An Assessment of Steinernema rarum as a Biocontrol Agent in Sugarcane with Focus on Sphenophorus levis, Host-Finding Ability, Compatibility with Vinasse and Field Efficacy
Abstract
1. Introduction
2. Material and Methods
2.1. Ability to Search for the Host
2.1.1. Galleria Mellonella as an Insect Host
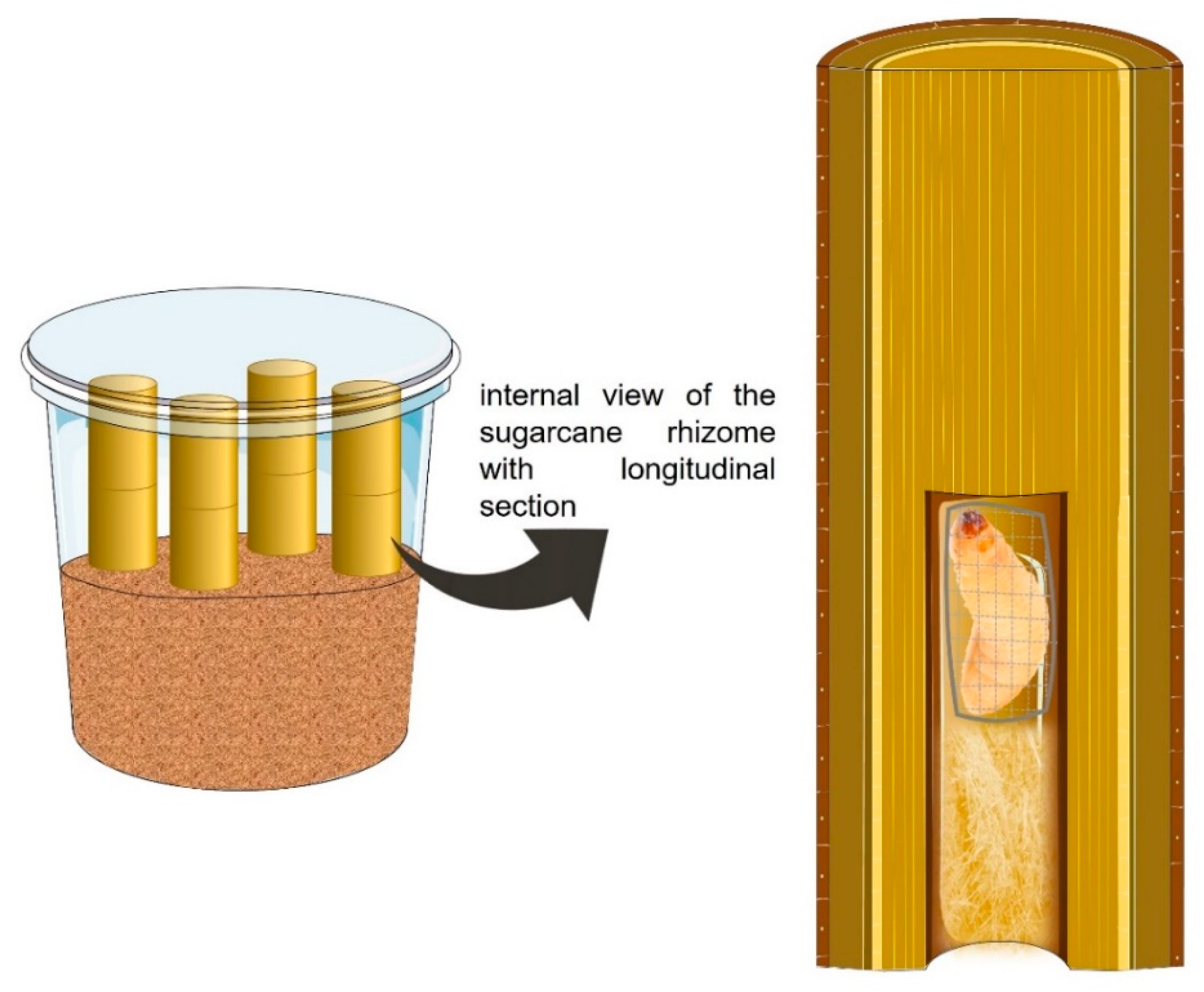
2.1.2. Sphenophorus levis Larvae as a Host
2.2. Performance at Different Application Rates
2.3. Survival in Vinasse
2.4. Field Test I—Efficacy of Nematodes
2.5. Field Test II—Rate Effects
2.6. Statistical Analyses
3. Results
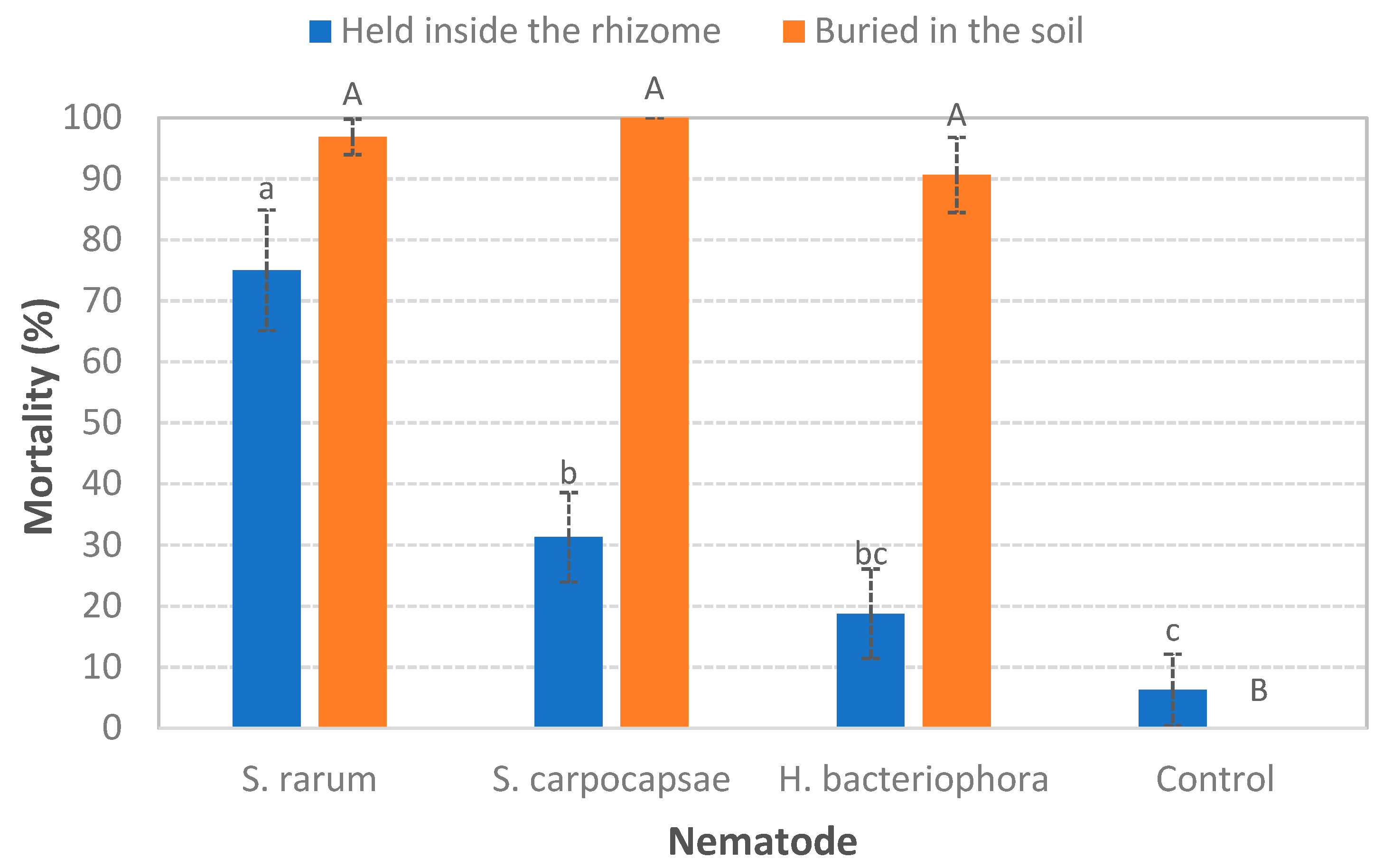
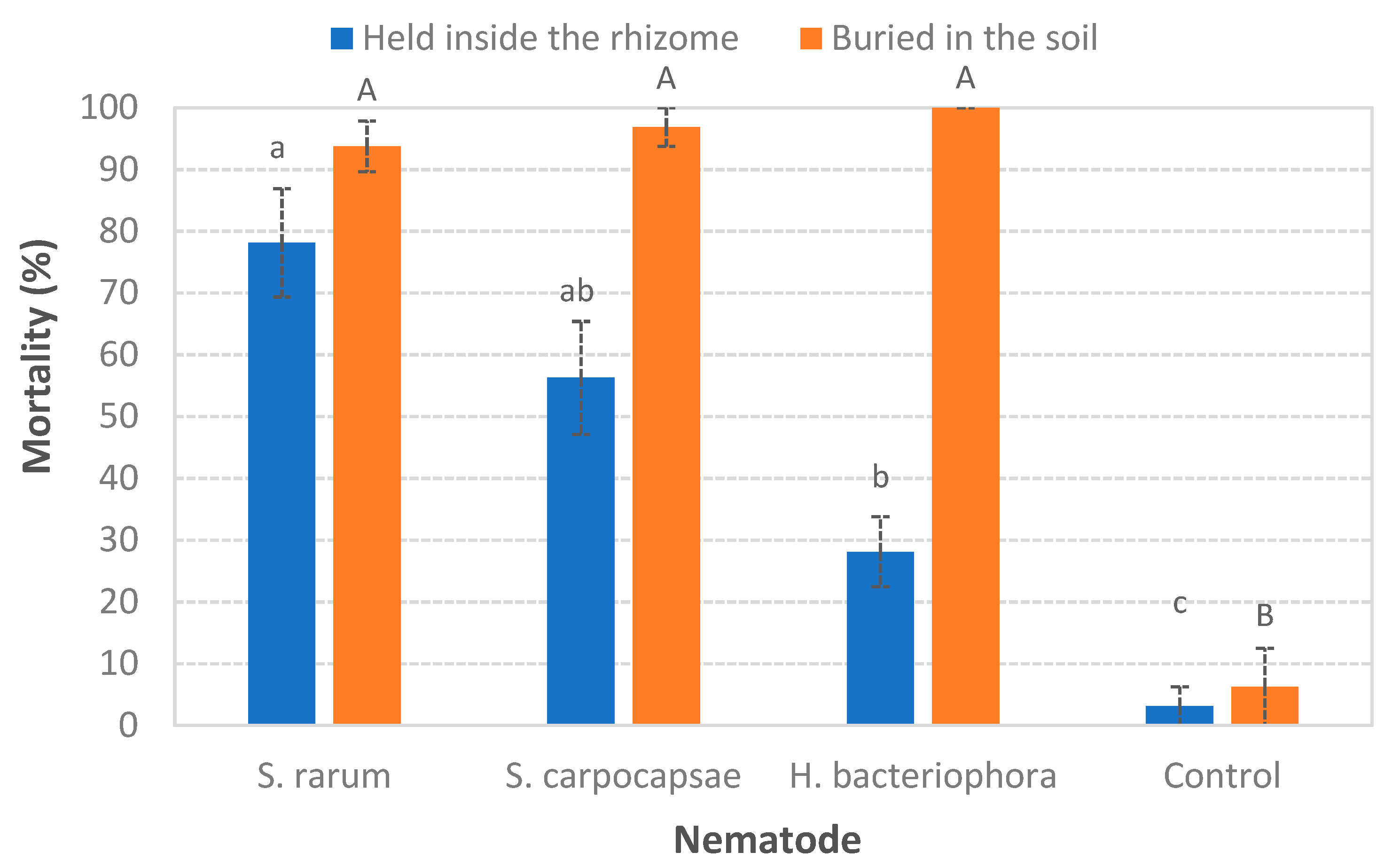
3.1. Ability to Search for the Host
3.2. Performance at Different Application Rates
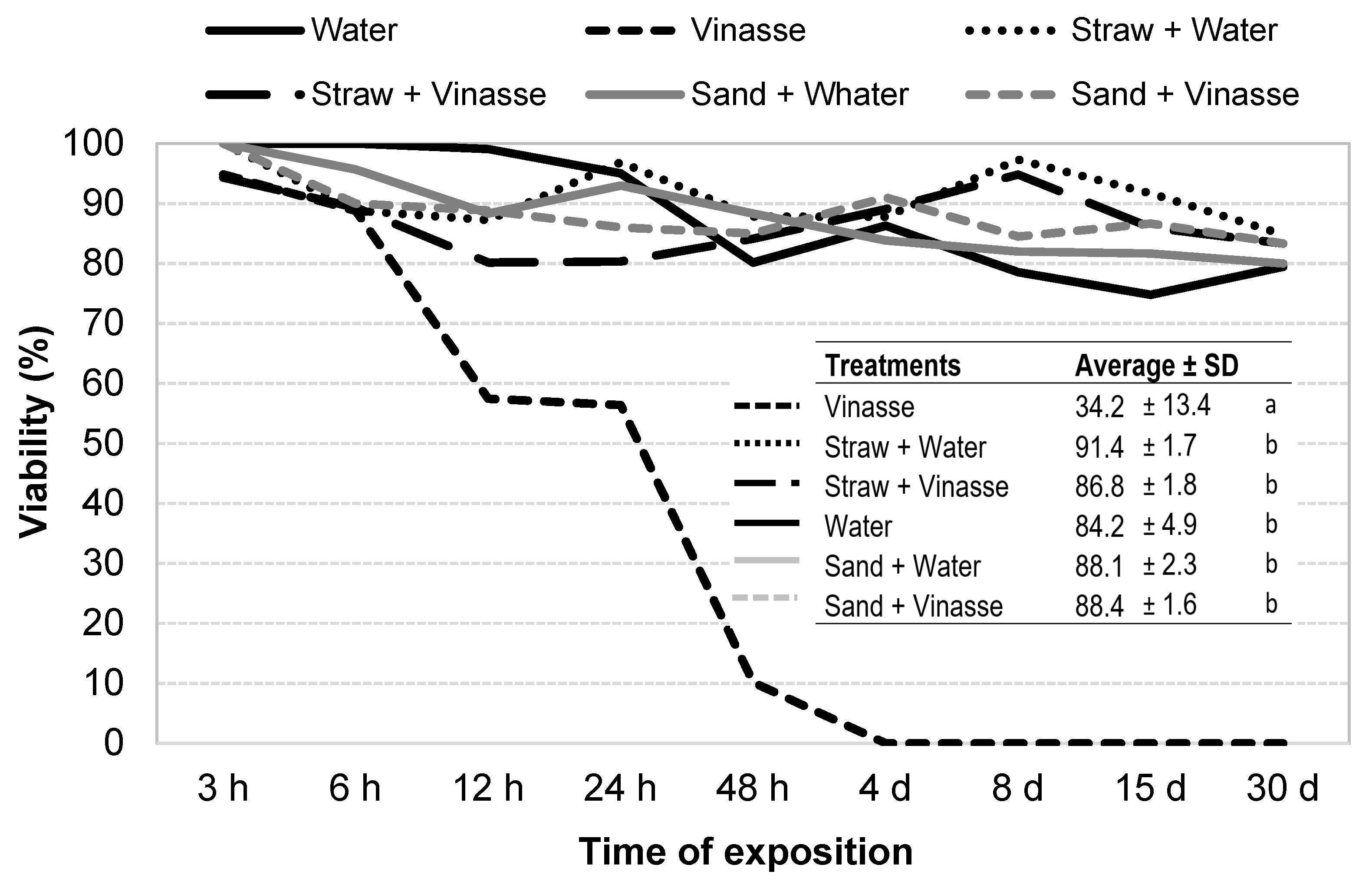
3.3. Survival in Vinasse
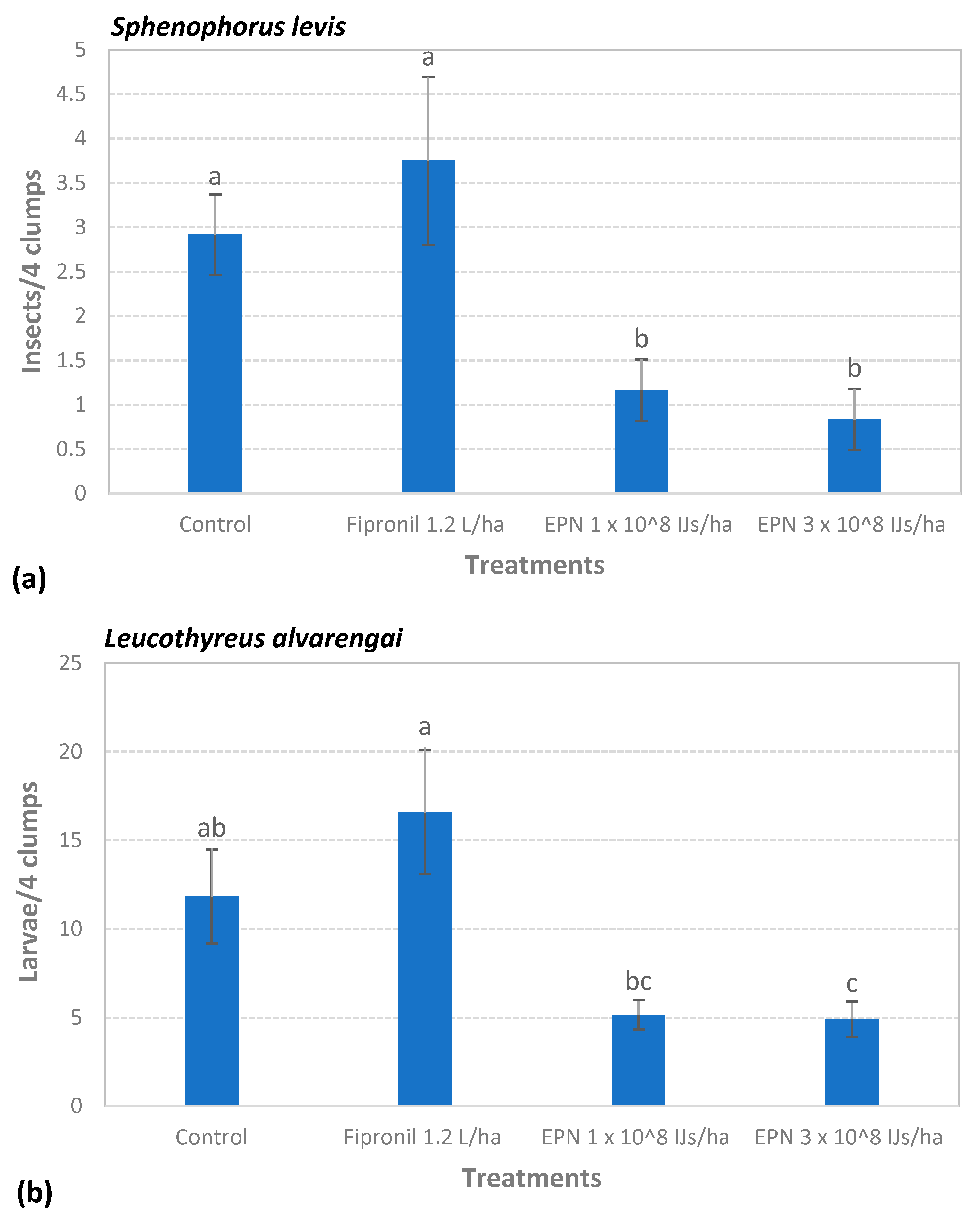
3.4. Field Test I—Efficacy of Nematodes
3.5. Field test II—Rate effects
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Companhia Nacional de Abastecimento. Available online: https://www.conab.gov.br/info-agro/safras/cana/boletim-da-safra-de-cana-de-acucar (accessed on 15 August 2019).
- Giometti, F.H.C.; Leite, L.G.; Tavares, F.M.; Schmit, F.S.; Batista Filho, A.; Dell’Acqua, R. Virulência de nematóides entomopatogênicos (Nematoda: Rhabditida) a Sphenophorus levis (Coleoptera: Curculionidae). Bragantia 2011, 70, 81–86. [Google Scholar] [CrossRef]
- Leite, L.G.; Tavares, F.M.; Botelho, P.S.M.; Batista Filho, A.; Polanczyk, R.A.; Schmidt, F.S. Eficiência de nematoides entomopatogênicos e inseticidas químicos contra Sphenophorus levis e Leucothyreus sp. em cana-de-açúcar. Pesqui. Agropecu. Trop. 2012, 42, 40–48. [Google Scholar] [CrossRef]
- Martins, L.F.; Tonelli, M.; Bento, J.M.S.; Bueno, C.J.; Leite, L.G. Attraction of the sugarcane billbug, Sphenophorus levis, to vinasse and its volatile composition. Chemoecology 2020, 30, 205–214. [Google Scholar] [CrossRef]
- Precetti, A.C.M.; Arrigone, E.B. Aspectos Bioecológicos do Besouro Sphenophorus levis Vaurie, 1978 (Coleoptera: Curculionidae) em Cana-de-Açúcar; Boletim Técnico COPERSUCAR: São Paulo, Brazil, 1990; pp. 3–15. [Google Scholar]
- Shapiro-Ilan, D.I.; Gouge, D.H.; Koppenhöfer, A.M. Factors affecting commercial success: Case studies in cotton, turf and citrus. In Entomopathogenic Nematology; Gaugler, R., Ed.; CABI: Wallingford, UK, 2002; pp. 333–355. [Google Scholar]
- Smith, K.A. Control of weevils with entomopathogenic nematodes. In Control of Insect Pests with Entomopathogenic Nematodes; Smith, K.A., Hatsukade, M., Eds.; Food and Fertilizer Technology Center: Taipei, Taiwan, 1994; pp. 1–13. [Google Scholar]
- Shapiro-Ilan, D.; Hazir, S.; Glazer, I. Basic and Applied Research. In Microbial Control of Insect and Mite Pests; Lacey, L.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 91–105. [Google Scholar]
- Dowds, B.C.A.; Peters, A. Virulence mechanisms. In Entomopathogenic Nematology; CABI: Wallingford, UK, 2002; pp. 79–98. [Google Scholar]
- Gaugler, R.; Brown, I.M. Temperature and humidity influence emergence and survival of entomopathogenic nematodes. Nematologica 1997, 43, 363–375. [Google Scholar] [CrossRef]
- Tavares, F.M.; Batista Filho, A.; Leite, L.G.; Almeida, L.C.; Silva, A.C.; Ambrós, C.M.G. Efeito de Heterorhabditis indica e Steinernema sp. (Nemata: Rhabditida) sobre larvas do bricudo da cana-de-açúcar, Sphenophorus levis (Coleoptera: Curculionidae), em laboratório e casa de vegetação. Nematol. Bras. 2007, 31, 12–19. [Google Scholar]
- Leite, L.G. Controle de pragas no Brasil com nematoides entomopatogênicos. In Experiencias com Nematodos Entomopatógenos. Retos y Oportunidades de su uso em Latinoamérica, 1st ed.; Aponte, A.S., Núnes, J.C.L., Leva, L.A.G., Eds.; CYTED: Bogota, Colombia, 2011; pp. 38–55. [Google Scholar]
- Nguyen, K.B.; Ambrós Ginarte, C.M.; Leite, L.G.; Santos, J.M.; Harakava, R. Steinernema brazilense n. sp. (Rhabditida: Steinernematidae), a new entomopathogenic nematode from Mato Grosso, Brazil. J. Invertebr. Pathol. 2010, 103, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, R.-U.; Niemann, I.; Hollmer, S.; Strauch, O.; Jende, D.; Shanmugasundaram, M.; Mehta, U.K.; Easwaramoorthy, S.K.; Burnell, A. Mass Production Potential of the Bacto-Helminthic Biocontrol Complex Heterorhabditis indica—Photorhabdus luminescens. Biocontrol Sci. Technol. 2000, 10, 607–616. [Google Scholar] [CrossRef]
- Doucet, M.M.A. A new species of Neoaplectana Steiner, 1929 (Nematoda: Steinernematidae) from Cordoba, Argentina. Rev. Nématol. 1986, 9, 317–323. [Google Scholar]
- Shapiro-Ilan, D.I.; Gardner, W.A.; Fuxa, J.R.; Wood, B.W.; Nguyen, K.B.; Adams, B.J.; Humber, R.A.; Hall, M.J. Survey of entomopathogenic nematodes and fungi endemic to pecan orchards of the southeastern United States and their virulence to the pecan weevil (Coleoptera: Curculionidae). Environ. Entomol. 2003, 32, 187–195. [Google Scholar] [CrossRef]
- de Brida, A.L.; Rosa, J.M.O.; de Oliveira, C.M.G.; de Castro e Castro, B.M.; Serrão, J.E.; Zanuncio, J.C.; Leite, L.G.; Wilcken, S.R.S. Entomopathogenic nematodes in agricultural areas in Brazil. Sci. Rep. 2017, 7, 45254. [Google Scholar] [CrossRef]
- Stock, S.P.; Goodrich-Blair, H. Nematode parasites, pathogens and associates of insects and invertebrates of economic importance. In Manual of Techniques in Invertebrate Pathology; Lacey, L.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 373–426. [Google Scholar]
- Leite, L.G.; Shapiro-Ilan, D.I.; Hazir, S.; Jackson, M.A. Effect of inoculum age and physical parameters on in vitro culture of the entomopathogenic nematode Steinernema feltiae. J. Helminthol. 2017, 91, 686–695. [Google Scholar] [CrossRef]
- Casteliani, A.; de Fatima Martins, L.; Maringoli Cardoso, J.F.; Oliveira Silva, M.S.; Abe da Silva, R.S.; Chacon-Orozco, J.G.; Barbosa Casteliani, A.G.; Půža, V.; Harakava, R.; Leite, L.G. Behavioral aspects of Sphenophorus levis (Coleoptera: Curculionidae), damage to sugarcane and its natural infection by Steinernema carpocapsae (Nematoda: Rhabditidae). Crop Prot. 2020, 137, 105262. [Google Scholar] [CrossRef]
- Cochran, W.G.; Cox, G.M. Experimental Designs, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1957. [Google Scholar]
- Southwood, T.R.E.; Henderson, P.A. Ecological Methods: With Particular Reference to the Study of Insect Populations, 3rd ed.; Blackwell Science: London, UK, 1978. [Google Scholar]
- Stock, S.P.; Hunt, D.J. Morphology and systematics of nematodes used in biocontrol. In Nematodes as Biocontrol Agents; Grewal, P., Ehlers, R., Shapiro-llan, D., Eds.; CABI: Wallingford, UK, 2005; pp. 3–43. [Google Scholar]
- Blinova, S.L.; Ivanova, E.S. Culturing the nematode-bacterial complex of Neoaplectana carpocapsae in insects. In Helminths of Insects; Sonin, M.D., Ed.; E.J. Brill: Leiden, The Netherlands, 1990; pp. 13–21. [Google Scholar]
- Shapiro, D.I.; Cate, J.R.; Pena, J.; Hunsberger, A.; McCoy, C.W. Effects of Temperature and Host Age on Suppression of Diaprepes abbreviatus (Coleoptera: Curculionidae) by Entomopathogenic Nematodes. J. Econ. Entomol. 1999, 92, 1086–1092. [Google Scholar] [CrossRef]
- Dolinski, C.; del Valle, E.E.; Burla, R.S.; Machado, I.R. Biological traits of two native brazilian entomopathogenic nematodes (Heterorhabditidae: Rhabditida). Nematol. Bras. 2007, 31, 180–185. [Google Scholar]
- Dias, P.V.C.; Dolinski, C.; Molina Acevedo, J.P. Influence of infective juvenile doses and Galleria mellonella (Lepidoptera: Pyralidae) larvae weight in the in vivo production of Heterorhabditis baujardi LPP7 (Rhabditida: Heterorhabditidae). Nematol. Bras. 2008, 32, 317–321. [Google Scholar]
- Wilson, M.J.; Ehlers, R.U.; Glaser, I. Entomopathogenic nematode foraging strategies—Is Steinernema carpocapsae really an ambush forager? Nematology 2012, 14, 389–394. [Google Scholar] [CrossRef]
- Koppenhöfer, A.M.; Kaya, H.K. Ecological Characterization of Steinernema rarum. J. Invertebr. Pathol. 1999, 73, 120–128. [Google Scholar] [CrossRef]
- Nguyen, K.B.; Shapiro-Ilan, D.I.; Fuxa, J.R.; Wood, B.W.; Bertolotti, M.A.; Adams, B.J. Taxonomic and biological characterization of Steinernema rarum found in the southeastern United States. J. Nematol. 2006, 38, 28–40. [Google Scholar]
- de Doucet, M.M.; Bertolotti, M.; Giayetto, A.; Miranda, M. Host Range, Specificity, and Virulence of Steinernema feltiae, Steinernema rarum and Heterorhabditis bacteriophora (Steinernematidae and Heterorhabditidae) from Argentina. J. Invertebr. Pathol. 1999, 73, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Walter, A.; Dolzan, P.; Quilodrán, O.; de Oliveira, J.G.; da Silva, C.; Piacente, F.; Segerstedtd, A. Sustainability assessment of bio-ethanol production in Brazil considering land use change, GHG emissions and socio-economic aspects. Energy Policy 2011, 39, 5703–5716. [Google Scholar] [CrossRef]
- Pedrosa, E.M.; Rolim, M.M.; Albuquerque, P.H.; Cunha, A.C. Supressividade de nematoides em cana-de-açúcar por adição de vinhaça ao solo. Rev. Bras. Eng. Agrícola e Ambient. 2005, 9, 197–201. [Google Scholar]
- Katase, M.; Kubo, C.; Ushio, S.; Ootsuka, E.; Takeuchi, T.; Mizukubo, T. Nematicidal activity of volatile fatty acids generated from wheat bran in reductive soil disinfestation. Jpn. J. Nematol. 2009, 39, 53–62. [Google Scholar] [CrossRef]
- Silva, M.d.A.; Gava, G.J.d.C.; Caputo, M.M.; Pincelli, R.P.; Jerônimo, E.M.; Cruz, J.C.S. Uso de reguladores de crescimento como potencializadores do perfilhamento e da produtividade em cana-soca. Bragantia 2007, 66, 545–552. [Google Scholar] [CrossRef]
- Rolim, M.M.; Lyra, M.R.C.C.; Duarte, A.D.S.; Fernandes, P.R.M.; De França e Silva, E.F.; Pedrosa, E.M.R. Influence of a vinasse-distributing lake on water quality of the groundwater. Ambiente e Agua Interdiscip. J. Appl. Sci. 2013, 8, 155–171. [Google Scholar] [CrossRef][Green Version]
- Edmunds, C.; Wilding, C.S.; Rae, R. Pathogenicity and environmental tolerance of commercial and UK native entomopathogenic nematodes (Steinernema and Heterorhabditis spp.) to the larvae of mosquitoes (Aedes aegypti and Ochlerotatus detritus). Int. J. Pest Manag. 2020, 1–9. [Google Scholar] [CrossRef]
- Ferreira, L.F.; Aguiar, M.; Pompeu, G.; Messias, T.G.; Monteiro, R.R. Selection of vinasse degrading microorganisms. World J. Microbiol. Biotechnol. 2010, 26, 1613–1621. [Google Scholar] [CrossRef]
- Kaya, H.K. Soil Ecology. In Entomopathogenic Nematodes in Biological Control; Gaugler, R., Ed.; CRC Press: Boca Raton, FL, USA, 1990; pp. 93–116. [Google Scholar]
- Parnaudeau, V.; Condom, N.; Oliver, R.; Cazevieille, P.; Recous, S. Vinasse organic matter quality and mineralization potential, as influenced by raw material, fermentation and concentration processes. Bioresour. Technol. 2008, 99, 1553–1562. [Google Scholar] [CrossRef]
- FitzGibbon, F.; Singh, D.; McMullan, G.; Marchant, R. The effect of phenolic acids and molasses spent wash concentration on distillery wastewater remediation by fungi. Process Biochem. 1998, 33, 799. [Google Scholar] [CrossRef]
- Choo, H.Y.; Koppenhöfer, A.M.; Kaya, H.K. Combination of Two Entomopathogenic Nematode Species for Suppression of an Insect Pest. J. Econ. Entomol. 1996, 89, 97–103. [Google Scholar] [CrossRef]
- Kaya, H.K.; Bedding, R.A.; Akhurst, R. An overview of insect-parasitic and entomopathogenic nematodes. In Nematodes and the Biological Control of Insect Pests; Bedding, R.A., Akhurst, R.J., Kaya, H.K., Eds.; CSIRO: East Melbourne, Australia, 1993; pp. 1–10. [Google Scholar]
- Georgis, R.; Poinar, G.O.J. Field effectiveness of entomophilic nematodes Neoaplectana and Heterorhabditis. In Integrated Pest Management for Turfgrass and Ornamentals; Leslie, A.R., Metcalf, R.L., Eds.; CRC Press: Boca Raton, FL, USA, 1989; pp. 213–224. [Google Scholar]
- Klein, M.G. Efficacy against Soil-Inhabiting Insect Pests. In Entomopathogenic Nematodes in Biological Control; Gaugler, R., Ed.; CRC Press: Boca Raton, FL, USA, 1990; pp. 193–214. [Google Scholar]
- Watschke, T.L.; Dernoeden, P.H.; Shetlar, D.J. Managing Turfgrass Pests; CRC Press: Boca Raton, FL, USA, 2013; ISBN 9780429097720. [Google Scholar]
- Jackson, T.A. Soil dwelling pests—Is specificity the key to successful microbial control. In Proceedings of the 3rd International Workshop on Microbial Control of Soil Dwelling Pests, Lincoln, New Zealand, 21–23 February 1996; AgResearch: Lincoln, New Zealand, 1996; pp. 1–6. [Google Scholar]
- Jackson, T. Factors in the Success and Failure of Microbial Controlagents for Soil Dwelling Pests. Integr. Pest Manag. Rev. 1999, 4, 281–285. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.S.O.; Cardoso, J.F.M.; Ferreira, M.E.P.; Baldo, F.B.; Silva, R.S.A.; Chacon-Orozco, J.G.; Shapiro-Ilan, D.I.; Hazir, S.; Bueno, C.J.; Leite, L.G. An Assessment of Steinernema rarum as a Biocontrol Agent in Sugarcane with Focus on Sphenophorus levis, Host-Finding Ability, Compatibility with Vinasse and Field Efficacy. Agriculture 2021, 11, 500. https://doi.org/10.3390/agriculture11060500
Silva MSO, Cardoso JFM, Ferreira MEP, Baldo FB, Silva RSA, Chacon-Orozco JG, Shapiro-Ilan DI, Hazir S, Bueno CJ, Leite LG. An Assessment of Steinernema rarum as a Biocontrol Agent in Sugarcane with Focus on Sphenophorus levis, Host-Finding Ability, Compatibility with Vinasse and Field Efficacy. Agriculture. 2021; 11(6):500. https://doi.org/10.3390/agriculture11060500
Chicago/Turabian StyleSilva, Mateus Salviano Oliveira, Jorge Franco Maringoli Cardoso, Maria Elizia Pacheco Ferreira, Fernando Berton Baldo, Raphael Satochi Abe Silva, Julie Giovanna Chacon-Orozco, David I. Shapiro-Ilan, Selcuk Hazir, Cesar Junior Bueno, and Luis Garrigós Leite. 2021. "An Assessment of Steinernema rarum as a Biocontrol Agent in Sugarcane with Focus on Sphenophorus levis, Host-Finding Ability, Compatibility with Vinasse and Field Efficacy" Agriculture 11, no. 6: 500. https://doi.org/10.3390/agriculture11060500
APA StyleSilva, M. S. O., Cardoso, J. F. M., Ferreira, M. E. P., Baldo, F. B., Silva, R. S. A., Chacon-Orozco, J. G., Shapiro-Ilan, D. I., Hazir, S., Bueno, C. J., & Leite, L. G. (2021). An Assessment of Steinernema rarum as a Biocontrol Agent in Sugarcane with Focus on Sphenophorus levis, Host-Finding Ability, Compatibility with Vinasse and Field Efficacy. Agriculture, 11(6), 500. https://doi.org/10.3390/agriculture11060500







