Abstract
(1) Background: Root traits play important roles in acclimating to water and phosphorus (P) shortages. However, the relative importance of root size and efficiency under these conditions is unknown. (2) Methods: This study investigated the role of root size and efficiency in acclimating to water- and P-limited environments. Three soybean genotypes with contrasting root sizes were grown in tall cylindrical pots to compare grain yield, root density, and water- and nutrient-uptake efficiencies under two water (well-watered and water-stressed) and three P levels (0 (P0), 60 (P60), and 120 (P120) mg P kg−1 dry soil). (3) Results: Water or P deficit, and combined water and P deficit significantly decreased grain yield, which was associated with greater P uptake per unit root dry weight (DW) under water stress. The genotype Zhonghuang 30 (ZH) with the greatest water, nitrogen, and P uptakes per unit root DW had the highest grain yield at P60 and P120 under water stress and P0 under well-watered conditions, but ZH had the lowest grain yield at P60 and P120 under well-watered conditions, due to its small root size. (4) Conclusions: High root efficiency—which was correlated with high root density—improved grain yield under P- and water-limited conditions, but restricted yield potential when P and water were not limited.
1. Introduction
Soybean is one of the most important oil crops worldwide and a main source of protein [1]. However, soybean grain yield is limited significantly under water shortage [2,3,4] and low phosphorus (P) availability [5,6]. Soybean yields are under threat with the predicted increase in frequency and severity of droughts [7] and the gradual depletion of global P reserves [8,9]. How soybean crops adapt to water and P availability is an important issue for plant physiologists and agronomists.
Water is absorbed from the soil by plant roots. Increasing root size, as measured by root dry weight (DW), can increase grain yield under water stress by improving soil water uptake, especially in rainfed areas where crop yields depend on stored soil water. Increasing root DW and/or length in the subsoil layer can increase grain yield [10,11,12,13]. However, in earlier glasshouse and field experiments, soybean genotypes with less root DW produced higher grain yields under water stress than those with more root DW [2,3]. Thus the role of root size in yield performance is needed to be determined.
Topsoil foraging, as proposed by Lynch and Brown [14], suggests that root traits play important roles in P uptake from the soil when soil P availability is low. More crown roots and greater lateral root density may enable more P capture and higher yields under P-limited conditions [15,16,17]. Adventitious (hypocotyl-borne) roots can increase P absorption and yield performance in soils with low P availability, due to their shallow root growth angle and low metabolic cost [18,19]. Increasing root DW may increase P uptake under P-limited conditions [20,21]. The nutrient-uptake efficiency of roots, or nutrient uptake per unit root DW, plays an important role in the response of plants to nutrient-limited conditions [21,22]. Phosphorus deficit can increase root P-uptake efficiency [22]. Higher root P-uptake efficiency means more P capture with the same root DW. In their study on rice, Mori et al. [21] proposed that root efficiency represents a new adaptive mechanism in soils with low P availability.
Both root size and morphology traits, such as the presence of adventitious roots and lateral root density, are important for yield performance under water-limited [2,10,11] or P-limited [14,21] conditions, but the relative contribution of root size or root efficiency to yield when both water and P are limiting, remains unknown. In this study, we compared grain yield, water-use efficiency for grain yield (WUEG), P and N contents, dry matter accumulation and partitioning, root density, and water- and nutrient- (P and N) uptake efficiencies of three soybean genotypes with large, medium, and small roots under water- and P-limited conditions. We investigated (1) the roles of root size and efficiency (water, P, and N) on yield under water- and P-limited conditions, and (2) root traits that contribute to root efficiency under water- and P-limited conditions.
2. Materials and Methods
2.1. Materials and Growing Conditions
A pot-based experiment using a long cylindrical PVC tube (length: 1.05 m; diameter: 0.16 m) was conducted during the 2015 growing season at the Yuzhong Experimental Station (35°51′ N, 104°07′ S, altitude 1620 m) of Lanzhou University in Yuzhong County, Gansu Province, China. The experiment was carried out in a rainout shelter and not repeated. The soil was a mixture of loess soil with vermiculite (v:v = 3:1), which was obtained from a field near the experimental station and had a silty-loam texture, similar to that of an Entisol [3]. Fifty-four cylinders containing soil to a depth of 1.0 m were used. The mixed soil (18.6 kg, 2.0 mg kg−1 plant-available soil P) was added to each cylinder. Phosphorus concentrations of 0 (P0), 60 (P60), or 120 (P120) mg kg−1 dry soil were applied to the top 0–0.4 m soil layer. NH4H2PO4 was used as the P fertilizer, which was mixed with the soil using an end-over-end shaker.
After soil filling and P application, the soil water content (SWC) in the cylinders was maintained at 100% field capacity (FC). Based on our previous study [3], we used genotypes Huandsedadou (HD) with a large root dry weight, Bailudou (BLD) with a medium-sized root dry weight, and Zhonghuang 30 (ZH) with a small root dry weight. Before sowing, the seeds were soaked for 10 min in water containing carbendazim (5 g L−1) to prevent disease. Two seeds were sown in each cylinder and thinned to one seedling after germination. Black plastic film (diameter: 0.17–0.18 m) was placed around the stem of each plant to prevent water evaporation from the soil. Two water treatments were imposed: (1) well-watered (WW), the SWC was maintained between 85–100% FC and (2) drought-rewatered cycle (WS), the water was withheld and rewatered to 100% FC when the SWC was decreased to 30% FC [9]. There were three replicates in each treatment combination.
2.2. Water Use and Yield at Physiological Maturity
In the WW treatment, each cylinder was weighed and watered every four days to maintain SWC at 80–100% FC until soybean maturity (136–147 days after sowing (DAS)), when 95% of the pods were brown [23]. In the WS treatment, cylinders were weighed every 4–5 days until maturity, and re-watered to 100% FC when SWC reached 30% FC. The water use from sowing to physiological maturity was recorded.
At physiological maturity, shoots were severed from the roots at soil level, divided into stems and pods, oven-dried at 80 °C for 48 h, and weighed. Grain yield was determined by weighing the dried seeds. The water-use efficiency of grain yield (WUEG, g L−1) = grain yield/water use. The combined dried leaves, stems, seeds, and hulls were stored in an airtight bin for later determination of N and P concentrations. Shoot dry weight (DW) = leaf DW + stem DW + pod DW.
2.3. Root Harvest and Root Density at Physiological Maturity
After removing the shoots, the polyethylene sleeves were pulled out of the cylinders, and the soil washed away from the roots using a sieve (0.2 mm). Root density was determined using the method described by He et al. [6,9]. The number of adventitious roots and their lengths were recorded. A 50 mm length of root below the first lateral root on the taproot was used to determine lateral root density. Three represented adventitious and lateral roots were chosen and used to determine the adventitious and lateral root branching density, the root number branching from the adventitious and lateral roots was counted within a 50 mm section of root from the base of the adventitious and lateral roots; then the equation, root density = number of lateral roots/root length, was used to determine the adventitious and lateral root branching density. Roots were oven-dried at 80 °C for 48 h after the root density was determined, weighed, and stored under dry conditions before the measurement of N and P concentrations. Water-uptake efficiency (kg g−1 DW) = total water use/root DW; shoot: root ratio (g g−1) = shoot DW/root DW.
2.4. P and N Concentrations
Shoot and root samples were ground to a fine powder using an Ultra Centrifugal Mill (ZM200, Retsch, GmbH, Düsseldorf, Germany). H2O2–H2SO4 was used to digest about 150 mg subsamples. The N and P concentrations of the shoot and root samples were determined using the Kjeldahl method (SKD-800, Shanghai Peiou Analytical Instruments Co. Ltd., Shanghai, China) and the molybdenum–stibium anti-spectrophotometry method (UV-1800 Spectrophotometer, Shanghai Meipuda Instrument Co. Ltd., Shanghai, China), respectively [9]. Shoot and root N and P contents were obtained by multiplying shoot and root DWs with the measured shoot and root N and P concentrations, respectively. P/N uptake efficiency (g g−1) = P/N content in the whole plant/root (DW).
2.5. Statistics
The data shown are means ± standard error. A three-way (water treatment, genotype, and soil P level) analysis of variance (ANOVA) was used to assess the measured parameters using the GenStat 19.0 statistical package (VSN International Ltd., Rothamsted, UK). Principal component analyses (PCA) were conducted by Origin (Pro 2018, OriginLab, Northampton, MA, USA) on grain yield, and shoot and root traits. The linear model was used to fit the curve between root efficiency traits (water uptake per root DW, shoot to root ratio, P and N uptake per root DW) and grain yield, WUEG, and root traits under two water conditions and three P levels.
3. Results
3.1. Grain Yield and Water-Use Efficiency under Water- and P-Limited Conditions
Water and P deficits significantly reduced grain yield, while the addition of P significantly increased grain yield under both WW and WS conditions (Table 1). Genotype ZH exhibited significantly higher grain yields at P60 and P120 under WS and P0 under WW than HD and BLD, but significantly lower grain yields at P60 and P120 under WW conditions (Table 1). Water stress significantly increased WUEG from 0.47 to 0.53, and ZH had significantly higher WUEG than HD and BLD in all treatment combinations (Table 1). The response of WUEG to P addition in all water treatments was dependent on soybean genotype; P addition significantly increased WUEG (P0 = 0.72, P60 = 0.80. P120 = 0.84) in ZH. Under WW conditions, P addition significantly increased WUEG in HD and BLD, but reduced WUEG in ZH (Table 1).

Table 1.
The grain yield (g plant−1), water use efficiency for grain yield (WUEG; g kg−1), shoot dry weight (DW, g plant−1), root DW (g plant−1), and shoot to root ratio (SRR, g g−1) at maturity of three soybean genotypes (Huandsedadou (HD), Bailudou (BLD), and Zhonghuang 30 (ZH)) exposed to three P concentrations (0 (P0), 60 (P60) and 120 (P120) mg P kg−1 dry soil) under water-stressed (WS) and well-watered (WW) conditions. n.s. not significant, * p < 0.05, ** p < 0.01, and *** p < 0.001. Values are means (n = 3). The values in parenthesis are LSD at p = 0.05.
3.2. Shoot to Root Ratio, Water- and Nutrient-Uptake Efficiencies and Root Density under Water and P Deficits
Shoot and root DW significantly decreased under water stress, and the addition of P significantly increased the shoot to root ratio and shoot and root DWs (Table 1). Genotype ZH produced the lowest root and shoot DWs, and HD produced the highest in all treatment combinations (Table 1). Across the P rates and genotypes, water stress significantly increased the shoot to root ratio (5.7 under WS and 4.7 under WW conditions). The shoot to root ratio varied between genotypes; across all treatment combinations, the mean shoot to root ratio was highest for ZH (6.6), mid-range for BLD (4.8), and lowest for HD (4.2) (Table 1).
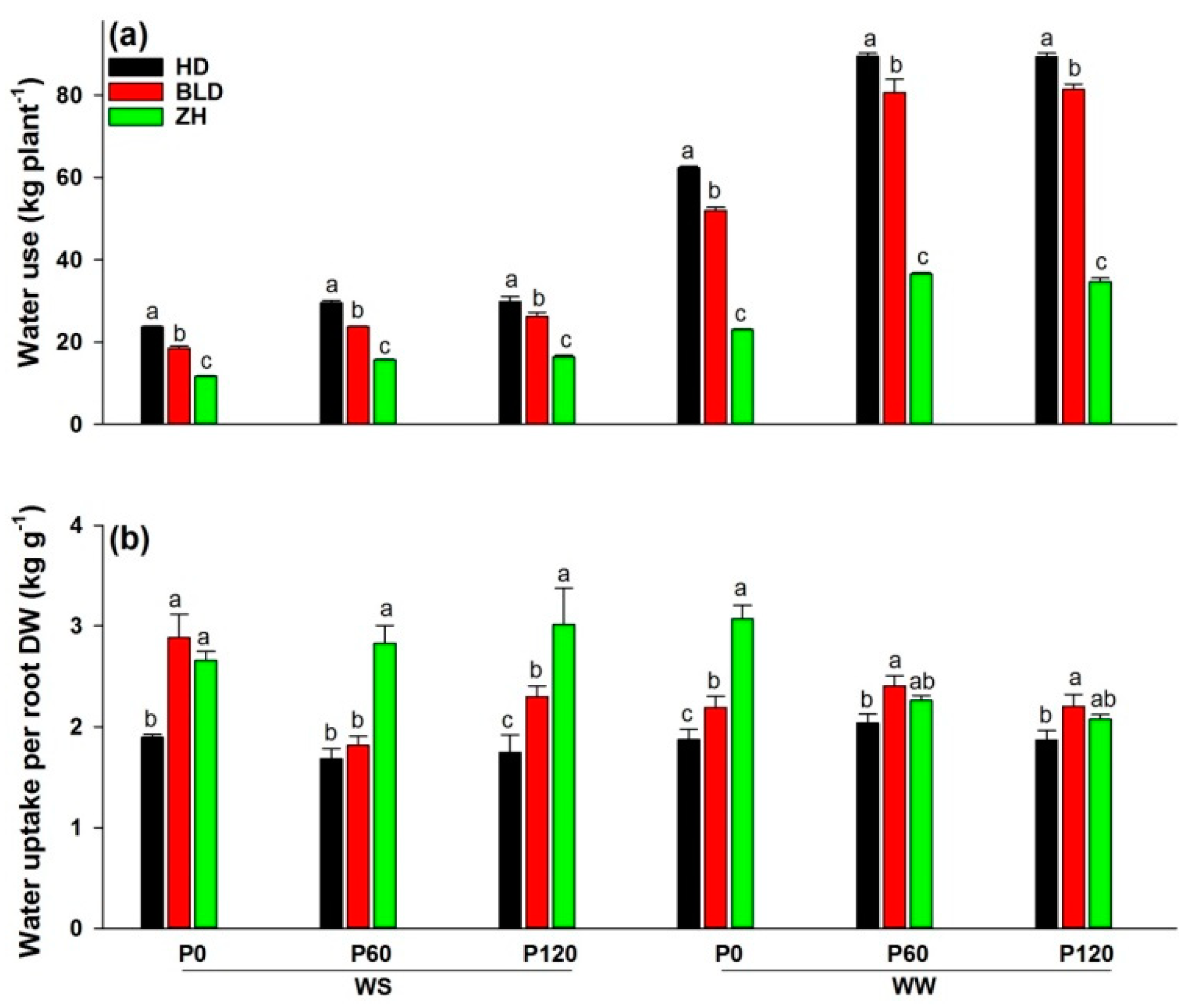
Both water and P deficit significantly reduced water use in soybean, and P addition significantly increased water use under WS and WW conditions; ZH had the lowest water use per plant and HD the highest in all treatment combinations (Figure 1a). Water uptake per unit root DW, or water-uptake efficiency, differed between genotypes. Genotype ZH had a significantly higher mean water-uptake efficiency than HD and BLD (ZH = 2.7, HD = 1.9, and BLD = 2.3; Table 1). Moreover, HD had the lowest water-uptake efficiency in all treatment combinations, and ZH had the highest at P60 and P120 under WS and P0 under WW conditions (Figure 1b).
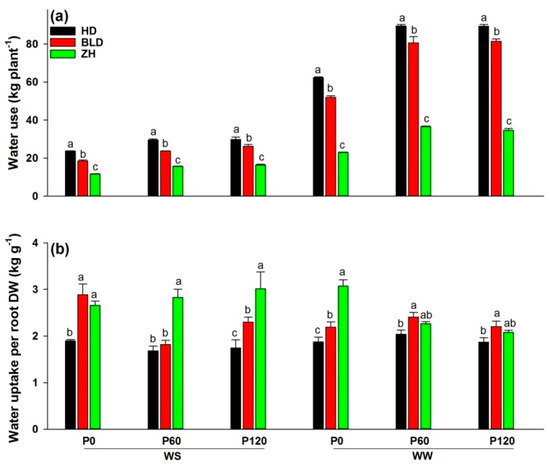
Figure 1.
(a) Water use (kg plant−1) and (b) water uptake per root DW (kg g−1) at maturity of three soybean genotypes (Huandsedadou (HD), Bailudou (BLD), and Zhonghuang 30 (ZH)) exposed to three P concentrations (0 (P0), 60 (P60) and 120 (P120) mg P kg−1 dry soil) under water-stressed (WS) and well-watered (WW) conditions. Means (n = 3) with different letters in any one set of three genotypes differ significantly at p = 0.05.
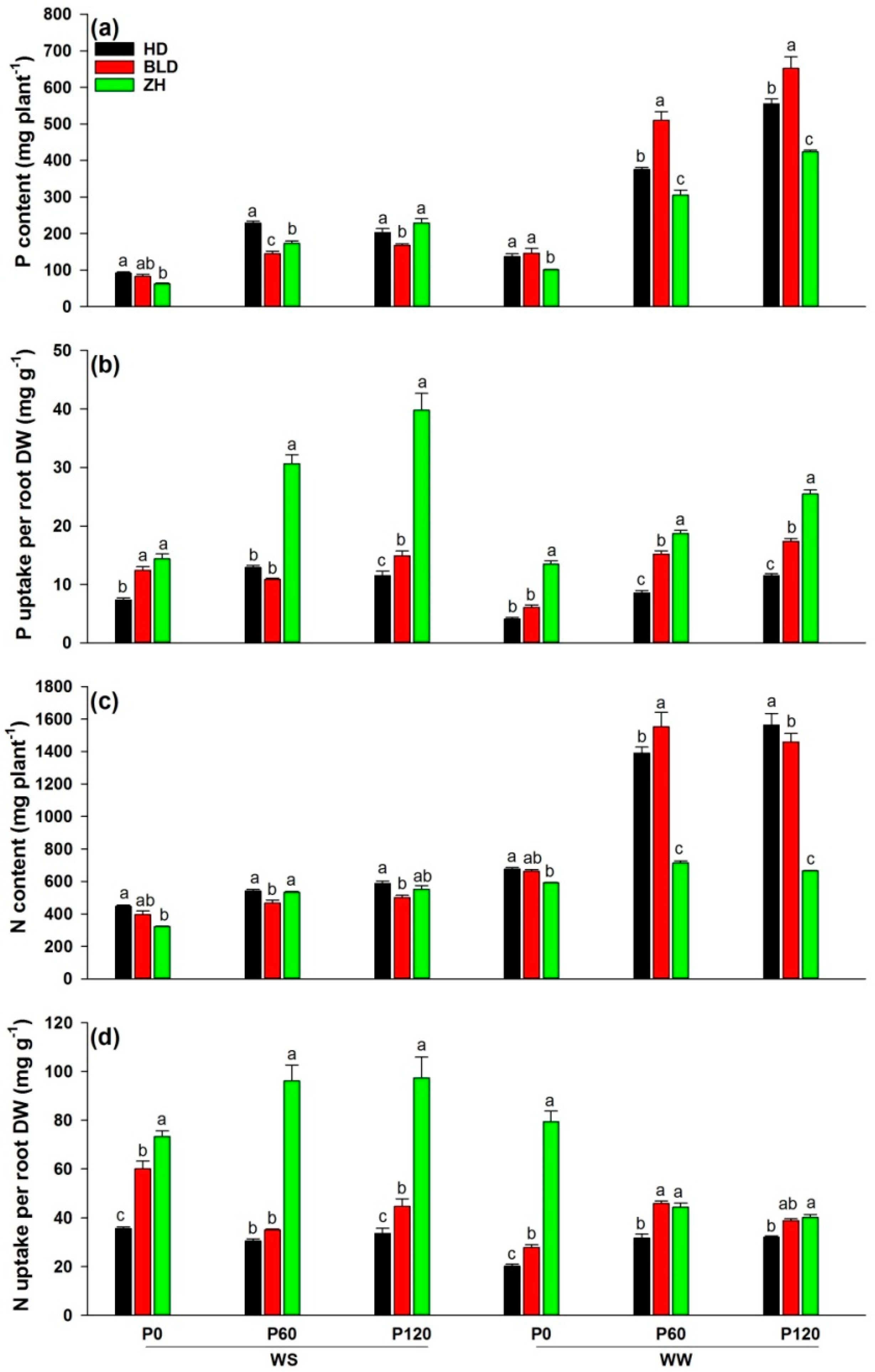
Although water and P deficits significantly reduced P and N contents, the addition of P significantly increased P and N contents under WW and WS conditions. Genotype ZH had the lowest P and N contents at P0 under WS and at three P levels under WW conditions (Table 2; Figure 2a,c). In all treatment combinations, ZH had the highest (mean = 23.8 for P and 71.7 for N) and HD had the lowest (mean = 9.3 for P and 30.6 for N) P and N uptake per unit root DW (except for P60 and P120 in WW), defined as P- and N-uptake efficiencies (Figure 2b,d).

Table 2.
Effect of genotype (G), water treatment (W), P level (P), and their interactions on water use (kg plant−1), water uptake per root DW (kg g−1 DW), P content (mg plant−1), P uptake per root DW (mg P g−1 DW), N content (mg plant−1), N uptake per root DW (mg N g−1 DW), adventitious root (AD) and root branching densities (cm−1), and lateral root (LR) and root branching densities (cm−1) of three soybean genotypes (Huandsedadou (HD), Bailudou (BLD) and Zhonghuang 30 (ZH)) exposed to two water treatments and three P levels. n.s. not significant, * p < 0.05, ** p < 0.01, and *** p < 0.001. Values are means (n = 3). The values in parenthesis are LSD at p = 0.05.
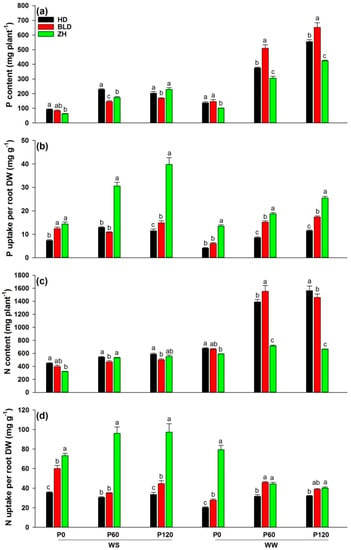
Figure 2.
(a) P content (mg plant−1), (b) P uptake per root DW (mg g−1), (c) N content (mg plant−1), and (d) N uptake per root DW (mg g−1) at maturity of three soybean genotypes (Huandsedadou (HD), Bailudou (BLD), and Zhonghuang 30 (ZH)) exposed to three P concentrations (0 (P0), 60 (P60) and 120 (P120) mg P kg−1 dry soil) under water-stressed (WS) and well-watered (WW) conditions. Means (n = 3) with different letters in any one set of three genotypes differ significantly at p = 0.05.
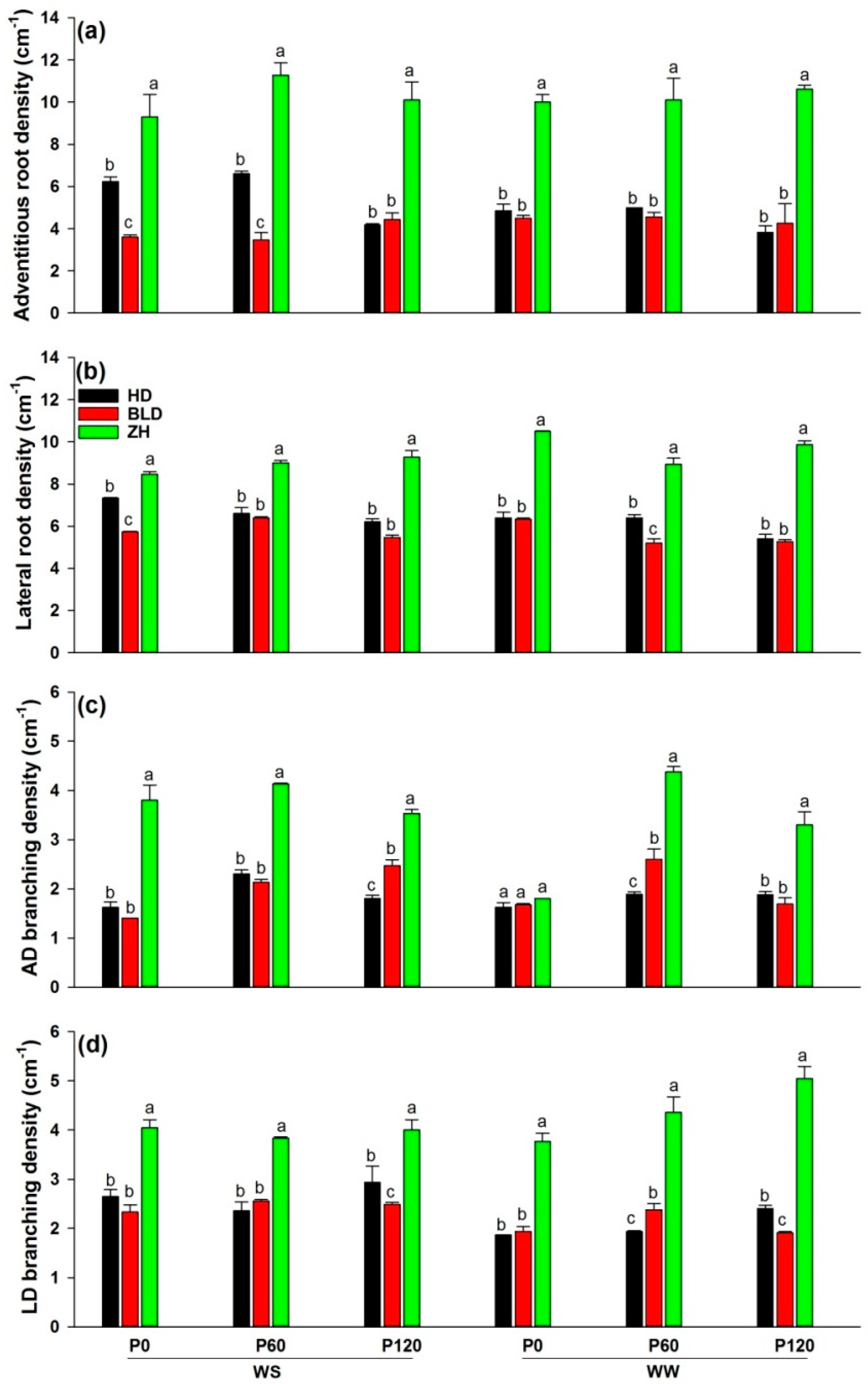
Adventitious root and lateral root branching densities varied between genotypes (Table 2). Genotype ZH had the smallest root DW but significantly more adventitious and lateral roots and higher branching densities than HD and BLD, both of which produced the same root density in all combinations (Figure 3).
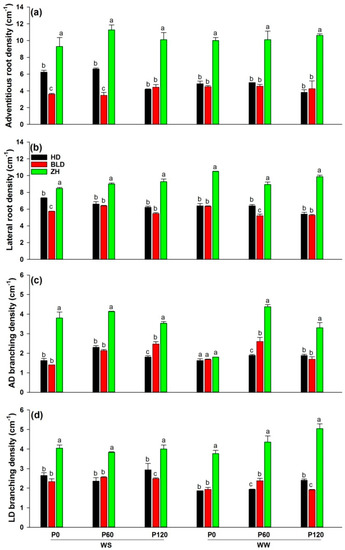
Figure 3.
(a) Adventitious root (AD) density (cm−1), (b) lateral root (LD) density (cm−1), (c) AD branching density (cm−1), and (d) LD branching density (cm−1) at maturity of three soybean genotypes (Huandsedadou (HD), Bailudou (BLD), and Zhonghuang 30 (ZH)) exposed to three P concentrations (0 (P0), 60 (P60) and 120 (P120) mg P kg−1 dry soil) under water-stressed (WS) and well-watered (WW) conditions. Means (n = 3) with different letters in any one set of three genotypes differ significantly at p = 0.05.
3.3. Correlation and Principal Component Analysis
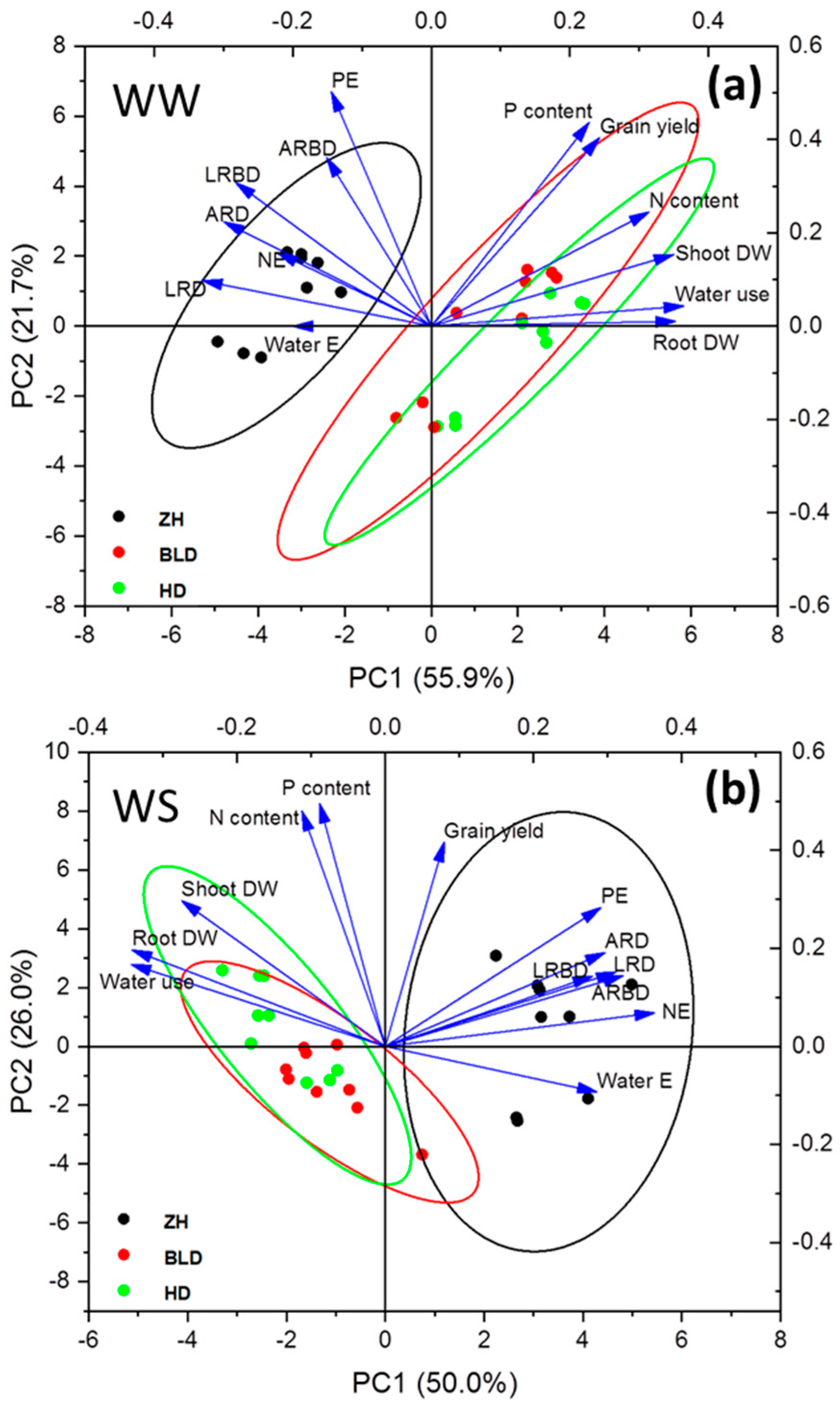
Grain yield had a positive correlation with shoot to root ratio and P uptake per root DW under WS (Figure S1). The WUEG was positively correlated with water uptake per unit root DW under WS, and shoot to root ratio and P and N uptake per root DW under WW and WS conditions (Figure S1). The shoot to root ratio was negatively correlated with root DW and positively correlated with adventitious root and adventitious root branching densities and lateral root and lateral root branching densities under WW and WS conditions (Figure S2). Water uptake per root DW had a significant negative correlation with root DW under WW and WS conditions (Figure S2). The P uptake per root DW was negatively correlated with root DW under WS, whereas P and N uptake per root DW were positively correlated with adventitious root and root branching densities and lateral root and root branching densities under WS (Figure S2). The PCA analysis showed that genotype ZH had high root density, and water- and nutrient- (N and P) uptake efficiencies, while the other two genotypes had high N and P contents, water use, and shoot and root DWs under WW and WS conditions (Figure 4).
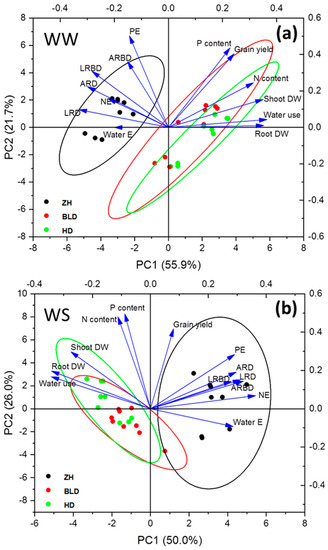
Figure 4.
Principal component analysis (PCA) results using grain yield and shoot and root traits under well-watered ((a), WW) and water-stressed ((b), WS) conditions. Shoot dry weight (shoot DW), root dry weight (root DW), water uptake per root DW (water E), P uptake per root DW (PE), N uptake per root DW (NE), adventitious root density (ARD), adventitious root branching density (ARBD), lateral root density (LRD), and lateral root branching density (LRBD).
4. Discussion
4.1. Root Efficiency vs. Root Size on Yield Performance
In this study, grain yield significantly decreased under water- and P-limited conditions, as observed in our previous studies [6,9]. Reduction in grain number was the main factor driving grain yield loss under water- or P-limitation and the combination of water- and P-limited conditions [8,9]. Here, we observed variation in the shoot to root ratio and water-, and P- and N-uptake efficiencies. The high grain yield was associated the high shoot to root ratio and P-uptake efficiency under WS, and ZH showed the highest root efficiency and had the highest grain yield at P0 under WW conditions. Although these results were not consistent with several previous studies which found increasing the biomass partitioned to the roots can pull up more water for survival under drought stress [7,12]. However, high root biomass could lead to quick water use which can lead to yield loss [24]; while the low root DW in ZH can conserve water use under water stress, which increased yield under water stress [6,25,26]. Water stress significantly increased the shoot to root ratio and this is not consistent with our previous study [2] and may be caused by the P application and/or the interaction between the P rates and genotypes [8,9]. The high shoot to root ratio in ZH showed that relatively more biomass was partitioned to shoots [27,28], and this was positively correlated with yield under water- and P-limited conditions [6]. A significantly positive relationship between shoot to root and water uptake per root DW (R2 = 0.63, data not shown), and a significantly negative relationship between shoot to root and water use (R2 = 0.30, data not shown) were found. These indicated reducing the root biomass partition could improve the water uptake efficiency and P addition could change the shoot traits such as increasing in leaf area to improve water uptake. More work is needed to investigate the response of transpiration factors under different water and P levels and their role in water uptake. Phosphorus content in ZH with low root DW (5.4 g plant−1) under WS was about 71% at P0, 97% at P60, and 125% at P120 of the mean phosphorus contents for HD and BLD which both presented high root DW (mean 13.7 g plant−1) under WS. Thus, the high P-uptake efficiency in this study demonstrates that it is cost-effective for soil P to improve grain yield [29], and that root efficiency plays a more important role than root DW in yield under water- and P-limited conditions.
Under WW conditions, genotype ZH only produced 74% and 65% grain yield at P60 and 65% and 74% grain yield at P120 of the two low root efficiency genotypes HD and BLD respectively, while ZH had significantly higher root efficiency and lower root DW than HD and BLD across all treatment combinations. These results indicate that root size, not root efficiency, might be the key determinant of yield performance. Our results showed that the P and N content in ZH were only 51% and 46% at P60, 43% and 46% at P120 of HD and BLD under WW, respectively, clearly showing that small roots which had high nutrition root efficiency could restrict P and N uptake when P and water are not limiting. Thus, a small root system restricts soil exploration [27,28] for water and nutrients (P and N), which was positively correlated with grain yield under P supplemented and WW conditions.
4.2. Root Traits and Root Efficiency
In this study, the shoot to root ratio and water- and N-uptake efficiencies declined with increasing root DW. The improving of P supply on the root DW (48% at P60 and 55% at P120) was lower than the increasing of P supply on the shoot to root ratios (24% at P60 and 20% at P120), water-uptake efficiencies (5% at P60 and 11% at P120), and N-uptake efficiencies (9% at P60 and 10% at P120), which resulted in a negative relationship between root DW and shoot to root ratio, and water- and N-uptake efficiencies. It is interesting to show that root density had no effect on water uptake, although root DW was negatively correlated with water-uptake efficiency. The possible reasons are as follows: (1) a high root density increases the acquisition of resources with low mobility in the soil, especially P [6,15,16,17,30,31]; (2) for the uptake of water, which is highly mobile, root length and root surface area (determined by root size) should be more important than root density.
Phosphorus is often a limiting nutrient for soybean because it affects not only plant growth, development, and yield [6,9], but also the N2 fixation process [32]. Low mobility and plant availability are the main factors limiting the acquisition of P [33], and soil drying further decreases P mobility [34,35]. Maximizing P acquisition is accompanied by changes in dry matter partitioning and root traits [14]. Phosphorus supply increased the shoot to root ratio in soybean (Glycine max L.), sunflower (Helianthus annuus L.), and maize (Zea mays L.) [22]. In the present study, the shoot to root ratio was positively correlated with P- uptake efficiency under WW and WS conditions, indicating reducing the partitioning of biomass to roots can increase P-uptake efficiency [30,31,36]. The proportion of the reduction in P content was lower than the proportion of reduction in the root biomass and can lead to improve the P-uptake efficiency. Besides, the high adventitious root and adventitious root branching density and lateral root and lateral root branching density were the other mechanisms to increase P acquisition efficiency [14].
In this study, a high root density was positively correlated with N-uptake efficiency. Possibly, a higher density of roots allows more N to be intercepted, and this improves N- uptake efficiency. We did not separate N uptake from N2-fixation; thus, the method used to calculate N-uptake efficiency is a limitation, and further research is required to separate the different N sources. The correlation analyses showed significant associations between traits under different conditions and a stronger relationship between root traits and root efficiency under WS than WW. Overall, the present findings demonstrate that numerous traits are involved in yield and root efficiency under water-limited conditions.
5. Conclusions
Root efficiency was more important than root size for yield when water and P were limiting, but we found the root size was more important than root efficiency in yield performance when water and P were not limiting. High root density increased P-uptake efficiency, but not water-uptake efficiency, which depends on root size. These findings proved that increasing the root efficiency which is associated with high root density could be considered as a way to improve the yield when water and P are limited.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/agriculture11060481/s1.
Author Contributions
Conceptualization, J.H. and Y.J.; formal analysis, J.H., Y.J., and K.H.M.S.; investigation, J.H. and Y.J.; writing—original draft preparation, J.H. and Y.J.; writing—review and editing, J.H., Y.J., K.H.M.S., and F.-M.L.; supervision, F.-M.L.; funding acquisition, J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (32060427), the Guizhou Science and Technology Support Program Project (Qiankehezhicheng (2019) 2399), the Guizhou Provincial Biology First-Class Subject Construction Project (GNYL (2017) 009), and the Provincial Nation-class Discipline of Biology Foundation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Lambers Hans for his useful comments on this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Manavalan, L.P.; Guttikonda, S.K.; Tran, L.S.P.; Nguyen, H.T. Physiological and molecular approaches to improve drought resistance in soybean. Plant Cell Physiol. 2009, 50, 1260–1276. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Du, Y.L.; Wang, T.; Turner, N.C.; Xi, Y.; Li, F.M. Old and new cultivars of soya bean (Glycine max L.) subjected to soil drying differ in abscisic acid accumulation, water relations characteristics and yield. J. Agron. Crop. Sci. 2016, 202, 372–383. [Google Scholar] [CrossRef]
- He, J.; Du, Y.L.; Wang, T.; Turner, N.C.; Yang, R.P.; Jin, Y.; Xi, Y.; Zhang, C.; Cui, T.; Fang, X.W.; et al. Conserved water use improves the yield performance of soybean (Glycine max (L. Merr.)) under drought. Agric. Water Manag. 2017, 179, 236–245. [Google Scholar] [CrossRef]
- Yang, M.H.; Jahufer, M.; He, J.; Dong, R.; Hofmann, R.; Siddique, K.H.M.; Li, F.M. Effect of traditional soybean breeding on water use strategy in arid and semi-arid areas. Eur. J. Agron. 2020, 120, 126128. [Google Scholar] [CrossRef]
- Xu, Q.P.; Luo, C.Y.; Liao, H.; Yan, X.L.; Nian, H. Study on the response of soybean varieties to P deficiency. Soybean Sci. 2003, 22, 108–114. [Google Scholar]
- He, J.; Du, Y.L.; Wang, T.; Turner, N.C.; Yang, R.P.; Siddique, K.H.M.; Li, F.M. Genotypic variation in yield, yield components, root morphology and architecture, in soybean in relation to water and phosphorus supply. Front. Plant Sci. 2017, 8, 1499. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Molyneux, N.; Yang, S.; Xiong, Y.C.; Siddique, K.H.M. Climate change in south-west Australia and north-west China: Challenges and opportunities for crop production. Crop. Pasture Sci. 2011, 62, 445–456. [Google Scholar] [CrossRef]
- Jin, J.; Wang, G.; Liu, X.; Pan, X.; Herbert, S.J.; Tang, C. Interaction between phosphorus nutrition and drought on grain yield, and assimilation of phosphorus and nitrogen in two soybean cultivars differing in protein concentration in grains. J. Plant Nutr. 2006, 29, 1433–1449. [Google Scholar] [CrossRef]
- He, J.; Jin, Y.; Turner, N.C.; Chen, Z.; Liu, H.Y.; Wang, X.L.; Siddique, K.H.M.; Li, F.M. Phosphorus application increases root growth, improves daily water use during the reproductive stage, and increases grain yield in soybean subjected to water shortage. Env. Exp. Bot. 2019, 166, 103816. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Johansen, C.; Slinkard, A.E.; Rao, R.C.N.; Saxena, N.P.; Chauhan, Y.S. Strategies for improving drought resistance in grain legumes. Cri.t Rev. Plant Sci. 1995, 14, 469–523. [Google Scholar] [CrossRef]
- Turner, N.C.; Wright, G.C.; Siddique, K.H.M. Adaptation of grain legumes (pulses) to water-limited environments. Adv. Agron. 2001, 71, 193–231. [Google Scholar]
- Kashiwagi, J.; Krishnamurthy, L.; Upadhyaya, H.D.; Krishna, H.; Chandra, S.; Vadez, V.; Serraj, R. Genetic variability of drought-avoidance root traits in the mini-core germplasm collection of chickpea (Cicer arietinum L.). Euphytica 2005, 146, 213–222. [Google Scholar] [CrossRef]
- He, J.; Jin, Y.; Turner, N.C.; Li, F.M. Irrigation during flowering improves subsoil water uptake and grain yield in rainfed soybean. Agronomy 2020, 10, 120. [Google Scholar] [CrossRef]
- Lynch, J.P.; Brown, K.M. Topsoil foraging–An architectural adaptation of plants to low phosphorus availability. Plant Soil 2001, 237, 225–237. [Google Scholar] [CrossRef]
- Postma, J.A.; Dathe, A.; Lynch, J.P. The optimal lateral root branching density for maize depends on nitrogen and phosphorus availability. Plant Physiol. 2014, 166, 590–602. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Liu, P.; Lynch, J.P. Greater lateral root branching density in maize (Zea mays L.) improves phosphorus acquisition from low phosphorus soil. J. Exp. Bot. 2018, 69, 4961–4970. [Google Scholar] [CrossRef]
- Sun, B.; Gao, Y.; Lynch, J. Large crown root number improves topsoil foraging and phosphorus acquisition. Plant Physiol. 2018, 177, 90–104. [Google Scholar] [CrossRef]
- Miller, C.R.; Ochoa, I.; Nielsen, K.L.; Beck, D.; Lynch, J.P. Genetic variation for adventitious rooting in response to low phosphorus availability: Potential utility for phosphorus acquisition from stratified soils. Funct. Plant Biol. 2003, 30, 973–985. [Google Scholar] [CrossRef]
- Rangarajan, H.; Postma, J.; Lynch, J.P. Co-optimization of axial root phenotypes for nitrogen and phosphorus acquisition in common bean. Ann. Bot. 2018, 122, 485–499. [Google Scholar] [CrossRef]
- Wissuwa, M.; Ae, N. Genotypic variation for tolerance to phosphorus deficiency in rice and the potential for its exploitation in rice improvement. Plant Breed. 2001, 120, 43–48. [Google Scholar] [CrossRef]
- Mori, A.; Fukuda, T.; Vejchasarn, P.; Nestler, J.; Pariasca-Tanaka, J.; Wissuwa, M. The role of root size versus root efficiency in phosphorus acquisition in rice. J. Exp. Bot. 2016, 67, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.C.; Rubio, G. Root morphological traits related to phosphorus-uptake efficiency of soybean, sunflower, and maize. J. Plant. Nutr. Soil Sci. 2015, 178, 807–815. [Google Scholar] [CrossRef]
- Fehr, W.; Caviness, C.; Burmood, D.; Pennington, J. Stage of development descriptions for soybeans, Glycine max (L.) Merrill. Crop. Sci. 1971, 11, 929–931. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Messina, C.D.; Beatty, A.; Samples, M. Assessment across the united states of the benefits of altered soybean drought traits. Agron. J. 2010, 102, 475–482. [Google Scholar] [CrossRef]
- Zaman-Allah, M.; Jenkinson, D.M.; Vadez, V.A. conservative pattern of water use, rather than deep or profuse rooting, is critical for the terminal drought tolerance of chickpea. J. Exp. Bot. 2011, 62, 4239–4252. [Google Scholar] [CrossRef]
- Vadez, V.; Kholova, J.; Zaman-Allah, M.; Belko, N. Water: The most important ‘molecular’ component of water stress tolerance research. Funct. Plant Biol. 2013, 40, 1310–1322. [Google Scholar] [CrossRef]
- Waddell, H.A.; Simpson, R.J.; Henderson, B.; Ryan, M.H.; Lambers, H.; Garden, D.L.; Richardson, A.E. Differential growth response of Rytidosperma species (wallaby grass) to phosphorus application and implications for grassland management. Grass Forage Sci. 2015, 71, 245–258. [Google Scholar] [CrossRef]
- Haling, R.E.; Yang, Z.; Shadwell, N.; Culvenor, R.A.; Stefanski, A.; Ryan, M.H.; Sandral, G.A.; Kidd, D.R.; Lambers, H.; Simpson, R. Growth and root dry matter allocation by pasture legumes and a grass with contrasting external critical phosphorus requirements. Plant Soil 2016, 407, 67–79. [Google Scholar] [CrossRef]
- Funayama-Noguchi, S.; Noguchi, K.O.; Terashima, I. Comparison of the response to phosphorus deficiency in two lupin species, Lupinus albus and L. angustifolius, with contrasting root morphology. Plant Cell Environ. 2015, 38, 399–410. [Google Scholar] [CrossRef]
- Lynch, J.P. Root phenes for enhanced soil exploration and phosphorus acquisition: Tools for future crops. Plant Physiol. 2011, 156, 1041–1049. [Google Scholar] [CrossRef]
- Richardson, A.E.; Lynch, J.P.; Ryan, P.R.; Delhaize, E.; Smith, A.F.; Smith, S.E.; Harvey, P.R.; Ryan, M.H.; Veneklaas, E.J.; Lambers, H.; et al. Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant Soil 2011, 349, 121–156. [Google Scholar] [CrossRef]
- Hernández, G.; Valdés-López, O.; Ramírez, M.; Goffard, N.; Weiller, G.; Aparicio-Fabre, R.; Fuentes, S.I.; Erban, A.; Kopka, J.; Udvardi, M.K.; et al. Global changes in the transcript and metabolic profiles during symbiotic nitrogen fixation in phosphorus-stressed common bean plants. Plant Physiol. 2009, 151, 1221–1238. [Google Scholar] [CrossRef] [PubMed]
- Schachtman, D.P.; Reid, R.J.; Ayling, S.M. Phosphorus uptake by plants: From soil to cell. Plant Physiol 1998, 116, 447–453. [Google Scholar] [CrossRef]
- Suriyagoda, L.D.; Ryan, M.H.; Renton, M.; Lambers, H. Multiple adaptive responses of Australian native perennial legumes with pasture potential to grow in phosphorus-and moisture-limited environments. Ann. Bot. 2010, 105, 755–767. [Google Scholar] [CrossRef]
- Suriyagoda, L.D.; Ryan, M.H.; Renton, M.; Lambers, H. Plant responses to limited moisture and phosphorus availability. Adv. Agron. 2014, 124, 143–200. [Google Scholar]
- Waddell, H.A.; Simpson, R.J.; Ryan, M.H.; Lambers, H.; Garden, D.L.; Richardson, A.E. Root morphology and its contribution to a large root system for phosphorus uptake by Rytidosperma species (wallaby grass). Plant Soil 2016, 412, 7–19. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).