Plant-Back Intervals of Imicyafos Based on Its Soil Dissipation and Plant Uptake for Rotational Cultivation of Lettuce and Spinach in Greenhouse
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
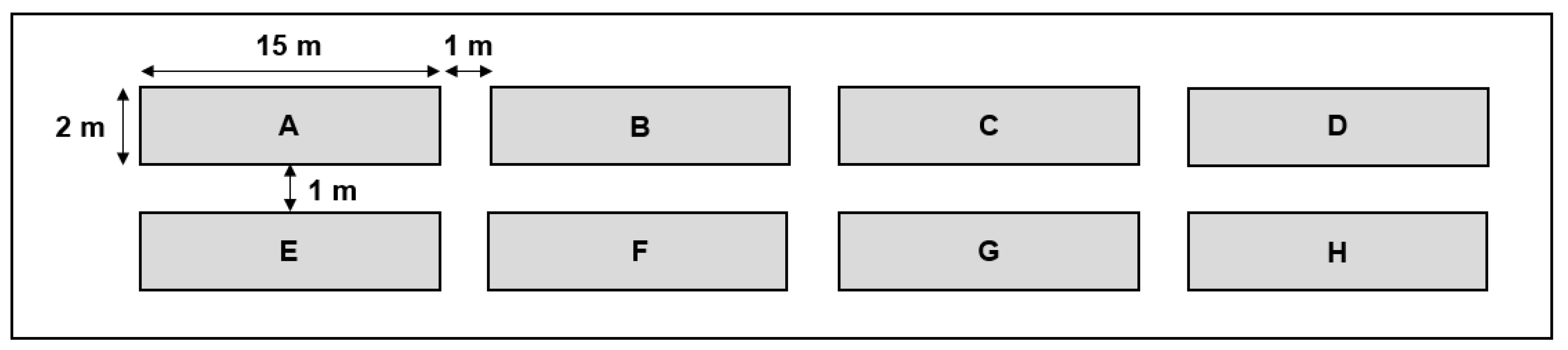
2.2. Greenhouse Experiment
2.3. Pesticide Application
2.4. Soil and Plant Sampling
2.5. Sample Preparation
2.6. Instrumental Validation
2.7. Instruments
2.8. Estimation of Plant-Back Intervals
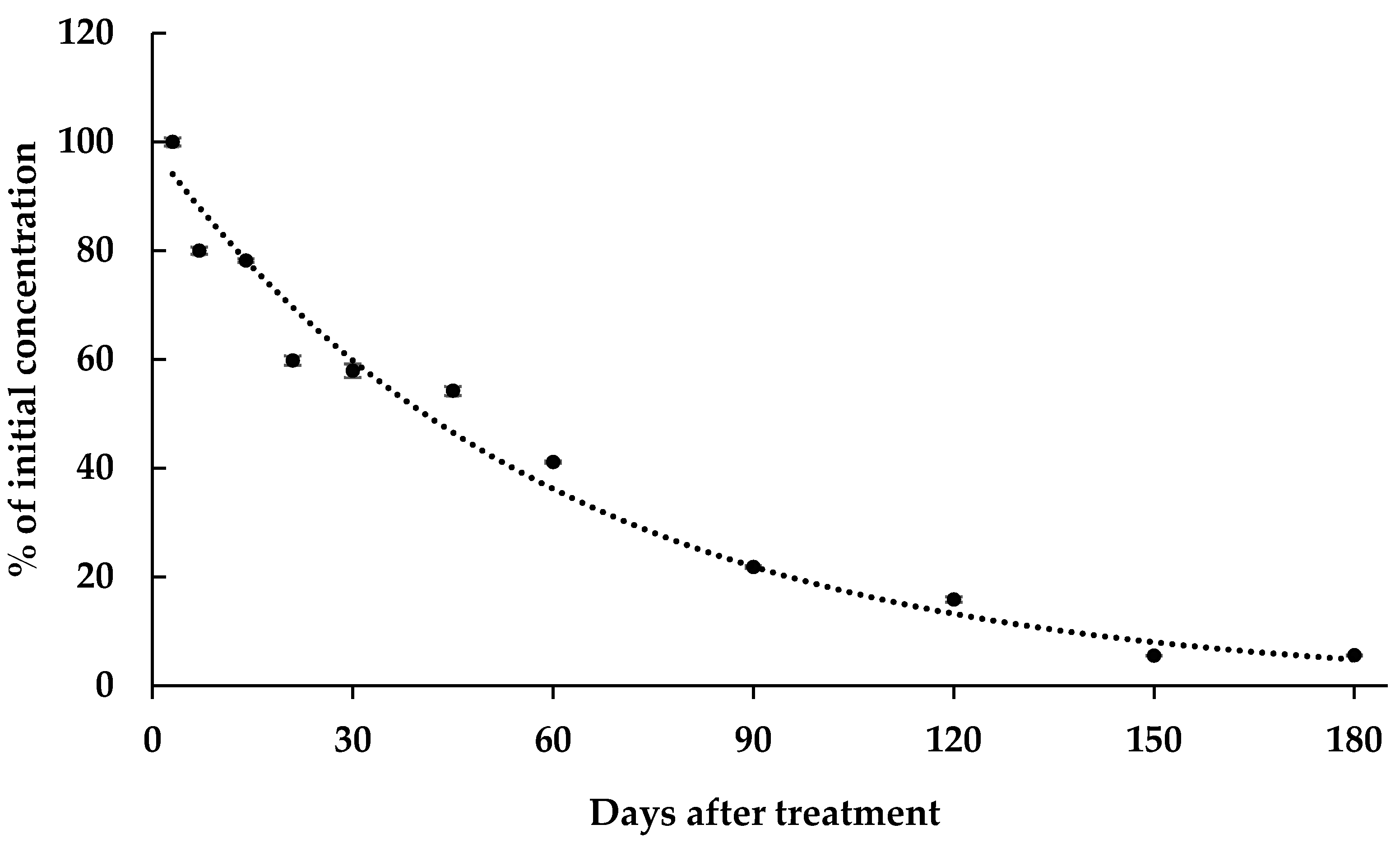
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lee, M. Management and regulation on the minor use of pesticides in Korea and foreign countries. Korean J. Pestic. Sci. 2013, 17, 231–235. [Google Scholar] [CrossRef]
- Mattina, M.I.; Eitzer, B.D.; Iannucci-Berger, W.; Lee, W.; White, J.C. Plant uptake and translocation of highly weathered, soil-bound technical chlordane residues: Data from field and rhizotron studies. Environ. Toxicol. Chem. 2004, 23, 2756–2762. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Ding, X.; Wu, H. Uptake and distribution characteristics of the novel fungicide pyraoxystrobin in cucumber plants. RSC Adv. 2018, 8, 27152–27156. [Google Scholar] [CrossRef]
- Lewandowska, A.; Walorczyk, S. Carbendazim residues in the soil and their bioavailability in four successive harvests. Pol. J. Environ. Stud. 2010, 19, 757–761. [Google Scholar]
- Panseri, S.; Bonerba, E.; Nobile, M.; Ceaare, F.D.; Mosconi, G.; Cecati, F.; Arioli, F.; Tantillo, G.; Chiesa, L. Pesticides and environmental contaminants in organic honeys according to their different productive areas toward food safety protection. Foods 2020, 9, 1863. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Linhart, C.; Niedrist, G.H.; Nagler, M.; Nagranl, R.; Temml, V.; Bardelli, T.; Wilhalm, T.; Riedl, A.; Zaller, J.G.; Clausing, P.; et al. Pesticide contamination and associated risk factors at public playgrounds near intensively managed apple and wine orchards. Environ Sci. Eur. 2019, 31, 28. [Google Scholar] [CrossRef]
- OECD. Draft Guidance Document on Residues in Rotational Crops. OECD Environ. Health Saf. Public 2016, 1, 1–46. Available online: https://www.oecd.org/env/ehs/testing/OECDRotationalCropGuidanceDraftVersion_19%20July2016_AE.pdf (accessed on 15 April 2021).
- Motoki, Y.; Iwafune, T.; Seike, N.; Otani, T.; Akiyama, Y. Relationship between plant uptake of pesticides and water-extractable residue in Japanese soils. Jpn. Pestic. Sci. 2015, 40, 175–183. [Google Scholar] [CrossRef]
- Kim, J.; Mwamula, A.O.; Kabir, F.; Shin, J.H.; Choi, Y.H.; Lee, J.; Lee, D.W. Efficacy of different nematicidal compounds on hatching and mortality of Heteodera schachtii infective juveniles. Korean J. Pestic. Sci. 2016, 20, 293–299. [Google Scholar] [CrossRef]
- Takagi, M.; Goto, M.; Wari, D.; Saito, M.; Perry, R.N.; Toyota, K. Screening of nematicides against the lotus root nematode, Hirschmanniella diversa Sher (Tylenchida Pratylenchidae) and the efficacy of a selected nematicide under lotus micro-field conditions. Agronomy 2020, 10, 373. [Google Scholar] [CrossRef]
- Kim, H.H.; Jung, Y.H.; Kim, D.H.; Ha, T.K.; Yoon, J.B.; Park, C.G.; Choo, H.Y. Control effects of imicyafos GR against two species of the root-know nematodes (Meloidogyne incognita and Meloidogyne hapla). Korean J Pestic. Sci. 2015, 19, 101–105. [Google Scholar] [CrossRef][Green Version]
- Smith, J.L.; Doran, J.W. Measurement and use of pH and electrical conductivity for soil quality analysis. In Methods for Assessing Soil Quality; Doran, J.W., Jones, A.J., Eds.; SSSA Special Publication 49; SSSA: Madison, WI, USA, 1996; pp. 169–185. [Google Scholar]
- Kim, Y.E.; Kim, S.W.; Kim, D.J.; Kim, I.S. Residue analysis of acetamiprid, imidacloprid and pyraclostrobin in the minor crop mustard green under greenhouse conditions for evaluation of their potentiality of PLS violation. Korean J. Environ. Agric. 2020, 39, 214–221. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and dispersive solid-phase extraction for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Lehotay, S.J.; Mśstovská, K.; Lightfield, A.R. Use of buffering and other means to improve results of problematic pesticides in a fast and easy method for residue analysis of fruits and vegetables. J. AOAC Int. 2005, 88, 615–629. [Google Scholar] [CrossRef]
- Dong, M.; Nie, D.; Tang, H.; Rao, Q.; Qu, M.; Wang, W.; Han, L.; Song, W.; Han, Z. Analysis of amicarbazone and its two metabolites in grains and soybeans by liquid chromatography with tandem mass spectrometry. J. Sep. Sci. 2015, 38, 2245–2252. [Google Scholar] [CrossRef]
- Kushwasha, M.; Verma, S.; Chatterjee, S. Profenofos, an acetylcholinesterase-inhibiting organophosphorus pesticide: A short review of its usage, toxicity, and biodegradation. J. Environ. Qual. 2016, 45, 1478–1489. [Google Scholar] [CrossRef]
- Hwang, J.; Lee, S.; Kim, J. Plant uptake and distribution of endosulfan and its sulfate metabolite persisted in soil. PLoS ONE 2015, 10, e0141728. [Google Scholar] [CrossRef]
- Singh, B.; Walker, A. Microbial degradation of organophosphorus compounds. FEMS Microbiol. Rev. 2006, 30, 428–471. [Google Scholar] [CrossRef]
- Anderson, J.J.; Bookhart, S.W.; Clark, J.M.; Jemberg, K.M.; Kingston, C.K.; Snyder, N.; Wallick, K.; Watson, L.J. Uptake of cyantraniliprole into tomato fruit and foliage under hydroponic conditions: Application to calibration of a plant/soil uptake model. J. Agric. Food Chem. 2013, 61, 9027–9035. [Google Scholar] [CrossRef]
- Hwang, K.; Yoo, S.C.; Le, S.; Moon, J.K. Residue level of chlorpyrifos in lettuces grown on chlorpyrifos-treated soils. Appl. Sci. 2018, 8, 2343. [Google Scholar] [CrossRef]
- Wu, X.; Ernst, F.; Conkle, J.L.; Gan, J. Comparative uptake and translocation of pharmaceutical and personal care products (PPCPs) by common vegetables. Environ. Int. 2013, 60, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, O. Evaluation of selected polychlorinated biphenyls (PCBs) congeners and dichlorodiphenyltrichloroethane (DDT) in fresh root and leafy vegetables using GC-MS. Sci. Rep. 2019, 9, 538. [Google Scholar] [CrossRef] [PubMed]
- Namiki, S.; Otani, T.; Motoki, Y.; Seiki, N.; Iwafune, T. Differential uptake and transformation of organic chemicals by several plant species from soil. J. Pestic. Sci. 2018, 43, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Zimmerman, A.; Kim, J. Bioconcentration factor-based management of soil pesticide residues: Endosulfan uptake by carrot and potato plants. Sci. Total Environ. 2018, 627, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Doucette, W.; Shunthirasingham, C.; Dettenmaier, E.M.; Zaleski, R.T.; Fantke, P.; Arnot, J.A. A review of measured bioaccumulation data on terrestrial plants for organic chemicals: Metrics, variability, and the need for standardized measurement protocols. Environ. Toxicol. Chem. 2018, 37, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Feng, Y.; Liu, Y.; Chang, H.; Li, Z.; Xue, J. Uptake and translocation of organic pollutants in plants: A review. J. Integr. Agric. 2017, 16, 1659–1668. [Google Scholar] [CrossRef]
- Park, S.; Yoo, J.; Oh, K.; Park, B.; Kim, S.; Chon, K.; Kwon, H.; Hong, S.; Moon, B.; Choi, H. Uptake and translocation of the soil residual pesticides into the vegetable crop. Korean J. Pestic. Sci. 2017, 21, 298–309. [Google Scholar] [CrossRef]
| Matrix | Linear Slope Equation | R2 | Matrix Effect (%) (1) | Ion Ratio Tolerance (%) (2) | LOQ (mg kg−1) (3) |
|---|---|---|---|---|---|
| Solvent | y = 869.0x + 1578.80 | 1 | - | - | - |
| Soil | y = 798.6x + 5.73 | 1 | −8.109 | −0.073 | 0.005 |
| Lettuce leaf | y = 276.8x + 73.70 | 0.997 | 24.002 | −1.44 | 0.002 |
| root | y = 214.1x − 7.05 | 0.999 | 11.02 | −2.217 | 0.002 |
| Spinach leaf | y = 240.8x + 38.17 | 1 | 5.13 | 4.746 | 0.002 |
| root | y = 252.1x + 60.48 | 0.999 | 8.376 | −0.23 | 0.002 |
| Samples | Recovery Values (%) (1) | ||
|---|---|---|---|
| 2 × LOQ (2) | 10 × LOQ | 50 × LOQ | |
| Soil | 91.7 ± 2.6 | 117.2 ± 0.6 | 118.9 ± 0.7 |
| Lettuce leaf | 109.9 ± 0.4 | 101.7 ± 0.6 | 110.1 ± 1.3 |
| root | 1003. ± 6.9 | 108.6 ± 4.1 | 101.7 ± 4.3 |
| Spinach leaf | 102.3 ± 4.6 | 106.4 ± 5.8 | 109.1 ± 3.1 |
| root | 102.4 ± 2.6 | 110.9 ± 4.6 | 104.3 ± 0.6 |
| Harvest Numbers (Days after Treatment) | Residues (mg kg−1) * | |||
|---|---|---|---|---|
| Lettuce | Spinach | |||
| Leaf | Root | Leaf | Root | |
| 1 (32) | 3.447 ± 0.075a | 0.793 ± 0.017e | 1.003 ± 0.027a | 0.351 ± 0.014c |
| 2 (35) | 3.510 ± 0.166a | 0.648 ± 0.028f | 0.828 ± 0.113b | 0.520 ± 0.027a |
| 3 (38) | 2.028 ± 0.041b | 1.178 ± 0.052c | 0.623 ± 0.134c | 0.451 ± 0.016b |
| 4 (41) | 1.444 ± 0.039c | 1.021 ± 0.047d | 0.292 ± 0.007d | 0.352 ± 0.007c |
| 5 (44) | 0.899 ± 0.036d | 2.069 ± 0.057a | 0.256 ± 0.004de | 0.440 ± 0.010b |
| 6 (47) | 0.598 ± 0.019e | 1.562 ± 0.092b | 0.136 ± 0.006ef | 0.243 ± 0.004d |
| 7 (50) | 0.306 ± 0.009f | 0.610 ± 0.049f | 0.045 ± 0.001f | 0.242 ± 0.005d |
| Harvest Numbers (Days after Treatment) | Imicyafos Amounts (μg Plant−1) * | |
|---|---|---|
| Lettuce | Spinach | |
| 1 (32) | 28.323 ±0.547e | 10.786 ± 0.243d |
| 2 (35) | 27.185 ± 1.126e | 8.829 ± 0.754d |
| 3 (38) | 80.359 ± 2.894c | 22.802 ± 3.716a |
| 4 (41) | 66.995 ± 1.591d | 21.331 ± 0.577ab |
| 5 (44) | 126.350 ± 1.229b | 16.682 ± 0.139c |
| 6 (47) | 180.014 ± 10.139a | 19.741 ± 0.303b |
| 7 (50) | 65.477 ± 3.679d | 22.318 ± 0.770ab |
| Harvest Numbers (Days after Treatment) | Bioconcentration Ratios (BCRs) * | |
|---|---|---|
| Lettuce | Spinach | |
| 1 (32) | 0.461a | 0.134a |
| 2 (35) | 0.469a | 0.111b |
| 3 (38) | 0.271b | 0.083c |
| 4 (41) | 0.193c | 0.039d |
| 5 (44) | 0.120d | 0.034de |
| 6 (47) | 0.080e | 0.018ef |
| 7 (50) | 0.041f | 0.006f |
| Plant | Factors | PBIs (days) (4) | ||
|---|---|---|---|---|
| BCRs (1) | PLS (mg kg−1) (2) | SARs (mg kg−1) (3) | ||
| Lettuce | 0.469 | 0.01 | 0.021 | 357.3 |
| Spinach | 0.134 | 0.01 | 0.075 | 283.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, D.-J.; Kim, S.-W.; Kim, Y.-E.; Yoon, J.-H.; Cho, H.-J.; Shin, B.-G.; Kim, H.-Y.; Kim, I.-S. Plant-Back Intervals of Imicyafos Based on Its Soil Dissipation and Plant Uptake for Rotational Cultivation of Lettuce and Spinach in Greenhouse. Agriculture 2021, 11, 495. https://doi.org/10.3390/agriculture11060495
Lim D-J, Kim S-W, Kim Y-E, Yoon J-H, Cho H-J, Shin B-G, Kim H-Y, Kim I-S. Plant-Back Intervals of Imicyafos Based on Its Soil Dissipation and Plant Uptake for Rotational Cultivation of Lettuce and Spinach in Greenhouse. Agriculture. 2021; 11(6):495. https://doi.org/10.3390/agriculture11060495
Chicago/Turabian StyleLim, Da-Jung, Seon-Wook Kim, Young-Eun Kim, Ji-Hyun Yoon, Hyun-Jeong Cho, Byeung-Gon Shin, Hyo-Young Kim, and In-Seon Kim. 2021. "Plant-Back Intervals of Imicyafos Based on Its Soil Dissipation and Plant Uptake for Rotational Cultivation of Lettuce and Spinach in Greenhouse" Agriculture 11, no. 6: 495. https://doi.org/10.3390/agriculture11060495
APA StyleLim, D.-J., Kim, S.-W., Kim, Y.-E., Yoon, J.-H., Cho, H.-J., Shin, B.-G., Kim, H.-Y., & Kim, I.-S. (2021). Plant-Back Intervals of Imicyafos Based on Its Soil Dissipation and Plant Uptake for Rotational Cultivation of Lettuce and Spinach in Greenhouse. Agriculture, 11(6), 495. https://doi.org/10.3390/agriculture11060495






