Phosphate-Solubilizing Enterobacter ludwigii AFFR02 and Bacillus megaterium Mj1212 Rescues Alfalfa’s Growth under Post-Drought Stress
Abstract
1. Background
2. Materials and Methods
2.1. Isolation, Screening, and Identification
2.2. Plant Growth-Promoting Effect of Isolate AFFR02 and MJ1212 on Alfalfa under Normal and Post-drought Stress
2.3. Determination of Leaf Water Potential and Electrolyte Leakage in Alfalfa Plants under Normal and Post-Drought-Stressed Conditions
2.4. Endogenous Abscisic Acid Quantification in Alfalfa Plants under Normal and Post-Drought-Stressed Conditions
2.5. Antioxidant Enzyme and Nonenzymatic Activities in Alfalfa Plants under Normal and Post-Drought-Stressed Conditions
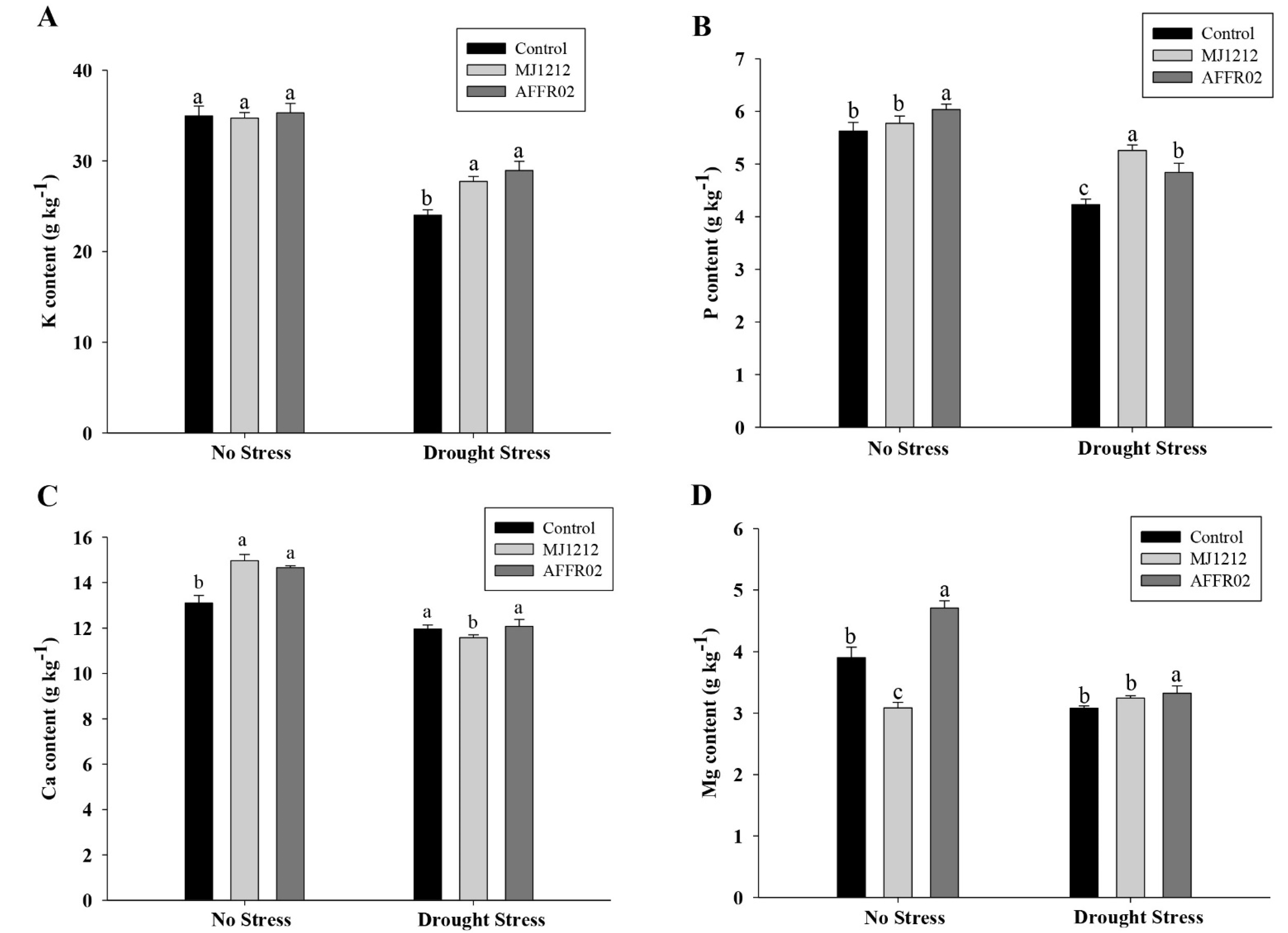
2.6. Determination of Mineral Uptake in Alfalfa Plants under Normal and Post-Drought-Stressed Conditions
2.7. Statistical Analysis
3. Results
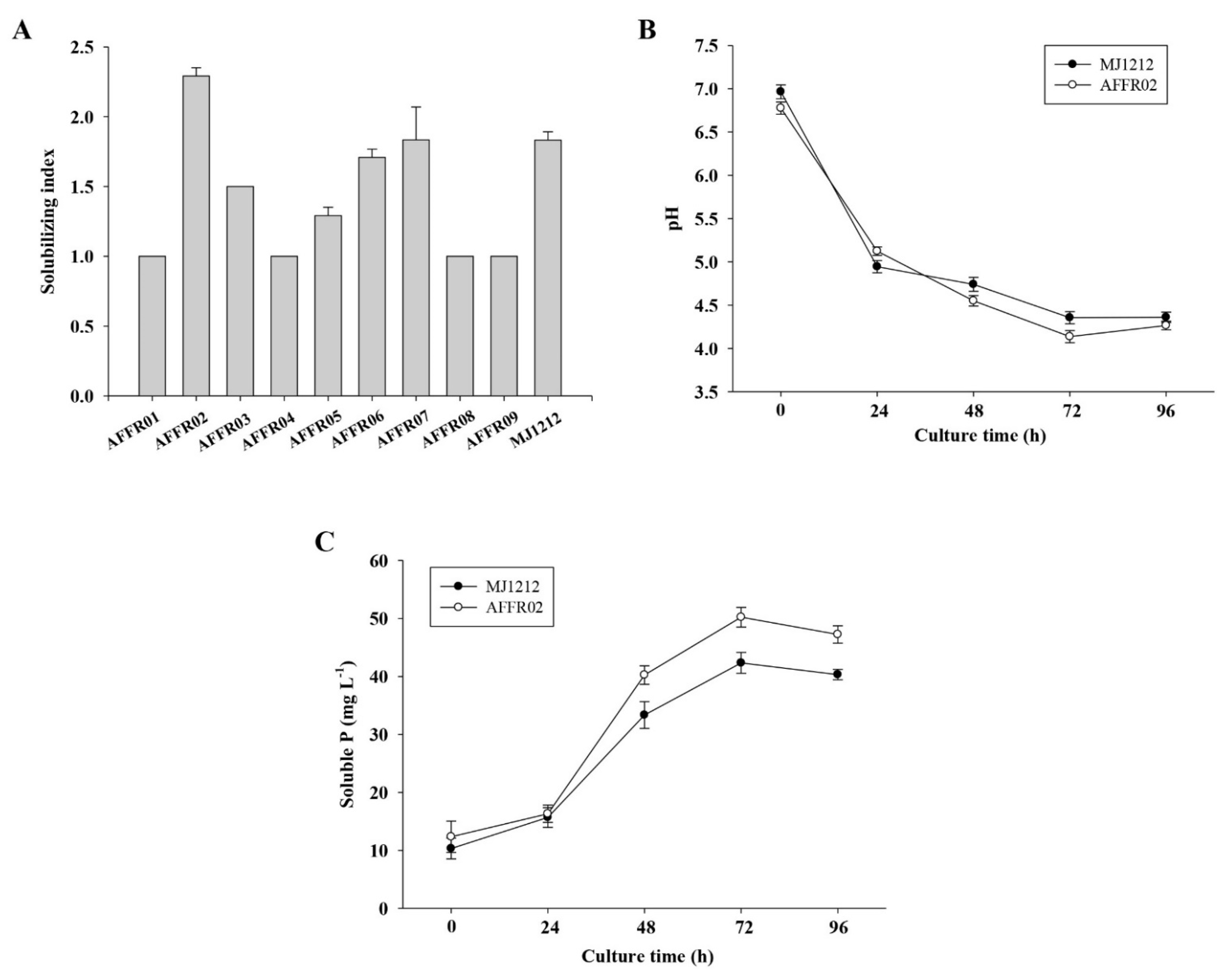
3.1. Isolation and Screening
3.2. Screening for Polyethylene Glycol (PEG) Tolerance
3.3. Phosphate-Solubilizing Ability of Isolate AFFR02 and Mj1212
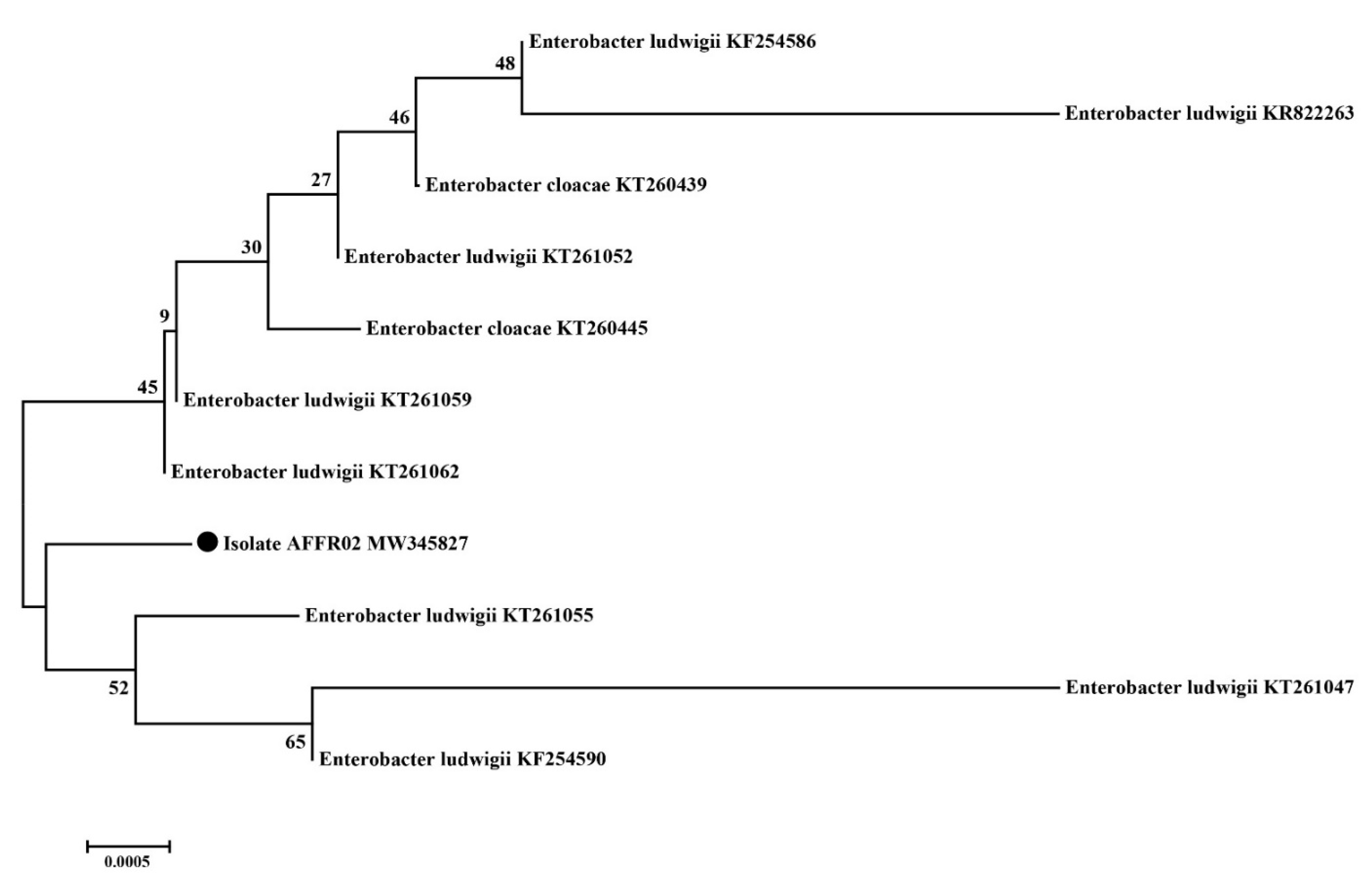
3.4. Identification of Effective Rhizospheric Isolate
3.5. Beneficial Effect of Isolate AFFR02 and Mj1212 on Alfalfa Plants under Normal and Post-Drought-Stressed Conditions
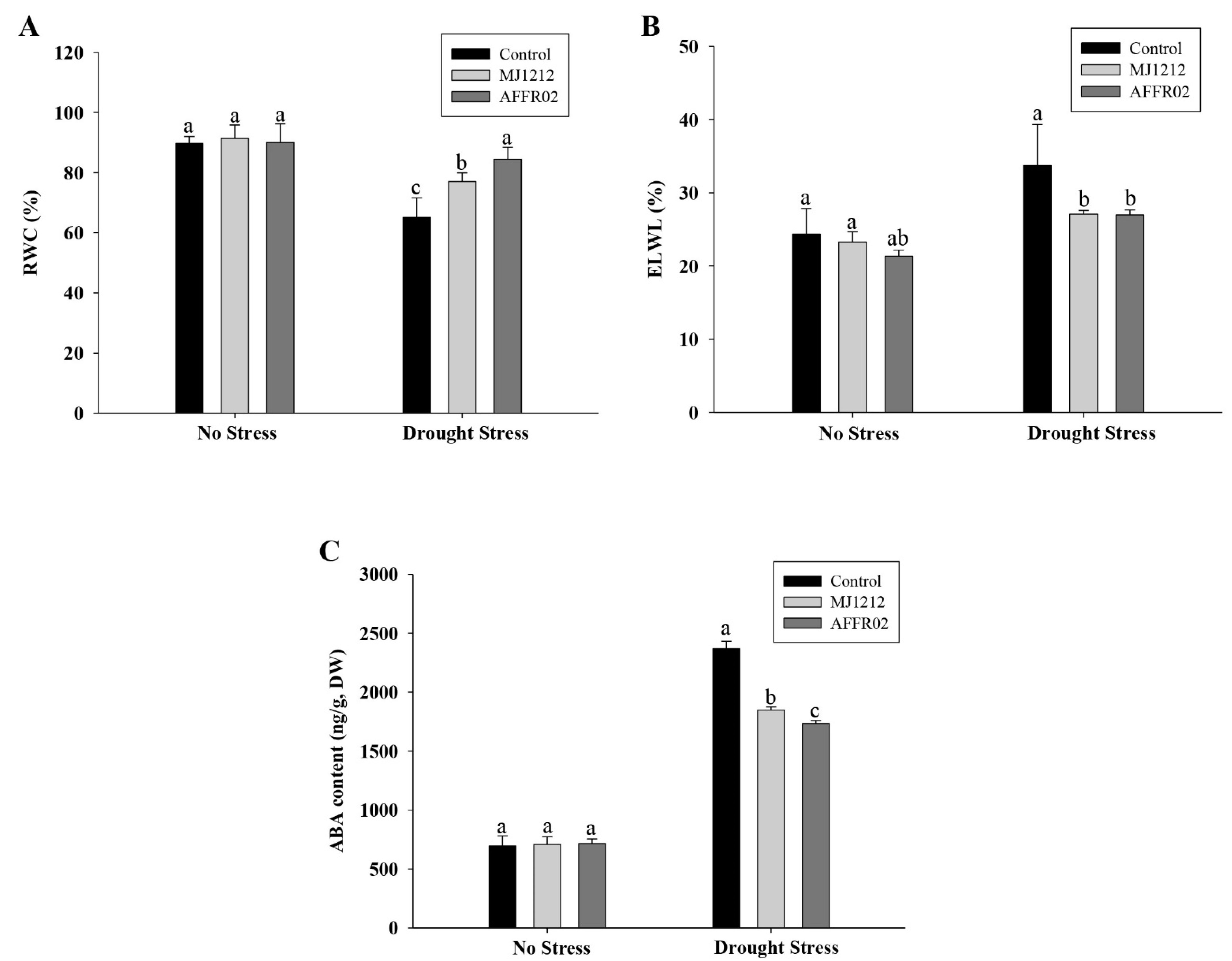
3.6. Effect of Relative Water Content, Electrolytic Leakage, and Endogenous Abscisic Acid Content on Alfalfa Plants under Normal and Post-Drought-Stressed Conditions
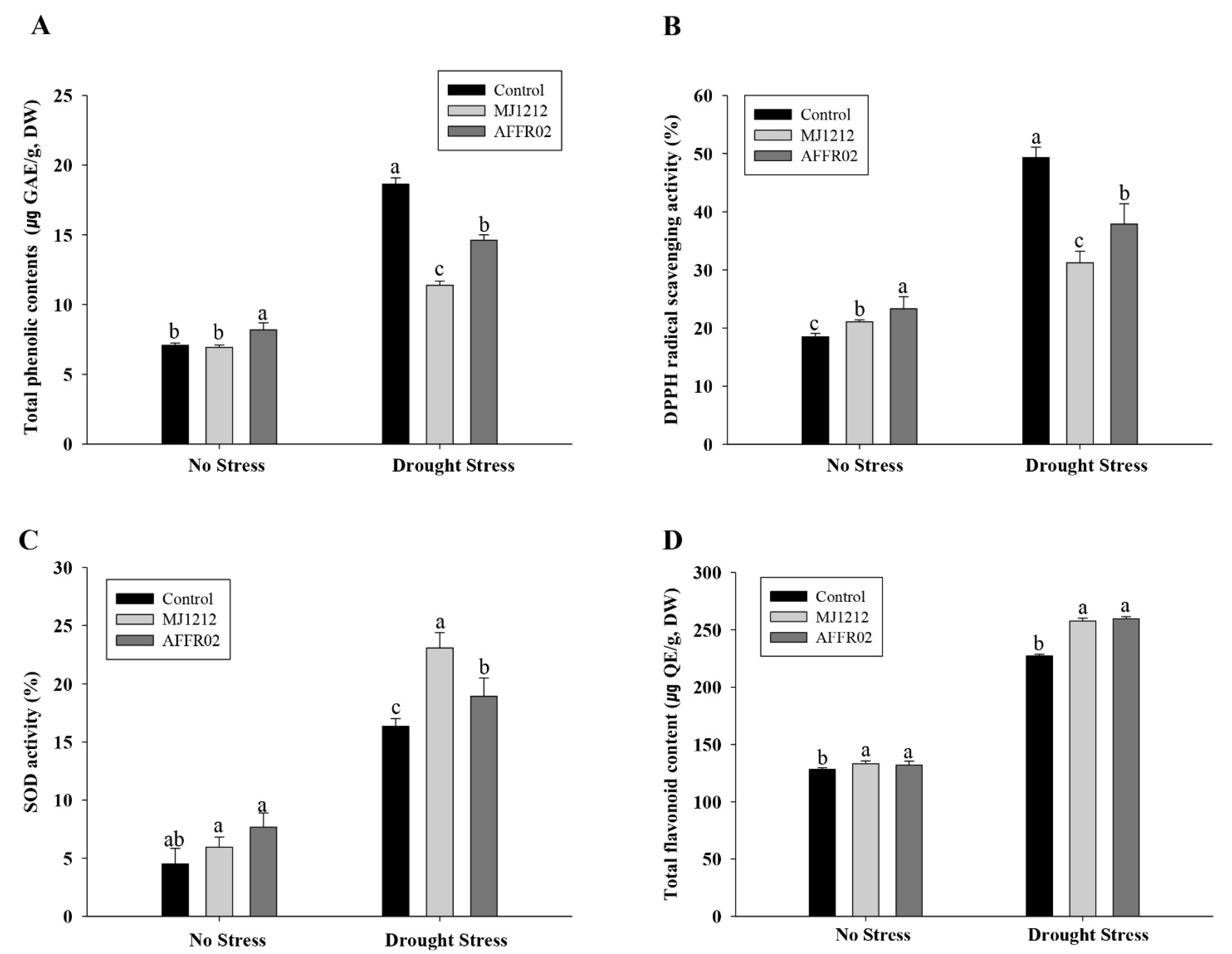
3.7. Regulation of Antioxidants in Alfalfa Plants Inoculated with Isolate AFFR02 and Mj1212 under Normal and Post-Drought-Stressed Conditions
3.8. Ion Uptake in Alfalfa Plants Inoculated with Isolate AFFR02 and Mj1212 under Normal and Post-Drought-Stressed Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Defez, R.; Andreozzi, A.; Dickinson, M.; Charlton, A.; Tadini, L.; Pesaresi, P.; Bianco, C. Improved Drought Stress Response in Alfalfa Plants Nodulated by an IAA Over-producing Rhizobium Strain. Front. Microbiol. 2017, 8, 2466. [Google Scholar] [CrossRef]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Jan, R.; Kang, S.-M.; Kim, K.-M.; Lee, I.-J. Rhizobacteria AK1 remediates the toxic effects of salinity stress via regulation of endogenous phytohormones and gene expression in soybean. Biochem. J. 2019, 476, 2393–2409. [Google Scholar] [CrossRef]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Jan, R.; Kang, S.-M.; Kim, K.-M.; Lee, I.-J. Extending thermotolerance to tomato seedlings by inoculation with SA1 isolate of Bacillus cereus and comparison with exogenous humic acid application. PLoS ONE 2020, 15, e0232228. [Google Scholar] [CrossRef]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Jan, R.; Kang, S.-M.; Kim, K.-M.; Lee, I.-J. Thermotolerance effect of plant growth-promoting Bacillus cereus SA1 on soybean during heat stress. BMC Microbiol. 2020, 20, 175. [Google Scholar] [CrossRef]
- Li, H.; Wang, Z.; Ke, Q.; Ji, C.Y.; Jeong, J.C.; Lee, H.S.; Lim, Y.P.; Xu, B.; Deng, X.P.; Kwak, S.S. Overexpression of codA gene confers enhanced tolerance to abiotic stresses in alfalfa. Plant. Physiol. Biochem. PPB 2014, 85, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Ismail, I.; Hamayun, M.; Hussain, A.; Iqbal, A.; Khan, S.A.; Khan, M.A.; Lee, I.-J. An Endophytic Fungus Gliocladium cibotii Regulates Metabolic and Antioxidant System of Glycine max and Helianthus annuus under Heat Stress. Pol. J. Environ. Stud. 2021, 30, 1631–1640. [Google Scholar] [CrossRef]
- Hamayun, M.; Sohn, E.-Y.; Afzal Khan, S.; Shinwari, Z.; Khan, A.; Lee, I.-J. Silicon alleviates the adverse effects of salinity and drought stress on growth and endogenous plant growth hormones of soybean (Glycine max L.). Abstr. Pap. 2010, 42, 1713–1722. [Google Scholar]
- Sahile, A.A.; Khan, M.A.; Hamayun, M.; Imran, M.; Kang, S.-M.; Lee, I.-J. Novel Bacillus cereus Strain, ALT1, Enhance Growth and Strengthens the Antioxidant System of Soybean under Cadmium Stress. Agronomy 2021, 11, 404. [Google Scholar] [CrossRef]
- Jan, R.; Khan, M.A.; Asaf, S.; Lubna; Lee, I.-J.; Kim, K.M. Metal Resistant Endophytic Bacteria Reduces Cadmium, Nickel Toxicity, and Enhances Expression of Metal Stress Related Genes with Improved Growth of Oryza Sativa, via Regulating Its Antioxidant Machinery and Endogenous Hormones. Plants 2019, 8, 363. [Google Scholar] [CrossRef]
- Azib, S.; Cheloufi, H.; Attab, S.; Bouras, N. Improvement of alfalfa growth under water stress by inoculation with Sinorhizobium meliloti strains from the Algerian Sahara. Int. Sci. Resh. J 2019, 75, 35–43. [Google Scholar] [CrossRef]
- Arzanesh, M.H.; Alikhani, H.A.; Khavazi, K.; Rahimian, H.A.; Miransari, M. Wheat (Triticum aestivum L.) growth enhancement by Azospirillum sp. under drought stress. World J. Microbiol. Biotechnol. 2011, 27, 197–205. [Google Scholar] [CrossRef]
- Yu-Na, K.; Muhammad Aaqil, K.; Sang-Mo, K.; Muhammad, H.; In-Jung, L. Enhancement of Drought-Stress Tolerance of Brassica oleracea var. italica L. by Newly Isolated Variovorax sp. YNA59. J. Microbiol. Biotechnol. 2020, 30, 1500–1509. [Google Scholar] [CrossRef]
- Sherameti, I.; Tripathi, S.; Varma, A.; Oelmüller, R. The Root-Colonizing Endophyte Pirifomospora indica Confers Drought Tolerance in Arabidopsis by Stimulating the Expression of Drought Stress–Related Genes in Leaves. Mol. Plant.-Microbe Interact.® 2008, 21, 799–807. [Google Scholar] [CrossRef]
- Ismail, M.H.; Hussain, A.; Khan, S.A.; Iqbal, A.; Lee, I.-J. An endophytic fungus Aspergillus violaceofuscus can be used as heat stress adaptive tool for Glycine max L. and Helianthus annuus L. J. Appl. Bot. Food Qual. 2020, 93, 112. [Google Scholar]
- Ciríaco da Silva, E.; Nogueira, R.J.; Silva, M.; Albuquerque, M. Drought Stress and Plant Nutrition. Plant. Stress 2010, 5, 32–41. [Google Scholar]
- Amtmann, A.; Blatt, M.R. Regulation of macronutrient transport. New Phytol. 2009, 181, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Tadayyon, A.; Nikneshan, P.; Pessarakli, M. Effects of drought stress on concentration of macro- and micro-nutrients in Castor (Ricinus communis L.) plant. J. Plant. Nutr. 2018, 41, 304–310. [Google Scholar] [CrossRef]
- Kour, D.; Rana, K.L.; Sheikh, I.; Kumar, V.; Yadav, A.N.; Dhaliwal, H.S.; Saxena, A.K. Alleviation of Drought Stress and Plant Growth Promotion by Pseudomonas libanensis EU-LWNA-33, a Drought-Adaptive Phosphorus-Solubilizing Bacterium. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 90, 785–795. [Google Scholar] [CrossRef]
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Tolerance to drought and salt stress in plants: Unraveling the signaling networks. Front. Plant. Sci. 2014, 5, 151. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Cruz De Carvalho, M.H.; Torres-Jerez, I.; Kang, Y.; Allen, S.N.; Huhman, D.V.; Tang, Y.; Murray, J.; Sumner, L.W.; Udvardi, M.K. Global reprogramming of transcription and metabolism in M edicago truncatula during progressive drought and after rewatering. Plant Cell Environ. 2014, 37, 2553–2576. [Google Scholar] [CrossRef]
- Wang, X.; Cai, X.; Xu, C.; Wang, Q.; Dai, S. Drought-responsive mechanisms in plant leaves revealed by proteomics. Int. J. Mol. Sci. 2016, 17, 1706. [Google Scholar] [CrossRef] [PubMed]
- Hamayun, M.; Hussain, A.; Khan, S.A.; Irshad, M.; Khan, A.L.; Waqas, M.; Shahzad, R.; Iqbal, A.; Ullah, N.; Rehman, G.; et al. Kinetin modulates physio-hormonal attributes and isoflavone contents of Soybean grown under salinity stress. Front. Plant. Sci. 2015, 6, 377. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Adhikari, A.; Jan, R.; Ali, S.; Imran, M.; Kim, K.-M.; Lee, I.-J. Halotolerant Rhizobacterial Strains Mitigate the Adverse Effects of NaCl Stress in Soybean Seedlings. BioMed Res. Int. 2019, 2019, 1–15. [Google Scholar] [CrossRef]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Ullah, I.; Ali, S.; Kang, S.-M.; Lee, I.-J. Alleviation of salt stress response in soybean plants with the endophytic bacterial isolate Curtobacterium sp. SAK1. Ann. Microbiol. 2019, 69, 797–808. [Google Scholar] [CrossRef]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant. Sci. 2016, 7, 571. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, R.; Bhalothia, P.; Bansal, P.; Basantani, M.K.; Bharti, V.; Mehrotra, S. Abscisic acid and abiotic stress tolerance–Different tiers of regulation. J. Plant. Physiol. 2014, 171, 486–496. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.-S. Response of plants to water stress. Front. Plant. Sci. 2014, 5, 86. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.L.; Waqas, M.; Hamayun, M.; Al-Harrasi, A.; Al-Rawahi, A.; Lee, I.-J. Co-synergism of endophyte Penicillium resedanum LK6 with salicylic acid helped Capsicum annuumin biomass recovery and osmotic stress mitigation. BMC Microbiol. 2013, 13, 51. [Google Scholar] [CrossRef]
- Timmusk, S.; Abd El-Daim, I.A.; Copolovici, L.; Tanilas, T.; Kännaste, A.; Behers, L.; Nevo, E.; Seisenbaeva, G.; Stenström, E.; Niinemets, Ü. Drought-Tolerance of Wheat Improved by Rhizosphere Bacteria from Harsh Environments: Enhanced Biomass Production and Reduced Emissions of Stress Volatiles. PLoS ONE 2014, 9, e96086. [Google Scholar] [CrossRef]
- Asaf, S.; Khan, A.; Khan, M.; Imran, Q.M.; Yun, B.-W.; Lee, I.-J. Osmoprotective functions conferred to soybean plants via inoculation with Sphingomonas sp. LK11 and exogenous trehalose. Microbiol. Res. 2017, 205, 135–145. [Google Scholar] [CrossRef]
- Timmusk, S.; Behers, L.; Muthoni, J.; Muraya, A.; Aronsson, A.-C. Perspectives and challenges of microbial application for crop improvement. Front. Plant. Sci. 2017, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Glick, B.R.; Bashan, Y.; Ryu, C.M. Enhancement of plant drought tolerance by microbes. In Plant responses to drought stress; Springer: Berlin/Heidelberg, Germany, 2012; pp. 383–413. [Google Scholar]
- Bae, H.; Sicher, R.C.; Kim, M.S.; Kim, S.-H.; Strem, M.D.; Melnick, R.L.; Bailey, B.A. The beneficial endophyte Trichoderma hamatum isolate DIS 219b promotes growth and delays the onset of the drought response in Theobroma cacao. J. Exp. Bot. 2009, 60, 3279–3295. [Google Scholar] [CrossRef]
- Leontidou, K.; Genitsaris, S.; Papadopoulou, A.; Kamou, N.; Bosmali, I.; Matsi, T.; Madesis, P.; Vokou, D.; Karamanoli, K.; Mellidou, I. Plant growth promoting rhizobacteria isolated from halophytes and drought-tolerant plants: Genomic characterisation and exploration of phyto-beneficial traits. Sci. Rep. 2020, 10, 14857. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, M.V.; Burity, H.A.; Martinez, C.R.; Chanway, C.P. Alleviation of drought stress in the common bean (Phaseolus vulgaris L.) by co-inoculation with Paenibacillus polymyxa and Rhizobium tropici. Appl. Soil Ecol. 2008, 40, 182–188. [Google Scholar] [CrossRef]
- Kang, S.M.; Adhikari, A.; Lee, K.E.; Khan, M.A.; Khan, A.L.; Shahzad, R.; Dhungana, S.K.; Lee, I.J. Inoculation with Indole-3-Acetic Acid-Producing Rhizospheric Rhodobacter sphaeroides KE149 Augments Growth of Adzuki Bean Plants Under Water Stress. J. Microbiol. Biotechnol. 2020, 30, 717–725. [Google Scholar] [CrossRef]
- Hamayun, M.; Hussain, A.; Khan, S.A.; Kim, H.-Y.; Khan, A.L.; Waqas, M.; Irshad, M.; Iqbal, A.; Rehman, G.; Jan, S.; et al. Gibberellins Producing Endophytic Fungus Porostereum spadiceum AGH786 Rescues Growth of Salt Affected Soybean. Front. Microbiol. 2017, 8, 686. [Google Scholar] [CrossRef]
- Kour, D.; Rana, K.L.; Yadav, A.N.; Yadav, N.; Kumar, V.; Kumar, A.; Sayyed, R.Z.; Hesham, A.E.-L.; Dhaliwal, H.S.; Saxena, A.K. Drought-Tolerant Phosphorus-Solubilizing Microbes: Biodiversity and Biotechnological Applications for Alleviation of Drought Stress in Plants. In Plant Growth Promoting Rhizobacteria for Sustainable Stress Management: Volume 1: Rhizobacteria in Abiotic Stress Management; Sayyed, R.Z., Arora, N.K., Reddy, M.S., Eds.; Springer: Singapore, 2019; pp. 255–308. [Google Scholar]
- Abid, M.; Mansour, E.; Yahia, L.B.; Bachar, K.; Ben Khaled, A.; Ferchichi, A. Alfalfa nutritive quality as influenced by drought in South-Eastern Oasis of Tunisia. Ital. J. Anim. Sci. 2016, 15, 334–342. [Google Scholar] [CrossRef]
- Annicchiarico, P.; Barrett, B.; Brummer, E.C.; Julier, B.; Marshall, A.H. Achievements and Challenges in Improving Temperate Perennial Forage Legumes. Crit. Rev. Plant. Sci. 2015, 34, 327–380. [Google Scholar] [CrossRef]
- Putnam, D.; Russelle, M.; Orloff, S.; Kuhn, J.; Fitzhugh, L.; Godfrey, L.; Kiess, A.; Long, R. Alfalfa Wildlife and the Environment—The Importance and Benefits of Alfalfa in the 21st Century. CA Forage Assoc. Novato CA. 2001, 1–24. Available online: http://agric.ucdavis.edu/files/242006.pdf (accessed on 23 May 2021).
- Kumar, T.; Bao, A.-K.; Bao, Z.; Wang, F.; Gao, L.; Wang, S.-M. The progress of genetic improvement in alfalfa (Medicago sativa L.). Czech. J. Genet. Plant. Breed. 2018, 54, 41–51. [Google Scholar] [CrossRef]
- Jia, H.; Wang, X.; Shi, Y.; Wu, X.; Wang, Y.; Liu, J.; Fang, Z.; Li, C.; Dong, K. Overexpression of Medicago sativa LEA4-4 can improve the salt, drought, and oxidation resistance of transgenic Arabidopsis. PLoS ONE 2020, 15, e0234085. [Google Scholar] [CrossRef]
- Zheng, G.; Fan, C.; Di, S.; Wang, X.; Xiang, C.; Pang, Y. Over-Expression of Arabidopsis EDT1 Gene Confers Drought Tolerance in Alfalfa (Medicago sativa L.). Front. Plant. Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Bouizgaren, A.; Farissi, M.; Ghoulam, C.; Kallida, R.; Faghire, M.; Barakate, M.; Al Feddy, M.N. Assessment of summer drought tolerance variability in Mediterranean alfalfa (Medicago sativa L.) cultivars under Moroccan fields conditions. Arch. Agron. Soil Sci. 2013, 59, 147–160. [Google Scholar] [CrossRef]
- Rao, S.; Qayyum, A.; Razzaq, A.; Ahmad, M.; Mahmood, I.; Sher, A. Role of foliar application of salicylic acid and L-tryptophan in drought tolerance of maize. J. Anim. Plant. Sci. 2012, 22, 768–772. [Google Scholar]
- Tariq, A.; Pan, K.; Olatunji, O.A.; Graciano, C.; Li, Z.; Sun, F.; Zhang, L.; Wu, X.; Chen, W.; Song, D.; et al. Phosphorous fertilization alleviates drought effects on Alnus cremastogyne by regulating its antioxidant and osmotic potential. Sci. Rep. 2018, 8, 5644. [Google Scholar] [CrossRef] [PubMed]
- Usmani, M.M.; Nawaz, F.; Majeed, S.; Shehzad, M.A.; Ahmad, K.S.; Akhtar, G.; Aqib, M.; Shabbir, R.N. Sulfate-mediated Drought Tolerance in Maize Involves Regulation at Physiological and Biochemical Levels. Sci. Rep. 2020, 10, 1147. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-M.; Khan, A.L.; Waqas, M.; You, Y.-H.; Kim, J.-H.; Kim, J.-G.; Hamayun, M.; Lee, I.-J. Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J. Plant. Interact. 2014, 9, 673–682. [Google Scholar] [CrossRef]
- Bharti, N.; Yadav, D.; Barnawal, D.; Maji, D.; Kalra, A. Exiguobacterium oxidotolerans, a halotolerant plant growth promoting rhizobacteria, improves yield and content of secondary metabolites in Bacopa monnieri (L.) Pennell under primary and secondary salt stress. World J. Microbiol. Biotechnol. 2013, 29, 379–387. [Google Scholar] [CrossRef]
- Cakmakci, R.; Dönmez, M.F.; Erdoğan, Ü. The effect of plant growth promoting rhizobacteria on barley seedling growth, nutrient uptake, some soil properties, and bacterial counts. Turk. J. Agric. For. 2007, 31, 189–199. [Google Scholar]
- Kang, S.-M.; Hamayun, M.; Joo, G.-J.; Khan, A.L.; Kim, Y.-H.; Kim, S.-K.; Jeong, H.-J.; Lee, I.-J. Effect of Burkholderia sp. KCTC 11096BP on some physiochemical attributes of cucumber. Eur. J. Soil Biol. 2010, 46, 264–268. [Google Scholar] [CrossRef]
- Khan, M.A.; Ullah, I.; Waqas, M.; Hamayun, M.; Khan, A.L.; Asaf, S.; Kang, S.-M.; Kim, K.-M.; Jan, R.; Lee, I.-J. Halo-tolerant rhizospheric Arthrobacter woluwensis AK1 mitigates salt stress and induces physio-hormonal changes and expression of GmST1 and GmLAX3 in soybean. Symbiosis 2019, 77, 9–21. [Google Scholar] [CrossRef]
- Kang, S.-M.; Asaf, S.; Khan, A.L.; Khan, A.; Mun, B.-G.; Khan, M.A.; Gul, H.; Lee, I.-J. Complete Genome Sequence of Pseudomonas psychrotolerans CS51, a Plant Growth-Promoting Bacterium, Under Heavy Metal Stress Conditions. Microorganisms 2020, 8, 382. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, A.; Khan, M.A.; Lee, K.E.; Kang, S.M.; Dhungana, S.K.; Bhusal, N.; Lee, I.J. The Halotolerant Rhizobacterium-Pseudomonas koreensis MU2 Enhances Inorganic Silicon and Phosphorus Use Efficiency and Augments Salt Stress Tolerance in Soybean (Glycine max L.). Microorganisms 2020, 8, 1256. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, A.; Lee, K.-E.; Khan, M.A.; Kang, S.-M.; Adhikari, B.; Imran, M.; Jan, R.; Kim, K.-M.; Lee, I.-J. Effect of Silicate and Phosphate Solubilizing Rhizobacterium Enterobacter ludwigii GAK2 on Oryza sativa L. under Cadmium Stress. J. Microbiol. Biotechnol. 2020, 30, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Radhakrishnan, R.; You, Y.H.; Lee, K.E.; Kim, J.; Joo, G.J.; Kim, J.G.; Lee, I. Mustard and Chinese cabbage plant growth promotion by optimal-medium-cultured acinetobacter calcoaceticus SE370. J. Pure Appl. Microbiol. 2016, 10, 1693–1699. [Google Scholar]
- Kang, S.M.; Radhakrishnan, R.; You, Y.H.; Joo, G.J.; Lee, I.J.; Lee, K.E.; Kim, J.H. Phosphate Solubilizing Bacillus megaterium mj1212 Regulates Endogenous Plant Carbohydrates and Amino Acids Contents to Promote Mustard Plant Growth. Indian J. Microbiol. 2014, 54, 427–433. [Google Scholar] [CrossRef]
- Khan, M.A.; Sahile, A.A.; Jan, R.; Asaf, S.; Hamayun, M.; Imran, M.; Adhikari, A.; Kang, S.-M.; Kim, K.-M.; Lee, I.-J. Halotolerant bacteria mitigate the effects of salinity stress on soybean growth by regulating secondary metabolites and molecular responses. BMC Plant. Biol. 2021, 21, 176. [Google Scholar] [CrossRef] [PubMed]
- Asaf, S.; Hamayun, M.; Khan, A.L.; Waqas, M.; Khan, M.A.; Jan, R.; Lee, I.-J.; Hussain, A. Salt tolerance of Glycine max.L induced by endophytic fungus Aspergillus flavus CSH1, via regulating its endogenous hormones and antioxidative system. Plant. Physiol. Biochem. 2018, 128, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Hamayun, M.; Iqbal, A.; Khan, S.A.; Hussain, A.; Asaf, S.; Khan, A.L.; Yun, B.-W.; Lee, I.-J. Gibberellin application ameliorates the adverse impact of short-term flooding on Glycine max L. Biochem. J. 2018, 475, 2893–2905. [Google Scholar] [CrossRef]
- Asaf, S.; Khan, M.; Khan, A.; Waqas, M.; Shahzad, R.; Kim, A.-Y.; Kang, S.-M.; Lee, I.-J. Bacterial endophytes from arid land plants regulate endogenous hormone content and promote growth in crop plants: An example of Sphingomonas sp. and Serratia marcescens. J. Plant. Interact. 2017, 12, 31–38. [Google Scholar] [CrossRef]
- Adhikari, B.; Dhungana, S.K.; Ali, M.W.; Adhikari, A.; Kim, I.-D.; Shin, D.-H. Resveratrol, total phenolic and flavonoid contents, and antioxidant potential of seeds and sprouts of Korean peanuts. Food Sci. Biotechnol. 2018, 27, 1275–1284. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Kang, S.-M.; Shahzad, R.; Khan, M.A.; Hasnain, Z.; Lee, K.-E.; Park, H.-S.; Kim, L.-R.; Lee, I.-J. Ameliorative effect of indole-3-acetic acid- and siderophore-producing Leclercia adecarboxylata MO1 on cucumber plants under zinc stress. J. Plant. Interact. 2021, 16, 30–41. [Google Scholar] [CrossRef]
- Marulanda, A.; Azcon, R.; Ruiz-Lozano, J.M. Contribution of six arbuscular mycorrhizal fungal isolates to water uptake by Lactuca sativa plants under drought stress. Physiol. Plant. 2003, 119, 526–533. [Google Scholar] [CrossRef]
- Kusaka, M.; Lalusin, A.G.; Fujimura, T. The maintenance of growth and turgor in pearl millet (Pennisetum glaucum [L.] Leeke) cultivars with different root structures and osmo-regulation under drought stress. Plant. Sci. 2005, 168, 1–14. [Google Scholar] [CrossRef]
- Chimenti, C.A.; Marcantonio, M.; Hall, A. Divergent selection for osmotic adjustment results in improved drought tolerance in maize (Zea mays L.) in both early growth and flowering phases. Field Crop. Res. 2006, 95, 305–315. [Google Scholar] [CrossRef]
- Erice, G.; Louahlia, S.; Irigoyen, J.J.; Sanchez-Diaz, M.; Avice, J.-C. Biomass partitioning, morphology and water status of four alfalfa genotypes submitted to progressive drought and subsequent recovery. J. Plant Physiol. 2010, 167, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-J.; Yang, W.; Wang, C.; Gu, C.; Niu, D.-D.; Liu, H.-X.; Wang, Y.-P.; Guo, J.-H. Induction of Drought Tolerance in Cucumber Plants by a Consortium of Three Plant Growth-Promoting Rhizobacterium Strains. PLoS ONE 2012, 7, e52565. [Google Scholar] [CrossRef]
- Mayak, S.; Tirosh, T.; Glick, B.R. Plant growth-promoting bacteria that confer resistance to water stress in tomatoes and peppers. Plant. Sci. 2004, 166, 525–530. [Google Scholar] [CrossRef]
- Vardharajula, S.; Zulfikar Ali, S.; Grover, M.; Reddy, G.; Bandi, V. Drought-tolerant plant growth promoting Bacillus spp.: Effect on growth, osmolytes, and antioxidant status of maize under drought stress. J. Plant. Interact. 2011, 6, 1–14. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The Critical Role of Potassium in Plant Stress Response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, U.; Çakmakçi, R.; Varmazyarı, A.; Turan, M.; Erdogan, Y.; Kıtır, N. Role of inoculation with multi-trait rhizobacteria on strawberries under water deficit stress. Zemdirb. Agric. 2016, 103, 67–76. [Google Scholar] [CrossRef]
- Armada, E.; Leite, M.F.A.; Medina, A.; Azcón, R.; Kuramae, E.E. Native bacteria promote plant growth under drought stress condition without impacting the rhizomicrobiome. FEMS Microbiol. Ecol. 2018, 94. [Google Scholar] [CrossRef]
- Abass, M.; Moradtalab, N.; Abd-Allah, E.; Ahmad, P.; Hajiboland, R. Plant growth under drought stress: Significance of mineral nutrients. Water Stress Crop. Plants Sustain. Approach 2016, 2, 649–668. [Google Scholar]
- Slamka, P.; Krček, M.; Golisová, A. Concentration of magnesium and its uptake by aboveground phytomass of spring barley (Hordeum vulgare L.) grown under drought stress condition. Res. J. Agric. Sci. 2011, 43, 198–205. [Google Scholar]
- Kim, T.-H.; Böhmer, M.; Hu, H.; Nishimura, N.; Schroeder, J.I. Guard cell signal transduction network: Advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu. Rev. Plant. Biol. 2010, 61, 561–591. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Adhikari, A.; Jan, R.; Ali, S.; Imran, M.; Kim, K.M.; Lee, I.J. Plant growth-promoting endophytic bacteria augment growth and salinity tolerance in rice plants. Plant. Biol. 2020, 22, 850–862. [Google Scholar] [CrossRef] [PubMed]
- Roger, M.J.R. Handbook of Plant Ecophysiology Techniques; Springer, Kluwer Academic Publishers: Berlin/Heidelberg, Germany, 2001; pp. 207–212. [Google Scholar]
- Khan, A.L.; Hamayun, M.; Ahmad, N.; Waqas, M.; Kang, S.-M.; Kim, Y.-H.; Lee, I.-J. Exophiala sp. LHL08 reprograms Cucumis sativus to higher growth under abiotic stresses. Physiol. Plant. 2011, 143, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Porcel, R.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal influence on leaf water potential, solute accumulation, and oxidative stress in soybean plants subjected to drought stress. J. Exp. Bot. 2004, 55, 1743–1750. [Google Scholar] [CrossRef]
- Khalvati, M.; Bartha, B.; Dupigny, A.; Schröder, P. Arbuscular mycorrhizal association is beneficial for growth and detoxification of xenobiotics of barley under drought stress. J. Soils Sediments 2010, 10, 54–64. [Google Scholar] [CrossRef]
- Cho, S.M.; Kang, B.R.; Han, S.H.; Anderson, A.J.; Park, J.-Y.; Lee, Y.-H.; Cho, B.H.; Yang, K.-Y.; Ryu, C.-M.; Kim, Y.C. 2R, 3R-butanediol, a bacterial volatile produced by Pseudomonas chlororaphis O6, is involved in induction of systemic tolerance to drought in Arabidopsis thaliana. Mol. Plant.-Microbe Interact. 2008, 21, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Zhu, J.K. Regulation of abscisic acid biosynthesis. Plant Physiol. 2003, 133, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-M.; Khan, A.L.; Waqas, M.; Asaf, S.; Lee, K.-E.; Park, Y.-G.; Kim, A.-Y.; Khan, M.A.; You, Y.-H.; Lee, I.-J. Integrated phytohormone production by the plant growth-promoting rhizobacterium Bacillus tequilensis SSB07 induced thermotolerance in soybean. J. Plant. Interact. 2019, 14, 416–423. [Google Scholar] [CrossRef]
- Kang, S.-M.; Shahzad, R.; Bilal, S.; Khan, A.L.; Park, Y.-G.; Lee, K.-E.; Asaf, S.; Khan, M.A.; Lee, I.-J. Indole-3-acetic-acid and ACC deaminase producing Leclercia adecarboxylata MO1 improves Solanum lycopersicum L. growth and salinity stress tolerance by endogenous secondary metabolites regulation. BMC Microbiol. 2019, 19, 80. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant. Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Waqas, M.; Khan, M.A.; Lee, I.-J.; Kim, W.-C. Exogenous ascorbic acid mitigates flood stress damages of Vigna angularis. Appl. Biol. Chem. 2017, 60, 603–614. [Google Scholar] [CrossRef]
- Mastouri, F.; Björkman, T.; Harman, G.E. Trichoderma harzianum enhances antioxidant defense of tomato seedlings and resistance to water deficit. Mol. Plant.-Microbe Interact. MPMI 2012, 25, 1264–1271. [Google Scholar] [CrossRef]
- Habib, S.H.; Kausar, H.; Saud, H.M. Plant Growth-Promoting Rhizobacteria Enhance Salinity Stress Tolerance in Okra through ROS-Scavenging Enzymes. Biomed. Res. Int. 2016, 2016, 6284547. [Google Scholar] [CrossRef]
- Saikia, J.; Sarma, R.K.; Dhandia, R.; Yadav, A.; Bharali, R.; Gupta, V.K.; Saikia, R. Alleviation of drought stress in pulse crops with ACC deaminase producing rhizobacteria isolated from acidic soil of Northeast India. Sci. Rep. 2018, 8, 3560. [Google Scholar] [CrossRef]
- Tiepo, A.N.; Constantino, L.V.; Madeira, T.B.; Gonçalves, L.S.A.; Pimenta, J.A.; Bianchini, E.; de Oliveira, A.L.M.; Oliveira, H.C.; Stolf-Moreira, R. Plant growth-promoting bacteria improve leaf antioxidant metabolism of drought-stressed Neotropical trees. Planta 2020, 251, 83. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A. Exopolysaccharide producing rhizobacteria and their impact on growth and drought tolerance of wheat grown under rainfed conditions. PLoS ONE 2019, 14, e0222302. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.P.; Singh, V.; Gupta, V.K.; Shukla, R.; Prabha, R.; Sarma, B.K.; Patel, J.S. Microbial inoculation in rice regulates antioxidative reactions and defense related genes to mitigate drought stress. Sci. Rep. 2020, 10, 4818. [Google Scholar] [CrossRef] [PubMed]
- Asghari, B.; Khademian, R.; Sedaghati, B. Plant growth promoting rhizobacteria (PGPR) confer drought resistance and stimulate biosynthesis of secondary metabolites in pennyroyal (Mentha pulegium L.) under water shortage condition. Sci. Hortic. 2020, 263, 109132. [Google Scholar] [CrossRef]
- Mirzaei, M.; Ladan Moghadam, A.; Hakimi, L.; Danaee, E. Plant growth promoting rhizobacteria (PGPR) improve plant growth, antioxidant capacity, and essential oil properties of lemongrass (Cymbopogon citratus) under water stress. Iran. J. Plant. Physiol. 2020, 10, 3155–3166. [Google Scholar]

| Shoot Length (cm) | Root Length (cm) | Shoot Weight (g) | Root Weight (g) | Stalk Diameter (mm) | |
|---|---|---|---|---|---|
| Control | |||||
| Control | 20.9 ± 1.64a | 16.46 ± 0.53b | 1.33 ± 0.04b | 0.51 ± 0.025b | 2.46 ± 0.029b |
| MJ1212 | 23.6 ± 0.61a | 18.08 ± 0.61b | 1.81 ± 0.06b | 0.80 ± 0.026b | 2.88 ± 0.024b |
| AFFR02 | 22.9 ± 1.12a | 18.42 ± 0.37a | 1.73 ± 0.18a | 0.67 ± 0.028a | 2.78 ± 0.037a |
| Post-drought Stress | |||||
| Control | 15.7 ± 0.33b | 10.75 ± 0.39b | 0.67 ± 0.032c | 0.25 ± 0.03b | 1.16 ± 0.024c |
| MJ1212 | 17.92 ± 0.86a | 13.4 ± 0.25b | 1.03 ± 0.018b | 0.43 ± 0.01b | 2.08 ± 0.029b |
| AFFR02 | 17.82 ± 0.82a | 14.5 ± 0.21a | 1.08 ± 0.016a | 0. 37 ± 0.01a | 2.52 ± 0.028a |
| Chlorophyll (SPAD) | Chl a | Chl b | CART | |
|---|---|---|---|---|
| Control | ||||
| Control | 37.5 ± 1.43b | 26.1 ± 0.96b | 22.04 ± 0.90b | 1.1 ± 0.06b |
| MJ1212 | 39.1 ± 1.16a | 31.1 ± 0.54a | 27.06 ± 0.54a | 1.18 ± 0.02b |
| AFFR02 | 39.8 ± 1.76a | 31.7 ± 0.96a | 27.7 ± 0.35a | 1.3 ± 0.04a |
| Post-Drought Stress | ||||
| Control | 30.3 ± 0.61c | 14.08 ± 0.88b | 12.08 ± 0.51b | 0.51 ± 0.07b |
| MJ1212 | 35.4 ± 1.71b | 17.1 ± 0.63a | 15.7 ± 0.57a | 0.89 ± 0.08a |
| AFFR02 | 37.1 ± 1.72a | 17.8 ± 0.37a | 15.86 ± 0.06a | 0.82 ± 0.06a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.-M.; Khan, M.-A.; Hamayun, M.; Kim, L.-R.; Kwon, E.-H.; Kang, Y.-S.; Kim, K.-Y.; Park, J.-J.; Lee, I.-J. Phosphate-Solubilizing Enterobacter ludwigii AFFR02 and Bacillus megaterium Mj1212 Rescues Alfalfa’s Growth under Post-Drought Stress. Agriculture 2021, 11, 485. https://doi.org/10.3390/agriculture11060485
Kang S-M, Khan M-A, Hamayun M, Kim L-R, Kwon E-H, Kang Y-S, Kim K-Y, Park J-J, Lee I-J. Phosphate-Solubilizing Enterobacter ludwigii AFFR02 and Bacillus megaterium Mj1212 Rescues Alfalfa’s Growth under Post-Drought Stress. Agriculture. 2021; 11(6):485. https://doi.org/10.3390/agriculture11060485
Chicago/Turabian StyleKang, Sang-Mo, Muhammad-Aaqil Khan, Muhammad Hamayun, Lee-Rang Kim, Eun-Hae Kwon, Yo-Sep Kang, Ki-Yong Kim, Jae-Jeong Park, and In-Jung Lee. 2021. "Phosphate-Solubilizing Enterobacter ludwigii AFFR02 and Bacillus megaterium Mj1212 Rescues Alfalfa’s Growth under Post-Drought Stress" Agriculture 11, no. 6: 485. https://doi.org/10.3390/agriculture11060485
APA StyleKang, S.-M., Khan, M.-A., Hamayun, M., Kim, L.-R., Kwon, E.-H., Kang, Y.-S., Kim, K.-Y., Park, J.-J., & Lee, I.-J. (2021). Phosphate-Solubilizing Enterobacter ludwigii AFFR02 and Bacillus megaterium Mj1212 Rescues Alfalfa’s Growth under Post-Drought Stress. Agriculture, 11(6), 485. https://doi.org/10.3390/agriculture11060485






