Screening of Functional Compounds in Supercritical Carbon Dioxide Extracts from Perennial Herbaceous Crops
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Harvesting of the Plant Material
2.3. Extraction Conditions
2.4. Determination of Total Polyphenol Content
2.5. Determination of Total Flavonoid Content
2.6. Antioxidant Capacity Assay (DPPH Test)
2.7. Fourier Transform Infrared Spectroscopy (QATR-FTIR) Analysis
2.8. Statistical Analysis
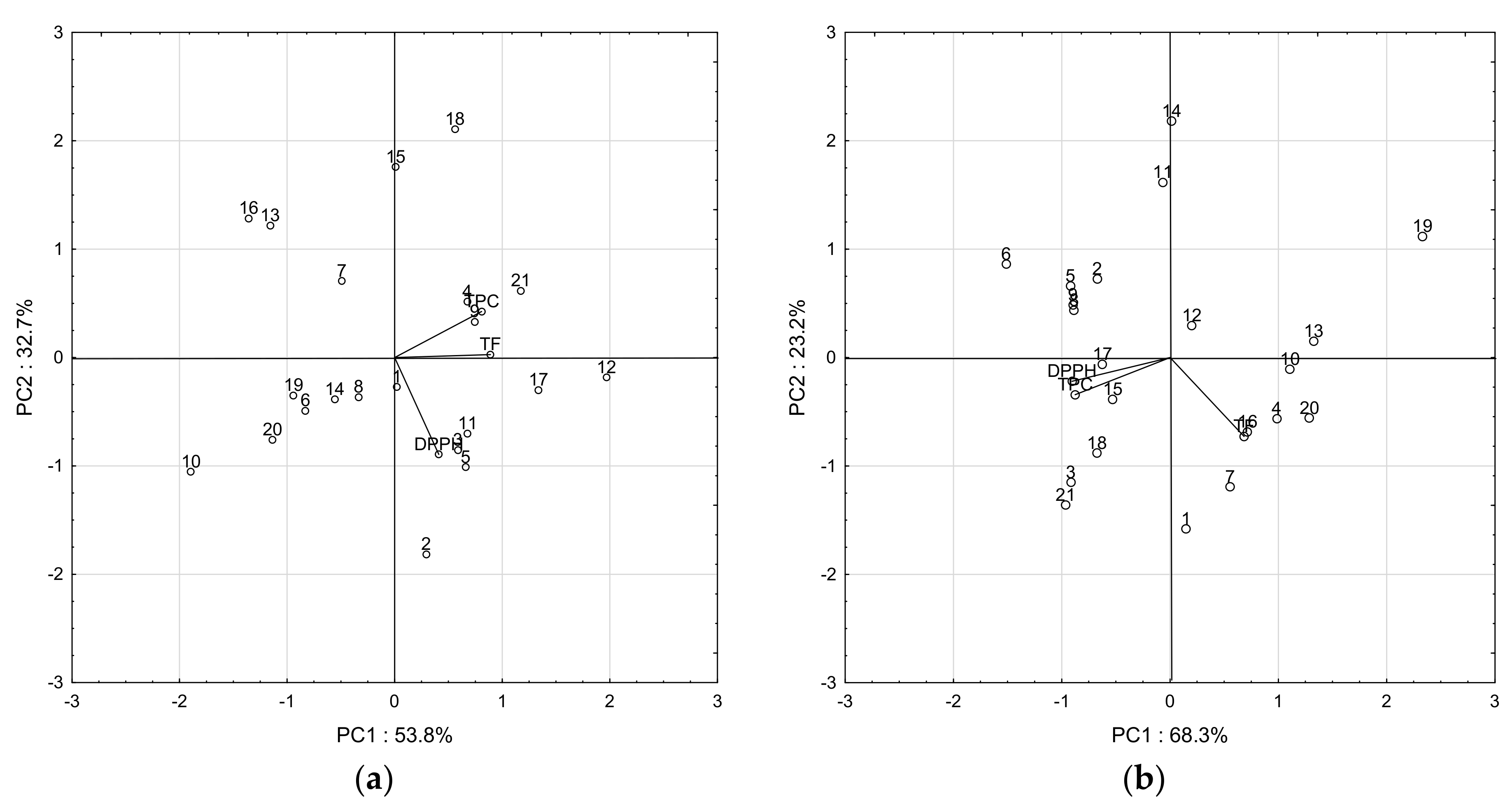
3. Results and Discussion
3.1. Total Polyphenols and Flavonoids
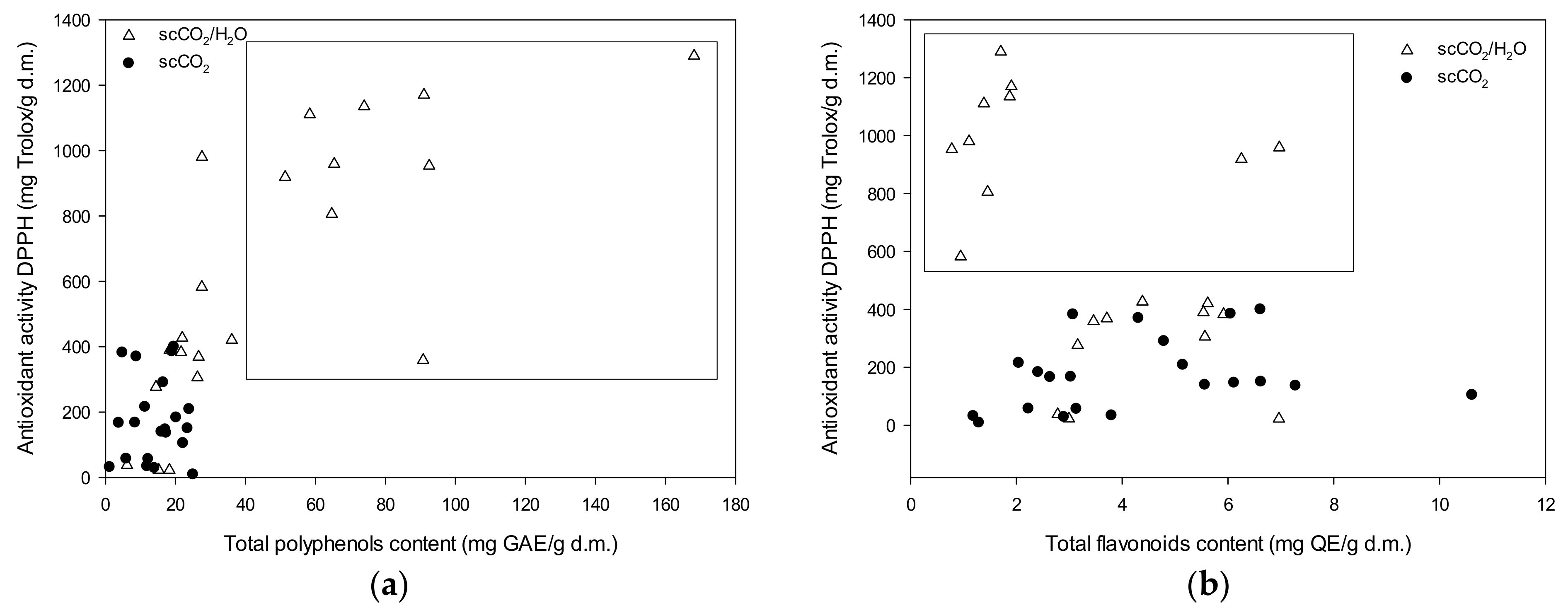
3.2. Antioxidant Activity
3.3. FTIR Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Keoleian, G.A.; Volk, T.A. Renewable energy from willow biomass crops: Life cycle energy, environmental and economic performance. Crit. Rev. Plant Sci. 2005, 24, 385–406. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Warmiński, K.; Krzyżaniak, M. Energy value of yield and biomass quality of poplar grown in two consecutive 4-year harvest rotations in the north-east of Poland. Energies 2020, 13, 1495. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Niksa, D.; Krzyżaniak, M.; Tworkowski, J.; Szczukowski, S. Willow productivity from small- and large-scale experimental plantations in Poland from 2000 to 2017. Renew. Sustain. Energy Rev. 2019, 101, 461–475. [Google Scholar] [CrossRef]
- James, L.K.; Swinton, S.M.; Thelen, K.D. Profitability analysis of cellulosic energy crops compared with corn. Agron. J. 2010, 102, 675–687. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Madunić, J.; Madunić, I.V.; Gajski, G.; Popić, J.; Garaj-Vrhovac, V. Apigenin: A dietary flavonoid with diverse anticancer properties. Cancer Lett. 2018, 413, 11–22. [Google Scholar] [CrossRef]
- Anhê, F.F.; Desjardins, Y.; Pilon, G.; Dudonné, S.; Genovese, M.I.; Lajolo, F.M.; Marette, A. Polyphenols and type 2 diabetes: A prospective review. PharmaNutrition 2013, 1, 105–114. [Google Scholar] [CrossRef]
- Marranzano, M.; Rosa, R.L.; Malaguarnera, M.; Palmeri, R.; Tessitori, M.; Barbera, A.C. Polyphenols: Plant sources and food industry applications. Curr. Pharm. Des. 2018, 24, 4125–4130. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Milinčić, D.D.; Popović, D.A.; Lević, S.M.; Kostić, A.Ž.; Tešić, Ž.L.; Nedović, V.A.; Pešić, M.B. Application of polyphenol-loaded nanoparticles in food industry. Nanomaterials 2019, 9, 1629. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011, 89, 217–233. [Google Scholar] [CrossRef]
- Gessner, D.K.; Ringseis, R.; Eder, K. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J. Anim. Physiol. Anim. Nutr. 2017, 101, 605–628. [Google Scholar] [CrossRef]
- González de Llano, D.; Liu, H.; Khoo, C.; Moreno-Arribas, M.V.; Bartolomé, B. Some new findings regarding the antiadhesive activity of cranberry phenolic compounds and their microbial-derived metabolites against uropathogenic bacteria. J. Agric. Food Chem. 2019, 67, 2166–2174. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, X.; Zhang, F.; Feng, L.; Li, J. Inhibition of quorum sensing, biofilm, and spoilage potential in Shewanella baltica by green tea polyphenols. J. Microbiol. 2015, 53, 829–836. [Google Scholar] [CrossRef]
- Fleitas Martínez, O.; Cardoso, M.H.; Ribeiro, S.M.; Franco, O.L. Recent advances in anti-virulence therapeutic strategies with a focus on dismantling bacterial membrane microdomains, toxin neutralization, quorum-sensing interference and biofilm inhibition. Front. Cell. Infect. Microbiol. 2019, 9, 74. [Google Scholar] [CrossRef]
- Kowalska, G.; Pankiewicz, U.; Kowalski, R. Evaluation of chemical composition of some Silphium, L. species as alternative raw materials. Agriculture 2020, 10, 132. [Google Scholar] [CrossRef]
- Pan, L.; Sinden, M.R.; Kennedy, A.H.; Chai, H.; Watson, L.E.; Graham, T.L.; Kinghorn, A.D. Bioactive constituents of Helianthus tuberosus (Jerusalem artichoke). Phytochem. Lett. 2009, 2, 15–18. [Google Scholar] [CrossRef]
- Nizioł-Łukaszewska, Z.; Furman-Toczek, D.; Zagórska-Dziok, M. Antioxidant activity and cytotoxicity of Jerusalem artichoke tubers and leaves extract on HaCaT and BJ fibroblast cells. Lipids. Health Dis. 2018, 17, 280. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, R.; Kędzia, B. Antibacterial activity of Silphium perfoliatum extracts. Pharm. Biol. 2007, 45, 494–500. [Google Scholar] [CrossRef]
- Kowalski, R. Silphium, L. extracts–composition and protective effect on fatty acids content in sunflower oil subjected to heating and storage. Food Chem. 2009, 112, 820–830. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Warmiński, K.; Krzyżaniak, M.; Tyśkiewicz, K.; Olba-Zięty, E.; Graban, Ł.; Lajszner, W.; Załuski, D.; Wiejak, R.; Kamiński, P.; et al. How does extraction of biologically active substances with supercritical carbon dioxide affect lignocellulosic biomass properties? Wood Sci. Technol. 2020, 54, 519–546. [Google Scholar] [CrossRef]
- Malm, A.; Grzegorczyk, A.; Biernasiuk, A.; Baj, T.; Rój, E.; Tyśkiewicz, K.; Dębczak, A.; Stolarski, M.J.; Krzyżaniak, M.; Olba-Zięty, E. Could supercritical extracts from the aerial parts of Helianthus salicifolius A. Dietr. and Helianthus tuberosus L. be regarded as potential raw materials for biocidal purposes? Agriculture 2021, 11, 10. [Google Scholar] [CrossRef]
- Ostolski, M.; Adamczak, M.; Brzozowski, B.; Wiczkowski, W. Antioxidant activity and chemical characteristics of supercritical CO2 and water extracts from willow and poplar. Molecules 2021, 26, 545. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Lamaison, J.L.; Carnat, A. The amount of main flavonoids in flowers and leaves of Crataegus monogyna Jacq. and Crataegus laevigata (Poiret) DC. (Rosaceae). Pharm. Acta Helv. 1990, 65, 315–320. [Google Scholar]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Rasouli, H.; Farzaei, M.H.; Mansouri, K.; Mohammadzadeh, S.; Khodarahmi, R. Plant cell cancer: May natural phenolic compounds prevent onset and development of plant cell malignancy? A literature review. Molecules 2016, 21, 1104. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Aggarwal, S.; Johnson, S.; Hakovirta, M.; Sastri, B.; Banerjee, S. Removal of water and extractives from softwood with supercritical carbon dioxide. Ind. Eng. Chem. Res. 2019, 58, 3170–3174. [Google Scholar] [CrossRef]
- Trubetskaya, A.; Budarin, V.; Arshadi, M.; Magalhães, D.; Kazanç, F.; Hunt, A.J. Supercritical extraction of biomass as an effective pretreatment step for the char yield control in pyrolysis. Renew. Energy 2021, 170, 107–117. [Google Scholar] [CrossRef]
- Attard, T.M.; Bukhanko, N.; Eriksson, D.; Arshadi, M.; Geladi, P.; Bergsten, U.; Budarin, V.L.; Clark, J.H.; Hunt, A.J. Supercritical extraction of waxes and lipids from biomass: A valuable first step towards an integrated biorefinery. J. Clean. Prod. 2018, 177, 684–698. [Google Scholar] [CrossRef]
- Showkat, M.M.; Falck-Ytter, A.B.; Strætkvern, K.O. Phenolic acids in Jerusalem artichoke (Helianthus tuberosus L.): Plant organ dependent antioxidant activity and optimized extraction from leaves. Molecules 2019, 24, 3296. [Google Scholar] [CrossRef] [PubMed]
- Balcerek, M.; Rąk, I.; Majtkowska, G.; Majtkowski, W. Antioxidant activity and total phenolic compounds in extracts of selected grasses (Poaceae). Herba Pol. 2009, 55, 215–220. [Google Scholar]
- Parveen, I.; Wilson, T.; Donnison, I.S.; Cookson, A.R.; Hauck, B.; Threadgill, M.D. Potential sources of high value chemicals from leaves, stems and flowers of Miscanthus sinensis ‘Goliath’ and Miscanthus sacchariflorus. Phytochem 2013, 92, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Uwineza, P.A.; Waśkiewicz, A. Recent advances in supercritical fluid extraction of natural bioactive compounds from natural plant materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef] [PubMed]
- Tyśkiewicz, K.; Konkol, M.; Rój, E. The application of supercritical fluid extraction in phenolic compounds isolation from natural plant materials. Molecules 2018, 23, 2625. [Google Scholar] [CrossRef]
- Da Porto, C.; Decorti, D.; Natolino, A. Water and ethanol as co-solvent in supercritical fluid extraction of proanthocyanidins from grape marc: A comparison and a proposal. J Supercrit. Fluids 2014, 87, 1–8. [Google Scholar] [CrossRef]
- He, J.-Z.; Shao, P.; Liu, J.-H.; Ru, Q.-M. Supercritical carbon dioxide extraction of flavonoids from pomelo (Citrus grandis (L.) Osbeck) peel and their antioxidant activity. Int. J. Mol. Sci. 2012, 13, 13065–13078. [Google Scholar] [CrossRef]
- Miguez, F.E.; Villamil, M.B.; Long, S.P.; Bollero, G.A. Meta-analysis of the effects of management factors on Miscanthus×giganteus growth and biomass production. Agric. For. Meteorol. 2008, 148, 280–1292. [Google Scholar] [CrossRef]
- Lemos, M.F.; Lemos, M.F.; Pacheco, H.P.; Endringer, D.C.; Scherer, R. Seasonality modifies rosemary’s composition and biological activity. Ind. Crops Prod. 2015, 70, 41–47. [Google Scholar] [CrossRef]
- Cao, Y.; Fang, S.; Fu, X.; Shang, X.; Yang, W. Seasonal variation in phenolic compounds and antioxidant activity in leaves of Cyclocarya paliurus (Batal.) Iljinskaja. Forests 2019, 10, 624. [Google Scholar] [CrossRef]
- Scognamiglio, M.; D’Abrosca, B.; Fiumano, V.; Golino, M.; Esposito, A.; Fiorentino, A. Seasonal phytochemical changes in Phillyrea angustifolia L.: Metabolomic analysis and phytotoxicity assessment. Phytochem. Lett. 2014, 8, 163–170. [Google Scholar] [CrossRef]
- Pacifico, S.; Galasso, S.; Piccolella, S.; Kretschmer, N.; Pan, S.-P.; Marciano, S.; Bauer, R.; Monaco, P. Seasonal variation in phenolic composition and antioxidant and anti-inflammatory activities of Calamintha nepeta (L.) Savi. Food Res. Int. 2015, 69, 121–132. [Google Scholar] [CrossRef]
- Bujor, O.C.; Le Bourvellec, C.; Volf, I.; Popa, V.I.; Dufour, C. Seasonal variations of the phenolic constituents in bilberry (Vaccinium myrtillus L.) leaves, stems and fruits, and their antioxidant activity. Food Chem. 2016, 213, 58–68. [Google Scholar] [CrossRef]
- Dela, G.; Or, E.; Ovadia, R.; Nissim-Levi, A.; Weiss, D.; Oren-Shamir, M. Changes in anthocyanin concentration and composition in ‘Jaguar’ rose flowers due to transient high-temperature conditions. Plant Sci. 2003, 164, 333–340. [Google Scholar] [CrossRef]
- Fu, B.; Ji, X.; Zhao, M.; He, F.; Wang, X.; Wang, Y.; Liu, P.; Niu, L. The influence of light quality on the accumulation of flavonoids in tobacco (Nicotiana tabacum L.) leaves. J. Photochem. Photobiol. B. Biol. 2016, 162, 544–549. [Google Scholar] [CrossRef]
- Rathee, J.S.; Hassarajani, S.A.; Chattopadhyay, S. Antioxidant activity of Nyctanthes arbor-tristis leaf extract. Food Chem. 2007, 103, 1350–1357. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, Y.; Zhang, Y.; Liu, J.; Yu, J. Changes in bioactive compounds and antioxidant activities in pomegranate leaves. Sci. Hortic. 2010, 123, 543–546. [Google Scholar] [CrossRef]
- Vazquez-Olivo, G.; López-Martínez, L.X.; Contreras-Angulo, L.; Heredia, J.B. Antioxidant capacity of lignin and phenolic compounds from corn stover. Waste Biomass Valori. 2019, 10, 95–102. [Google Scholar] [CrossRef]
- Vanderghem, C.; Jacquet, N.; Richel, A. Can lignin wastes originating from cellulosic ethanol biorefineries act as radical scavenging agents? Aust. J. Chem. 2014, 67, 1693–1699. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra. In A Practical Approach; Meyers, R.A., Ed.; Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2006; pp. 1–23. [Google Scholar] [CrossRef]
- Hayat, J.; Akodad, M.; Moumen, A.; Baghour, M.; Skalli, A.; Ezrari, S.; Belmalha, S. Phytochemical screening, polyphenols, flavonoids and tannin content, antioxidant activities and FTIR characterization of Marrubium vulgare L. from 2 different localities of Northeast of Morocco. Heliyon 2020, 6, e05609. [Google Scholar] [CrossRef] [PubMed]
- Huck, C.W. Advances of infrared spectroscopy in natural product research. Phytochem. Lett. 2015, 11, 384–393. [Google Scholar] [CrossRef]
- Nogales-Bueno, J.; Baca-Bocanegra, B.; Rooney, A.; Hernández-Hierro, J.M.; Byrne, H.J.; Heredia, F.J. Study of phenolic extractability in grape seeds by means of ATR-FTIR and Raman spectroscopy. Food Chem. 2017, 232, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, V.H.; de Melo, M.M.R.; Portugal, I.; Silva, C.M. Extraction of Eucalyptus leaves using solvents of distinct polarity. Cluster analysis and extracts characterization. J. Supercrit. Fluids 2018, 135, 263–274. [Google Scholar] [CrossRef]
- Thummajitsakul, S.; Samaikam, S.; Tacha, S.; Silprasit, K. Study on FTIR spectroscopy, total phenolic content, antioxidant activity and anti-amylase activity of extracts and different tea forms of Garcinia schomburgkiana leaves. LWT 2020, 134, 110005. [Google Scholar] [CrossRef]
| Plant Material | TPC (mg GAE/g d.m.) | |||||
|---|---|---|---|---|---|---|
| scCO2 | scCO2/H2O | |||||
| A | B | C | A | B | C | |
| Helianthus salicifolius | 20.22 ± 0.20 d | 25.02 ± 0.31 a | 11.92 ± 0.16 i | 27.51 ± 0.11 h | 36.08 ± 0.15 g | 15.2 ± 0.62 j |
| Silphium perfoliatum | 11.33 ± 0.07 j | 13.99 ± 0.24 h | 17.37 ± 0.54 f | 58.37 ± 1.11 e | 21.59 ± 0.15 i | 18.25 ± 0.32 i,j |
| Helianthus tuberosus | 16.50 ± 0.06 g | 16.01 ± 0.23 g | 8.47 ± 0.11 k | 27.57 ± 0.38 h | 18.34 ± 0.23 i,j | 26.26 ± 0.36 h |
| Miscanthus × giganteus | 22.17 ± 0.18 c | 12.2 ± 0.19 i | 1.24 ± 0.06 o | 73.91 ± 0.96 c | 51.33 ± 0.69 f | 90.75 ± 0.71 b |
| Miscanthus sinensis | 19.01 ± 0.20 e | 17.11 ± 0.28 f | 8.84 ± 0.35 k | 90.98 ± 2.05 b | 65.36 ± 1.26 d | 26.61 ± 0.60 h |
| Miscanthus sacchariflorus | 19.55 ± 0.17 d,e | 5.95 ± 0.23 l | 4.86 ± 0.09 m | 64.64 ± 0.97 d | 21.91 ± 0.59 i | 14.43 ± 0.51 j |
| Spartina pectinata | 23.93 ± 0.14 b | 23.46 ± 0.22 b | 3.82 ± 0.16 n | 168.18 ± 6.26 a | 92.47 ± 1.46 b | 6.17 ± 0.07 k |
| Plant Material | TFC (mg QE/g d.m.) | |||||
|---|---|---|---|---|---|---|
| scCO2 | scCO2/H2O | |||||
| A | B | C | A | B | C | |
| Helianthus salicifolius | 2.41 ± 0.06 m | 1.29 ± 0.07 o | 3.80 ± 0.04 i | 0.95 ± 0.02 l,m | 5.62 ± 0.01 d | 2.99 ± 0.09 g,h |
| Silphium perfoliatum | 2.05 ± 0.05 n | 2.90 ± 0.03 k | 7.28 ± 0.09 b | 1.39 ± 0.02 k | 5.92 ± 0.05 c | 6.95 ± 0.11 a |
| Helianthus tuberosus | 4.79 ± 0.02 g | 5.57 ± 0.05 e | 3.03 ± 0.11 j,k | 1.11 ± 0.05 l | 5.54 ± 0.04 d | 5.56 ± 0.64 d |
| Miscanthus × giganteus | 10.62 ± 0.09 a | 3.14 ± 0.03 j | 1.19 ± 0.03 g | 1.87 ± 0.03 i | 6.25 ± 0.11 b | 3.46 ± 0.06 f |
| Miscanthus sinensis | 6.05 ± 0.05 d | 6.12 ± 0.03 d | 4.31 ± 0.01 h | 1.91 ± 0.03 i | 6.97 ± 0.11 a | 3.71 ± 0.12 f |
| Miscanthus sacchariflorus | 6.61 ± 0.07 c | 2.23 ± 0.02 m,n | 3.07 ± 0.03 j,k | 1.46 ± 0.02 j,k | 4.38 ± 0.06 e | 3.16 ± 0.07 g |
| Spartina pectinata | 5.15 ± 0.11 f | 6.62 ± 0.05 c | 2.64 ± 0.06 l | 1.71 ± 0.03 i,j | 0.78 ± 0.01 m | 2.78 ± 0.08 h |
| Antioxidant Activity Using the DPPH Method (mg Trolox/g d.m.) | ||||||
|---|---|---|---|---|---|---|
| Plant Material | scCO2 | scCO2/H2O | ||||
| A | B | C | A | B | C | |
| Helianthus salicifolius | 183.04 ± 7.77 f | 9.04 ± 1.95 n | 34.02 ± 0.21 m | 582.50 ± 34.76 h | 420.78 ± 2.94 i | 23.01 ± 0.13 n |
| Silphium perfoliatum | 215.55 ± 4.54 e | 28.62 ± 1.01 m | 136.47 ± 0.59 j | 1110.64 ± 47.27 c | 383.25 ± 3.17 j,k | 22.85 ± 0.62 n |
| Helianthus tuberosus | 290.43 ± 2.25 d | 139.48 ± 4.82 i,j | 167.18 ± 2.25 g | 980.24 ± 25.15 d | 389.94 ± 0.51 j | 305.99 ± 0.67 l |
| Miscanthus × giganteus | 104.83 ± 2.97 k | 56.31 ± 1.52 i | 31.56 ± 0.24 m | 1134.86 ± 33.73 c | 919.02 ± 1.48 f | 359.16 ± 20.86 k |
| Miscanthus sinensis | 384.94 ± 5.52 b | 146.69 ± 1.20 h,i | 369.83 ± 3.76 c | 1170.19 ± 17.96 b | 958.81 ± 1.87 d,e | 368.78 ± 5.65 j,k |
| Miscanthus sacchariflorus | 399.41 ± 2.67 a | 57.20 ± 4.56 i | 382.05 ± 2.67 a | 805.72 ± 31.51 g | 426.86 ± 1.94 i | 276.91 ± 2.10 m |
| Spartina pectinata | 208.44 ± 5.21 e | 150.07 ± 2.74 h | 166.43 ± 0.85 g | 1289.50 ± 34.35 a | 953.15 ± 1.66 e | 37.85 ± 0.16 n |
| Group | Wavenumbers (cm−1) | Plant Extract | Bond Type | Functional Group | |
|---|---|---|---|---|---|
| scCO2 | scCO2/H2O | ||||
| 1 | 4000–3100 | H. salicifolius, H. tuberosus, M. × giganteus, M. sinensis | H. salicifolius, S. perfoliatum, H. tuberosus, M. sinensis, S. pectinata | O–H, N–H stretching | Polyphenolic, carbohydrates, proteins |
| 2 | 3100–2800 | H. salicifolius, S. perfoliatum, H. tuberosus, M. × giganteus, M. sinensis, M. sacchariflorus, S. pectinata | H. salicifolius, S. perfoliatum, H. tuberosus, M. × giganteus, M. sinensis, M. sacchariflorus, S. pectinata | C–H stretching in CH2 and CH3; C–H aromatic stretching; O–CH3 | Lipids, lignins, carbohydrates, esters |
| 3 | 1750–1650 | H. salicifolius, S. perfoliatum, H. tuberosus, M. × giganteus, M. sinensis, M. sacchariflorus, S. pectinata | H. salicifolius, S. perfoliatum, H. tuberosus, M. × giganteus, M. sinensis, M. sacchariflorus, S. pectinata | C=O ester stretching; C–N stretching; cis C=C unconjugated | Polyesters, lignins, proteins, alkenyl groups |
| 4 | 1610–1500 | - | H. salicifolius, S. perfoliatum, H. tuberosus, S. pectinata | C–C, C=C aromatic stretching; COO− stretching | Phenolic groups, pectins, lignins |
| 5 | 1500–1290 | H. salicifolius, S. perfoliatum, H. tuberosus, M. × giganteus, M. sinensis, M. sacchariflorus, S. pectinata | H. salicifolius, S. perfoliatum, H. tuberosus, M. × giganteus, M. sinensis, M. sacchariflorus, S. pectinata | CH2 scissoring, bending, out of plane; C–H in >CH–; C–H in –CH3; C–H, CH2, CH3 deformations; C=C–C aromatic ring stretching; O–H bending; N–H, C–N | triterpenoids, phenyl groups, polysaccharides, pectins, lipids, lignins, tertiary alcohols, proteins |
| 6 | 1260–1100 | H. salicifolius, S. perfoliatum, H. tuberosus, M. × giganteus, M. sinensis, M. sacchariflorus, S. pectinata | H. salicifolius, S. perfoliatum, H. tuberosus, M. × giganteus, M. sinensis, M. sacchariflorus, S. pectinata | C–O, C–N, C–C, >PO2 stretching; O–H bending; C–O ring vibrations | Phenyl groups, lignins, pectins, triterpenoids, polysaccharides, phospholipids, proteins |
| 7 | 1100–1000 | H. salicifolius, S. perfoliatum, H. tuberosus, M. × giganteus, M. sinensis, M. sacchariflorus, S. pectinata | H. salicifolius, S. perfoliatum, H. tuberosus, M. × giganteus, M. sinensis, M. sacchariflorus, S. pectinata | C–O, C–N stretching; C–H aromatic stretching; >PO2 stretching | Phenolic groups, phospholipids, polysaccharides, pectins, aliphatic amines |
| 8 | 1000–510 | H. salicifolius, S. perfoliatum, H. tuberosus, M. × giganteus, M. sinensis, M. sacchariflorus, S. pectinata | H. salicifolius, H. tuberosus, M. × giganteus, M. sinensis, M. sacchariflorus, S. pectinata | C–H aromatic stretching; –CH2–; –HC–; cis –CH; trans C–H out of plane | Isoprenoids |
| 9 | 510–400 | H. salicifolius, H. tuberosus | H. tuberosus, S. pectinata | S–S stretching; C–OH3 torsion | Aryl disulfides, polysulfides, methoxy groups |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostolski, M.; Adamczak, M.; Brzozowski, B.; Stolarski, M.J. Screening of Functional Compounds in Supercritical Carbon Dioxide Extracts from Perennial Herbaceous Crops. Agriculture 2021, 11, 488. https://doi.org/10.3390/agriculture11060488
Ostolski M, Adamczak M, Brzozowski B, Stolarski MJ. Screening of Functional Compounds in Supercritical Carbon Dioxide Extracts from Perennial Herbaceous Crops. Agriculture. 2021; 11(6):488. https://doi.org/10.3390/agriculture11060488
Chicago/Turabian StyleOstolski, Mateusz, Marek Adamczak, Bartosz Brzozowski, and Mariusz Jerzy Stolarski. 2021. "Screening of Functional Compounds in Supercritical Carbon Dioxide Extracts from Perennial Herbaceous Crops" Agriculture 11, no. 6: 488. https://doi.org/10.3390/agriculture11060488
APA StyleOstolski, M., Adamczak, M., Brzozowski, B., & Stolarski, M. J. (2021). Screening of Functional Compounds in Supercritical Carbon Dioxide Extracts from Perennial Herbaceous Crops. Agriculture, 11(6), 488. https://doi.org/10.3390/agriculture11060488








