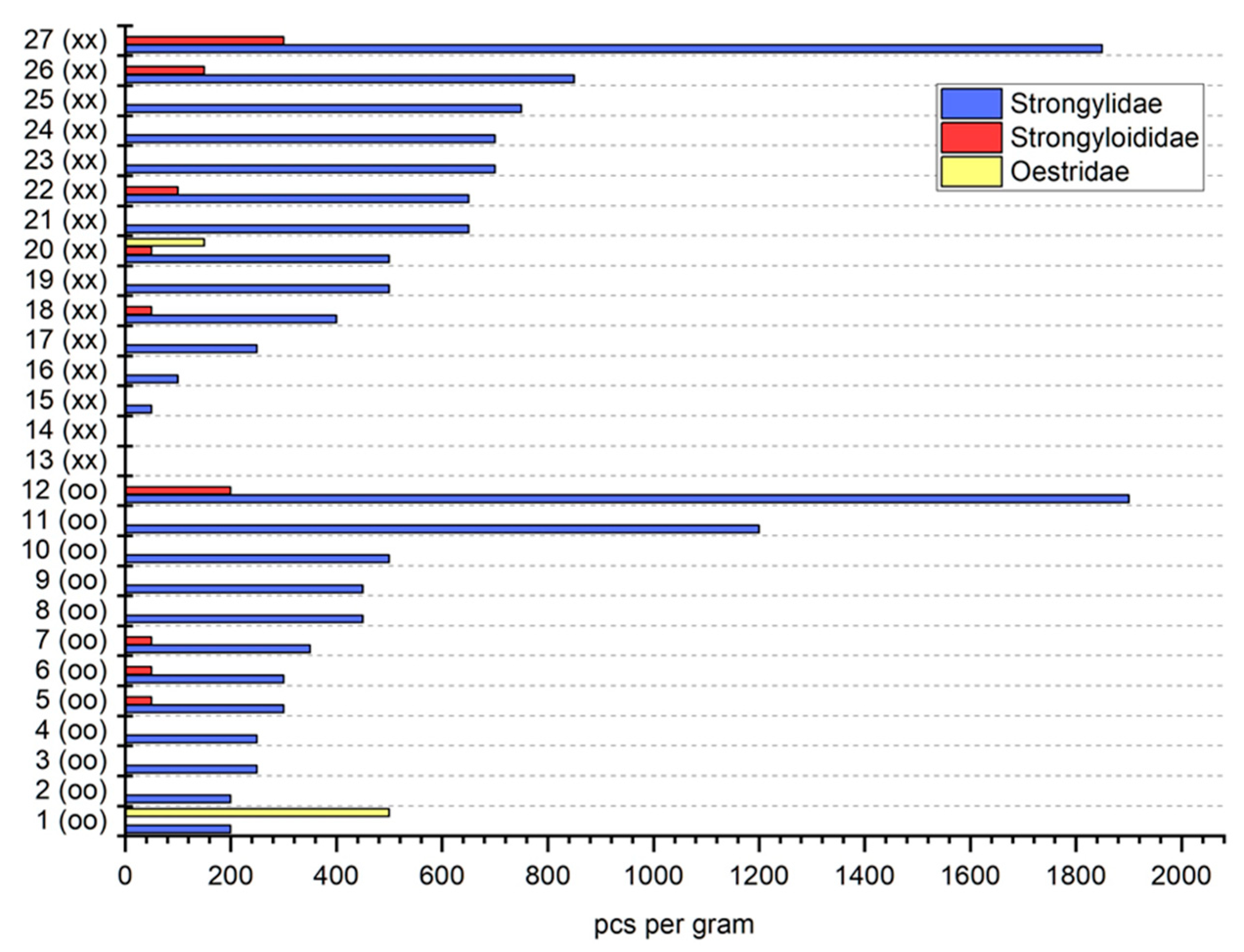
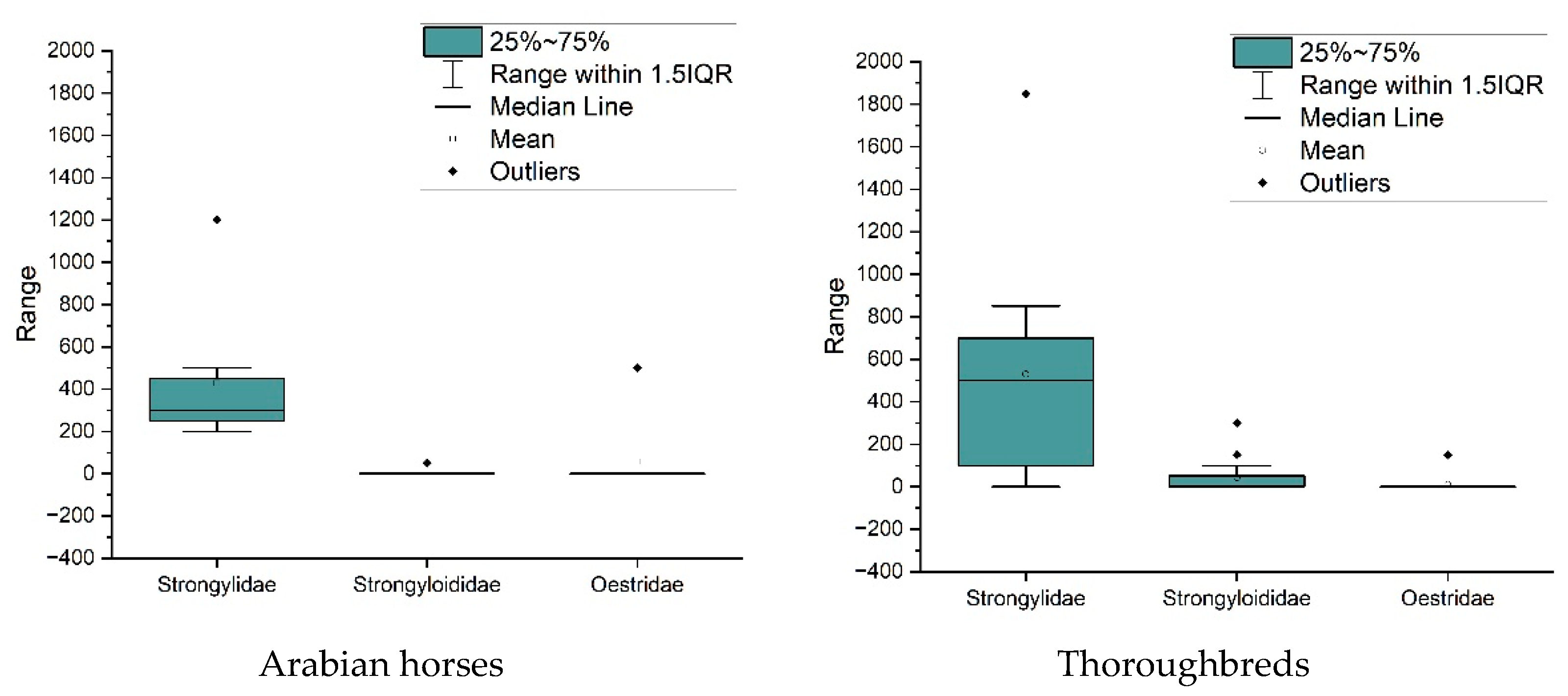
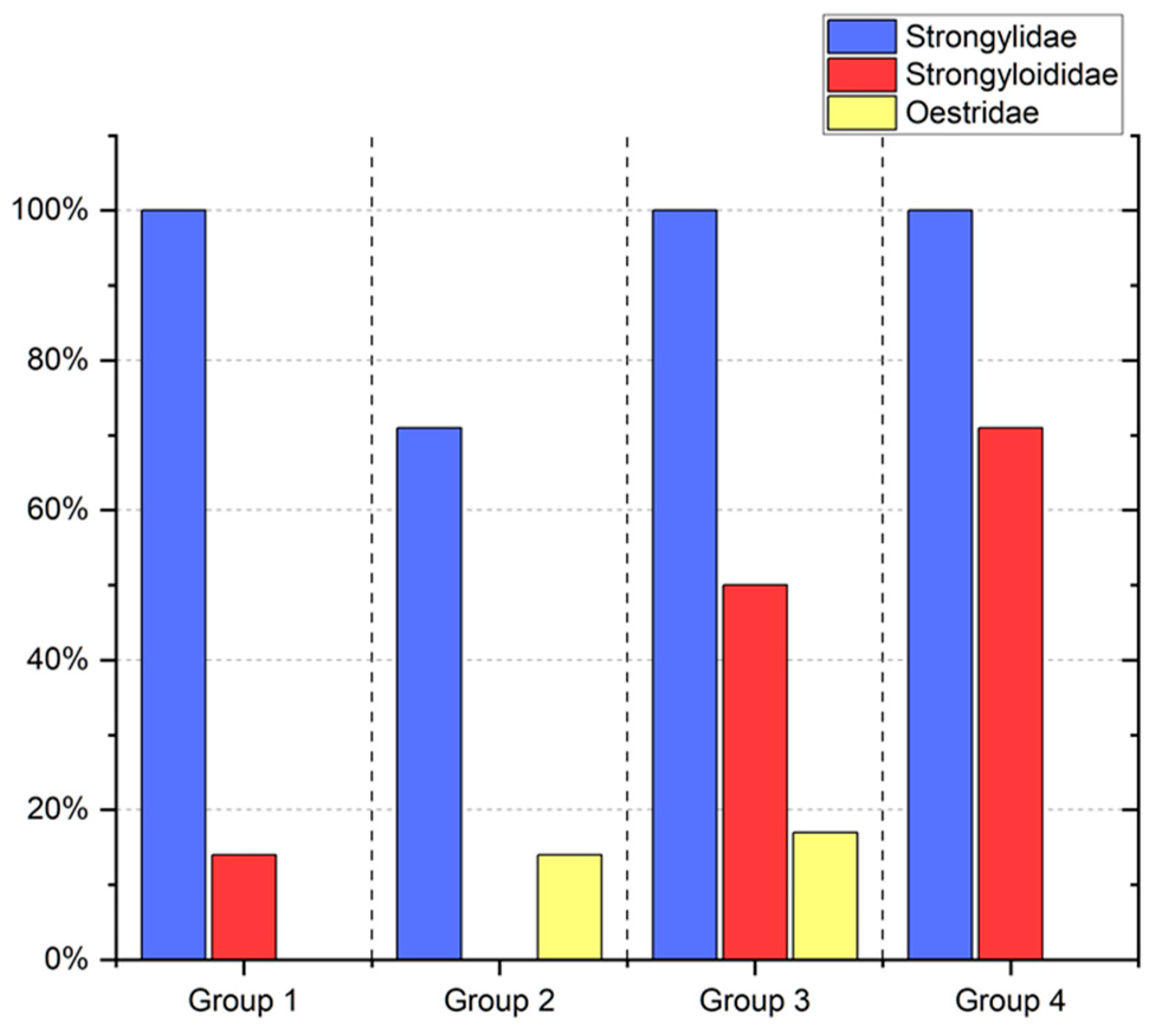
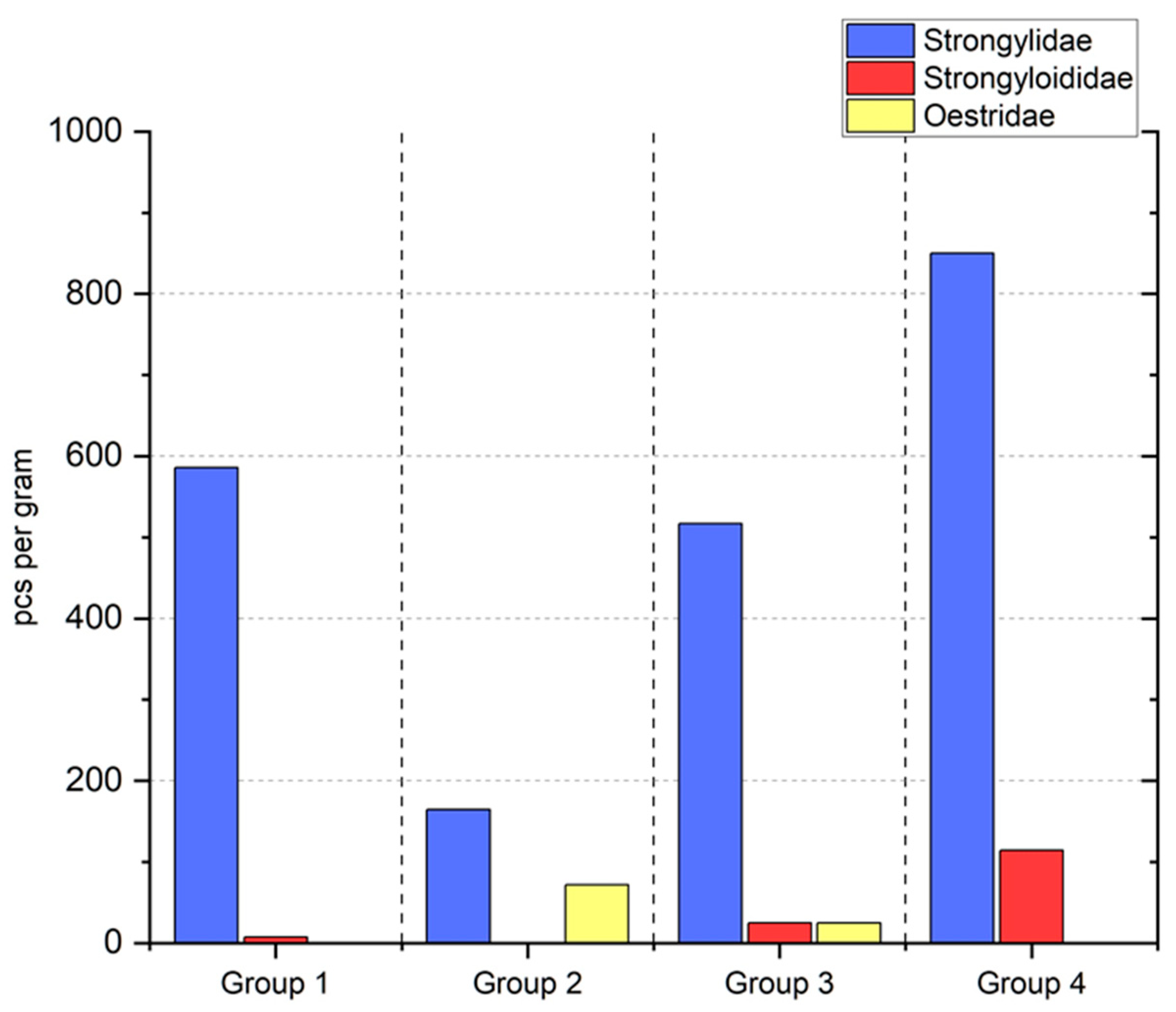
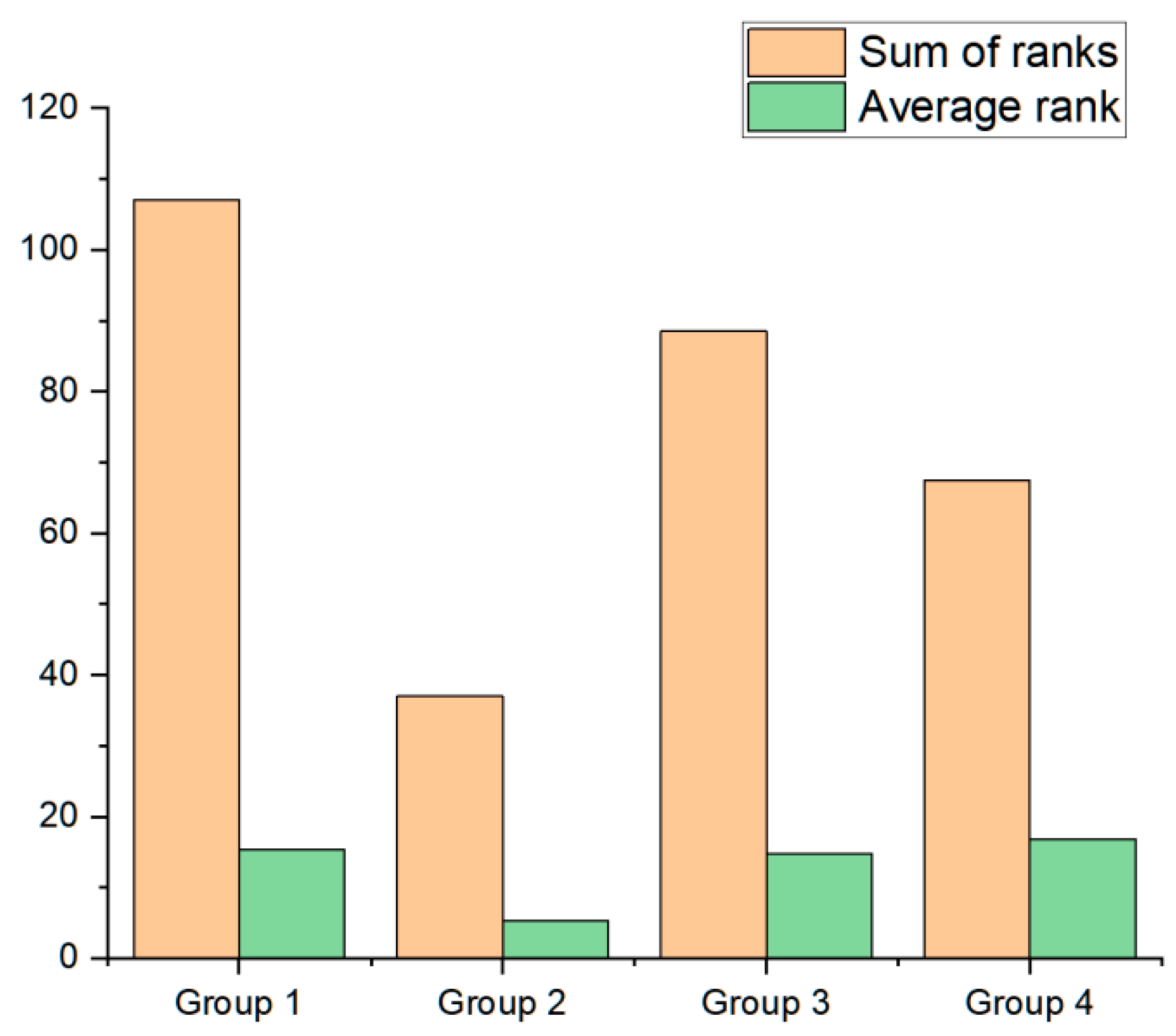
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Czapla, D.; Seremak, B.; Kruzhel, B.; Vovk, S. Parasitic fauna of gastrointestinal tract of horses and evaluation of deworming effectiveness. Acta Sci. Pol. Zootech. 2015, 14, 61–68. [Google Scholar]
- Traversa, D.; Castagna, G.; von Samson-Himmelstjerna, G.; Meloni, S.; Bartolini, R.; Geurden, T.; Pearce, M.C.; Woringer, E.; Besognet, B.; Milillo, P.; et al. Efficacy of major anthelmintics against horse cyathostomins in France. Vet. Parasitol. 2012, 188, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Raś-Noryńska, M.; Sokół, R. Controlling parasitic invasion in horses. Życie Wet. 2011, 86, 299–301. [Google Scholar]
- Comer, K.; Coles, G.C.; Hillyer, M.H.M. A national survey for anthelmintic resistant nematodes in thoroughbreds. In Proceedings of the Eighteenth International Conference of the World Association for the Advancement of Veterinary Parasitology, Stresa, Italy, 26–30 August 2001; Benedettina: Parma, Italy, 2001; p. 166. [Google Scholar]
- Lind, E.O.; Rautalinko, E.; Uggla, A.; Waller, P.J.; Morrison, D.A.; Höglund, J. Parasite control practices on Swedish horse farms. Acta Vet. Scand. 2007, 49, 25. [Google Scholar] [CrossRef] [PubMed]
- Jagła, E.; Śpiewak, J.; Zaleśny, G.; Popiołek, M. Effect of storage and preservation of horse faecal samples on the detectability and viability of strongylid nematode eggs and larvae. Bull. Vet. Inst. Pulawy 2013, 57, 161–165. [Google Scholar] [CrossRef]
- Lloyd, S.; Smith, J.; Connan, R.M.; Hatcher, M.A.; Hedges, T.R.; Humphrey, D.J.; Jones, A.C. Parasite control methods used by horse owners: Factors predisposing to the development of anthelmintic resistance in nematodes. Vet. Rec. 2000, 146, 487–492. [Google Scholar] [CrossRef]
- Kaplan, R.M. Drug resistance in nematodes of veterinary importance: A status report. Trends Parasitol. 2004, 20, 477–481. [Google Scholar] [CrossRef]
- Brady, H.A.; Nichols, W.T. Drug resistance in equine parasites: An emerging global problem. J. Equine Vet. Sci. 2009, 29, 285–295. [Google Scholar] [CrossRef]
- Daniels, S. Friend or foe? Intestinal parasites of horses and sustainable worm control mechanisms. Vet. Nurs. J. 2019, 34, 72–77. [Google Scholar] [CrossRef]
- Raza, A.; Qamar, A.G.; Hayat, K.; Ashraf, S.; Williams, A.R. Anthelmintic resistance and novel control options in equine gastrointestinal nematodes. Parasitology 2019, 146, 425–437. [Google Scholar] [CrossRef]
- Tydén, E.; Jansson, A.; Ringmark, S. Parasites in horses kept in a 2.5 year-round grazing system in nordic conditions without supplementary feeding. Animals 2019, 9, 1156. [Google Scholar] [CrossRef]
- Kuzmina, T.A.; Kharchenko, V.O. Anthelmintic resistance in cyathostomins of brood horses in Ukraine and influence of anthelmintic treatments on strongylid community structure. Vet. Parasitol. 2008, 154, 277–288. [Google Scholar] [CrossRef]
- Relf, V.E.; Lester, H.E.; Morgan, E.R.; Hodgkinson, J.E.; Matthews, J.B. Anthelmintic efficacy on UK Thoroughbred stud farms. Int. J. Parasitol. 2014, 44, 507–514. [Google Scholar] [CrossRef]
- Cogley, T.P.; Cogley, M.C. Inter-relationship between Gasterophilus larvae and the horse’s gastric and duodenal wall with special reference to penetration. Vet. Parasitol. 1999, 86, 127–142. [Google Scholar] [CrossRef]
- Lapointe, J.M.; Celeste, C.; Villeneuve, A. Septic peritonitis due to colonic perforation associated with aberrant migration of a Gasterophilus intestinalis larva in a horse. Vet. Pathol. 2003, 40, 338–339. [Google Scholar] [CrossRef]
- Morcos, Z. Prevalent diseases of race horses in Egypt; conclusion of 1927–1947 observations. Vet. Med. 1947, 42, 94–97. [Google Scholar]
- Lyons, E.T.; Tolliver, S.C.; Drudge, J.H. Historical perspective of cyathostomes: Prevalence, treatment and control programs. Vet. Parasitol. 1999, 85, 97–112. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathog. 2018, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Athanasiadou, S.; Githiori, J.; Kyriazakis, I. Medicinal plants for helminth parasite control: Facts and fiction. Animals 2007, 1, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Liu, X.; Zhao, G.; Hu, T.; Wang, Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim. Nutr. 2018, 4, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.P.; Fyfe, L.; Smith, H. Plant active components–a resource for antiparasitic agents? Trends Parasitol. 2005, 21, 462–468. [Google Scholar] [CrossRef]
- Williams, C.A.; Lamprecht, E.D. Some commonly fed herbs and other functional foods in equine nutrition: A review. Vet. J. 2008, 178, 21–31. [Google Scholar] [CrossRef]
- Waller, P.J.; Bernes, G.; Thamsborg, S.; Sukura, A.; Richter, S.H.; Ingerbrigsten, K.; Höglund, J. Plants as de-worming agents of livestock in the Nordic countries: Historical perspective, popular beliefs and prospects for the future. Acta Vet. Scand. 2001, 42, 31–44. [Google Scholar] [CrossRef] [PubMed]
- El Shenawy, N.; Soliman, M.F.M.; Reyad, S.I. The effect of antioxidant properties of aqueous garlic extract and Nigella sativa as anti-schistosomiasis agents in mice. Rev. Inst. Med. Trop. Sao Paulo 2008, 50, 29–36. [Google Scholar] [CrossRef]
- Szewc, M.; De Waal, T.; Zintl, A. Biological methods for control of gastrointestinal nematodes. Vet. J. 2021, 268, 105602. [Google Scholar] [CrossRef] [PubMed]
- Kronfeld, D.S.; Holland, J.L.; Rich, G.A.; Meacham, T.N.; Fontenot, J.P.; Sklan, D.J.; Harris, P.A. Fat digestibility in Equus caballus follows increasing first-order kinetics. J. Anim. Sci. 2004, 82, 1773–1780. [Google Scholar] [CrossRef]
- O’Neil, W.; McKee, S.; Clarke, A.F. Flaxseed (Linum usitatissimum) supplementation associated with reduced skin test lesional area in horses with Culicides hypersensitivity. Can. J. Vet. Res. 2002, 66, 272–277. [Google Scholar]
- de Almeida, F.Q.; de Godoi, F.N. Soybean oil in horses’ diets. In Soybean and Nutrition; El-Shemy, H., Ed.; IntechOpen: London, UK, 2011; pp. 251–268. [Google Scholar] [CrossRef][Green Version]
- Okai, K.; Taharaguchi, S.; Orita, Y.; Yokota, H.; Taniyama, H. Comparative endoscopic evaluation of normal and ulcerated gastric mucosae in Thoroughbred foals. J. Vet. Med. Sci. 2015, 77, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Elghandour, M.M.M.Y.; Reddy, P.R.K.; Salem, A.Z.M.; Reddy, P.P.R.; Hyder, I.; Barbabosa-Pliego, A.; Yasaswini, D. Plant bioactives and extracts as feed additives in horse nutrition. J. Equine Vet. Sci. 2018, 69, 66–77. [Google Scholar] [CrossRef]
- Saastamoinen, M.; Särkijärvi, S. Effect of linseed (Linum usitatissimum) groats-based mixed feed supplements on diet nutrient digestibility and blood parameters of horses. Animals 2020, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (NRC). Nutrient Requirements of Horses, 6th ed.; National Academy Press: Washington, DC, USA, 2007. [Google Scholar]
- Pereckiene, A.; Kaziūnaitė, V.; Vyšniauskas, A.; Petkevičius, S.; Malakauskas, A.; Šarkūnas, M.; Taylor, M.A. A comparison of modifications of the McMaster method for the enumeration of Ascaris suum eggs in pig faecal samples. Vet. Parasitol. 2007, 149, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Draber-Mońko, A. Klucze do Oznaczania Owadów Polski. Cz. XXVIII Muchówki—Diptera, zesz. 75. Gziki—Gastherophilidae; PWN: Warszawa, Poland, 1969. [Google Scholar]
- Li, X.Y.; Pape, T.; Zhang, D. Taxonomic review of Gasterophilus (Oestridae, Gasterophilinae) of the world, with updated nomenclature, keys, biological notes, and distributions. ZooKeys 2019, 891, 119–156. [Google Scholar] [CrossRef] [PubMed]
- Kyvsgaard, N.C.; Lindbom, J.; Andreasen, L.L.; Luna-Olivares, L.A.; Nielsen, M.K.; Monrad, J. Prevalence of strongyles and efficacy of fenbendazole and ivermectin in working horses in El Sauce, Nicaragua. Vet. Parasitol. 2011, 181, 248–254. [Google Scholar] [CrossRef]
- Kuzmina, T.A.; Dzeverin, I.; Kharchenko, V.A. Strongylids in domestic horses: Influence of horse age, breed and deworming programs on the strongyle parasite community. Vet. Parasitol. 2016, 227, 56–63. [Google Scholar] [CrossRef]
- Forteau, L.; Dumont, B.; Sallé, G.; Bigot, G.; Fleurance, G. Horses grazing with cattle have reduced strongyle egg count due to the dilution effect and increased reliance on macrocyclic lactones in mixed farms. Animals 2020, 14, 1076–1082. [Google Scholar] [CrossRef]
- Jenkins, E.; Backwell, A.L.; Bellaw, J.; Colpitts, J.; Liboiron, A.; McRuer, D.; Medill, S.; Parker, S.; Shury, T.; Smith, M.; et al. Not playing by the rules: Unusual patterns in the epidemiology of parasites in a natural population of feral horses (Equus caballus) on Sable Island, Canada. Int. J. Parasitol. Parasites Wildl. 2020, 11, 183–190. [Google Scholar] [CrossRef]
- Hamed, M.I.; El-Allawy, T.A.A.; Hassnein, E.A. Prevalence and anthelmintic resistance of strongyle infection of donkeys in El-Wadi El-Gadid, Egypt. J. Adv. Vet. Res. 2019, 9, 144–150. [Google Scholar]
- Kaur, S.; Singh, E.; Singla, L.D. Strongylosis in equine: An overview. J. Entomol. Zool. Stud. 2019, 7, 43–46. [Google Scholar]
- Ramey, D.W.; Nielsen, M.K. Limited strongyle parasite occurrence in horses kept in an arid environment. Equine Vet. Educ. 2020, 32, 37–40. [Google Scholar] [CrossRef]
- Eslami, A.; Bokai, S.; Tabatabai, V. Equine parasites in Iran. J. Equine Vet. Sci. 2005, 25, 143–144. [Google Scholar] [CrossRef]
- Bulgaru, A.; Tudor, P. The prevalence of helminth parasites in horses raised in modern conditions. Sci. Works Ser. C Vet. Med. 2016, 61, 271–274. [Google Scholar]
- Ola-Fadunsin, S.D.; Daodu, O.B.; Hussain, K.; Ganiyu, I.A.; Rabiu, M.; Sanda, I.M.; Adah, A.S.; Adah, A.D.; Aiyedun, J.O. Gastrointestinal parasites of horses (Equus caballus Linnaeus, 1758) and risk factors associated with equine coccidiosis in Kwara and Niger States, Nigeria. Sokoto J. Vet. Sci. 2019, 17, 35–43. [Google Scholar] [CrossRef][Green Version]
- Wannas, H.Y.; Dawood, K.A.; Gassem, G.A. Prevalence of gastro-intestinal parasites in horses and donkeys in Al Diwaniyah Governorate. Al-Qadisiyah J. Vet. Med. Sci. 2012, 11, 148–155. [Google Scholar] [CrossRef]
- Sultan, A.; Ayele, G.; Tadesse, B.; Ahmed, A. Prevalence of gastrointestinal parasites of horses and donkeys in Kurfa Chale District, East Hararghe, Ethiopia. Livest. Res. Rural Dev. 2014, 26, 119. [Google Scholar]
- Liu, S.H.; Hu, D.F.; Li, K. Oviposition site selection by Gasterophilus pecorum (Diptera: Gasterophilidae) in its habitat in Kalamaili Nature Reserve, Xinjiang, China. Parasite 2015, 22, 34. [Google Scholar] [CrossRef]
- Zaheri, B.A.; Ronaghi, H.; Youssefi, M.R.; Hoseini, S.M.; Omidzahir, S.; Dozouri, R.; Eshkevari, S.R.; Mousapour, A. Gasterophilus pecorum and Habronema muscae in Persian onager (Equus hemionus onager), histopathology and parasitology survey. Comp. Clin. Pathol. 2015, 24, 1009–1013. [Google Scholar] [CrossRef]
- Smith, M.A.; McGarry, J.W.; Kelly, D.F.; Proudman, C.J. Gasterophilus pecorum in the soft palate of a British pony. Vet. Rec. 2005, 156, 283–284. [Google Scholar] [CrossRef]
- Kornaś, S.; Gawor, J.; Skalska, M.; Nowosad, B. Occurrence of botfly in horses from small farms. Medycyna Wet. 2006, 26, 452–454. [Google Scholar]
- Zhang, K.; Huang, H.; Zhou, R.; Zhang, B.; Wang, C.; Ente, M.; Li, B.; Zhang, D.; Li, K. The impact of temperature on the life cycle of Gasterophilus pecorum in northwest China. Parasites Vectors 2021, 14, 129. [Google Scholar] [CrossRef]
- Tolliver, S.C.; Lyons, E.T.; Drudge, J.H. Prevalence of internal parasites in horses in critical tests of activity of parasiticides over a 28-year period (1956–1983) in Kentucky. Vet. Parasitol. 1987, 23, 273–284. [Google Scholar] [CrossRef]
- Sweeney, H.J. The prevalence and pathogenicity of Gasterophilus intestinalis larvae in horses in Ireland. Ir. Vet. J. 1990, 43, 67–73. [Google Scholar]
- Lyons, E.T.; Swerczek, T.W.; Tolliver, S.C.; Bair, H.D.; Drudge, J.H.; Ennis, L.E. Prevalence of selected species of internal parasites in equids at necropsy in central Kentucky (1995–1999). Vet. Parasitol. 2000, 92, 51–62. [Google Scholar] [CrossRef]
- Sallé, G.; Guillot, J.; Tapprest, J.; Foucher, N.; Sevin, C.; Laugier, C. Compilation of 29 years of postmortem examinations identifies major shifts in equine parasite prevalence from 2000 onwards. Int. J. Parasitol. 2020, 50, 125–132. [Google Scholar] [CrossRef]
- Pilo, C.; Altea, A.; Scala, A. Gasterophilosis in horses in Sardinia (Italy): Effect of meteorological variables on adult egg-laying activity and presence of larvae in the digestive tract, and update of species. Parasitol. Res. 2015, 114, 1693–1702. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, B.; Chu, H.; Zhang, D.; Li, K. Gasterophilus (Diptera, Gasterophilidae) infestation of equids in the Kalamaili Nature Reserve, China. Parasite 2016, 23, 36. [Google Scholar] [CrossRef][Green Version]
- Liu, S.H.; Li, K.; Hu, D.F. The incidence and species composition of Gasterophilus (Diptera, Gasterophilidae) causing equine myiasis in northern Xinjiang, China. Vet. Parasitol. 2016, 217, 36–38. [Google Scholar] [CrossRef]
- Moshaverinia, A.; Baratpour, A.; Abedi, V.; Mohammadi-Yekta, M. Gasterophilosis in Turkmen horses caused by Gasterophilus pecorum (Diptera, Oestridae). Sci. Parasitol. 2016, 17, 49–52. [Google Scholar]
- Blackwell, A.D.; Martin, M.; Kaplan, H.; Gurven, M. Antagonism between two intestinal parasites in humans: The importance of co-infection for infection risk and recovery dynamics. Proc. Biol. Sci. 2013, 280, 20131671. [Google Scholar] [CrossRef] [PubMed]
- De Wolf, B.M.; Zajac, A.M.; Hoffer, K.A.; Sartini, B.L.; Bowdridge, S.; LaRoith, T.; Petersson, K.H. The effect of vitamin E supplementation on an experimental Haemonchus contortus infection in lambs. Vet. Parasitol. 2014, 205, 140–149. [Google Scholar] [CrossRef]
| Group | Oil | Numbers of Horses |
|---|---|---|
| 1 | Soybean | n = 7 (4 oo, 3 xx) |
| 2 | Linseed | n = 7 (3 oo, 4 xx) |
| 3 | linseed + vitamin E | n = 6 (2 oo, 4 xx) |
| 4 | - | n = 7 (3 oo, 4 xx) |
| Group 1 R: 15.286 | Group 2 R: 5.2857 | Group 3 R: 14.750 | Group 4 R: 16.875 | |
|---|---|---|---|---|
| Group 1 | p = 0.048906 z = 2.6457 | p = 1 z = 0.1361 | p = 1 z = 0.3585 | |
| Group 2 | p = 0.048906 z = 2.6457 | p = 0.096828 z = 2.4057 | p = 0.053553 z = 2.6148 | |
| Group 3 | p = 1 z = 0.1361 | p = 0.096828 z = 2.4057 | p = 1 z = 0.4655 | |
| Group 4 | p = 1 z = 0.3585 | p = 0.053553 z = 2.6148 | p = 1 z = 0.4655 |
| Strongylidae | Strongyloididae | Oestridae | |
|---|---|---|---|
| Strongylidae | 1.000000 | 0.346247 | −0.106779 |
| Strongyloididae | 0.346247 | 1.000000 | 0.083236 |
| Oestridae | −0.106779 | 0.083236 | 1.000000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Górniak, W.; Moniuszko, H.; Wojnarowski, K.; Górniak, A.; Cholewińska, P.; Waliczek, A.; Soroko, M.; Szeligowska, N. Effect of Vegetable Oils Feed Additives on Endoparasites Associated with Dewormed Racing Horses. Agriculture 2021, 11, 525. https://doi.org/10.3390/agriculture11060525
Górniak W, Moniuszko H, Wojnarowski K, Górniak A, Cholewińska P, Waliczek A, Soroko M, Szeligowska N. Effect of Vegetable Oils Feed Additives on Endoparasites Associated with Dewormed Racing Horses. Agriculture. 2021; 11(6):525. https://doi.org/10.3390/agriculture11060525
Chicago/Turabian StyleGórniak, Wanda, Hanna Moniuszko, Konrad Wojnarowski, Aleksander Górniak, Paulina Cholewińska, Agnieszka Waliczek, Maria Soroko, and Natalia Szeligowska. 2021. "Effect of Vegetable Oils Feed Additives on Endoparasites Associated with Dewormed Racing Horses" Agriculture 11, no. 6: 525. https://doi.org/10.3390/agriculture11060525
APA StyleGórniak, W., Moniuszko, H., Wojnarowski, K., Górniak, A., Cholewińska, P., Waliczek, A., Soroko, M., & Szeligowska, N. (2021). Effect of Vegetable Oils Feed Additives on Endoparasites Associated with Dewormed Racing Horses. Agriculture, 11(6), 525. https://doi.org/10.3390/agriculture11060525









