Effects of Exogenous Trans-Zeatin and Lovastatin on Abortion of Small Seeds in ‘Dawuxing’ Loquat (Eriobotrya Japonica Lindl.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatment Conditions
2.2. Extraction and Determination of Soluble Sugar
2.3. Extraction and Determination of Starch
2.4. Extraction and Determination of Soluble Protein
2.5. Extraction and Determination of Trans-Zeatin
2.6. Extraction and Determination of Antioxidant Enzymes
2.7. Total RNA Extraction, cDNA Synthesis and Relative Expression Analysis
3. Results
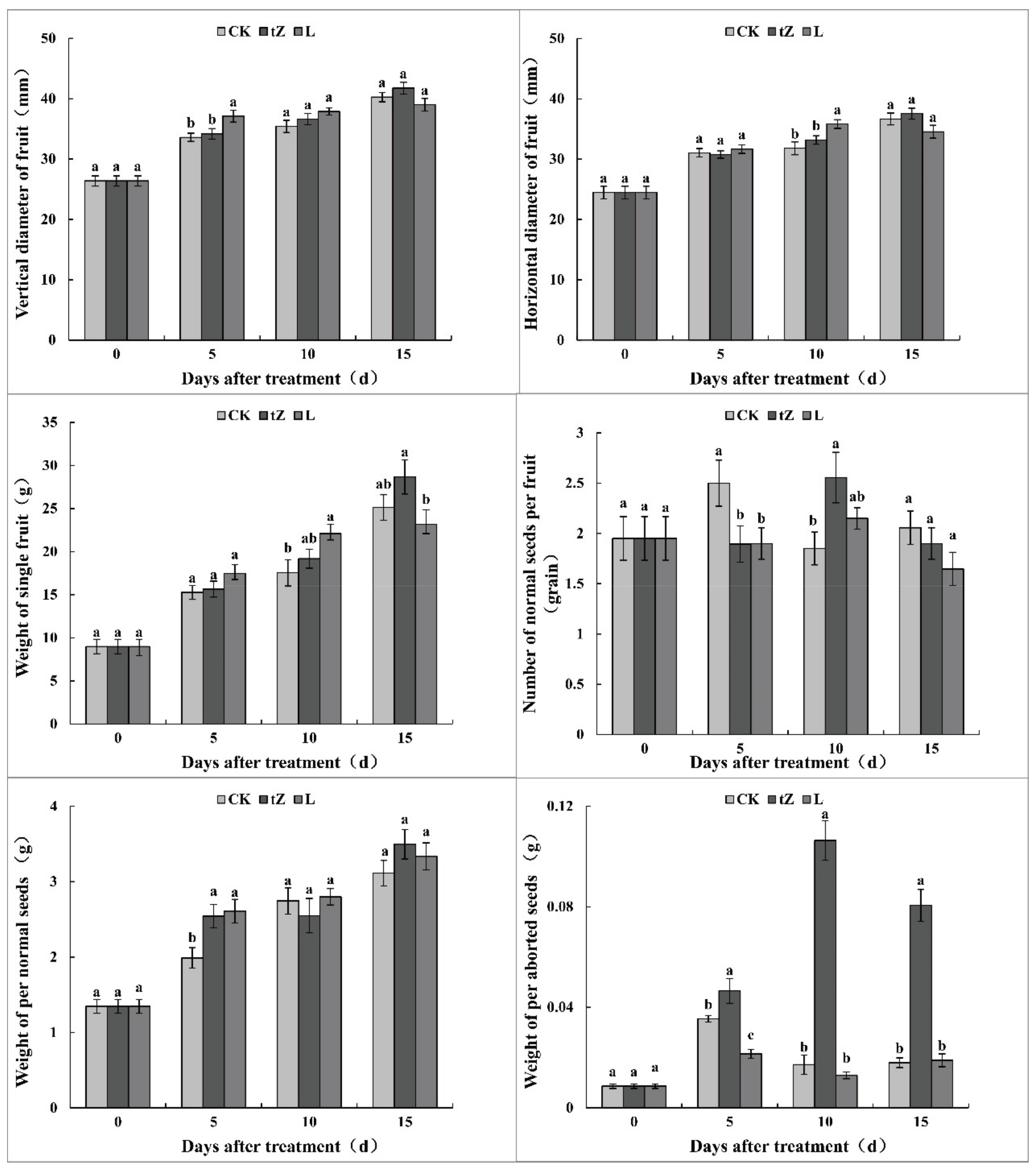
3.1. Morphological Indexes of Loquat Treated with Exogenous Hormones
3.2. Effects of Exogenous Trans-Zeatin and Lovastatin on Nutrients during Seed Abortion in Loquat
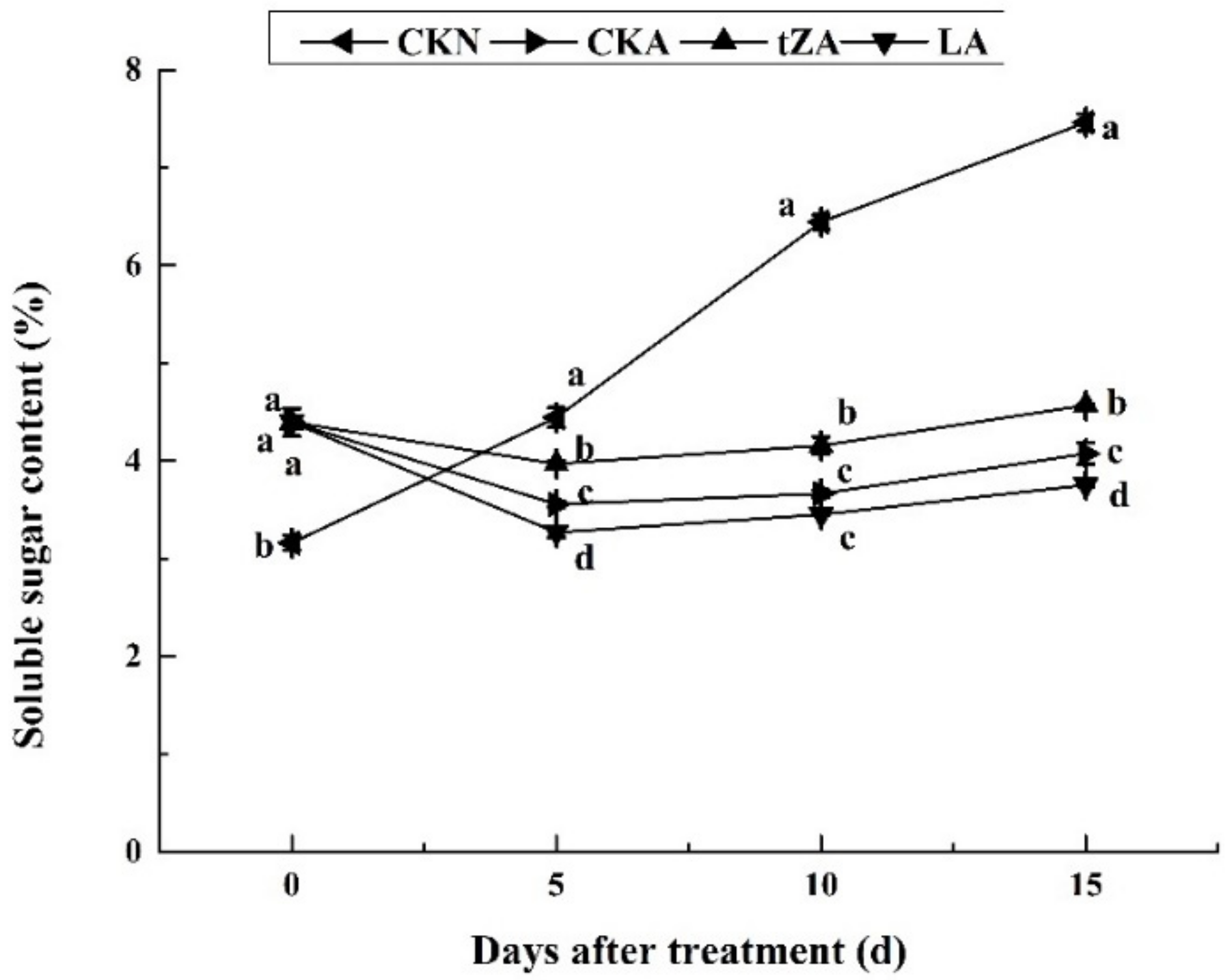
3.2.1. Effects of Exogenous Trans-Zeatin and Lovastatin on Soluble Sugar during Seed Abortion in Loquat
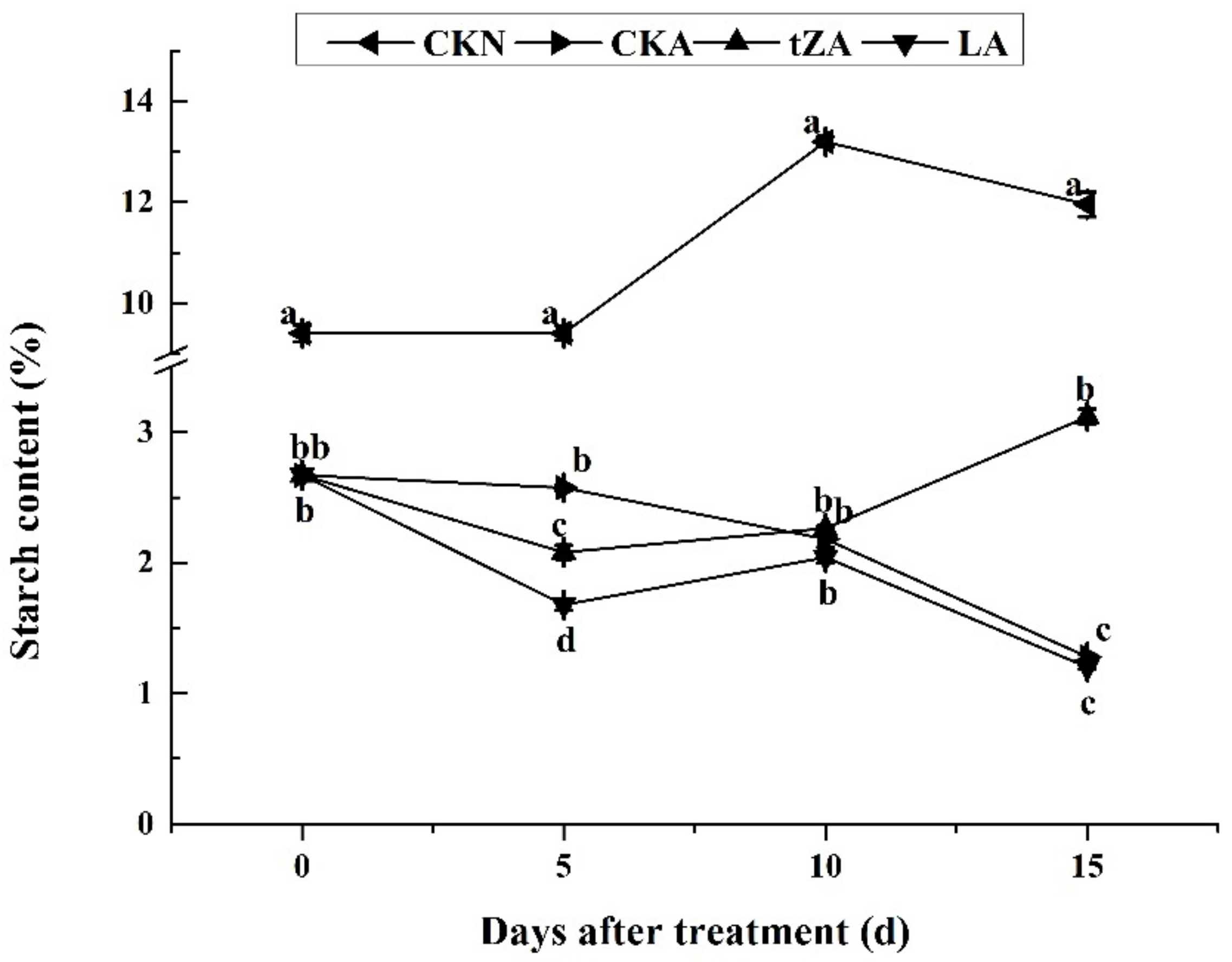
3.2.2. Effects of Exogenous Trans-Zeatin and Lovastatin on Starch during Seed Abortion in Loquat
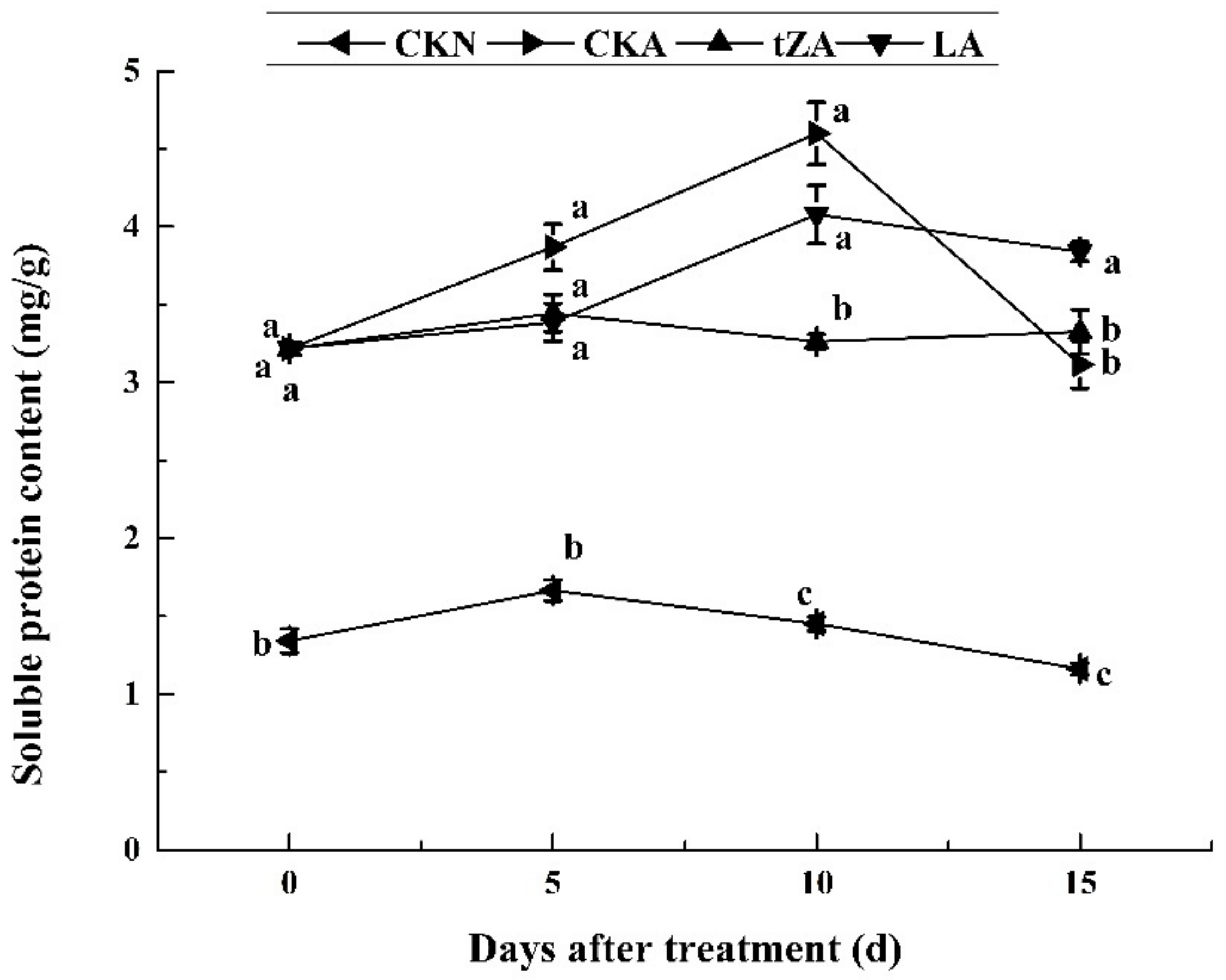
3.2.3. Effects of Exogenous Trans-Zeatin and Lovastatin on the Soluble Protein during Seed Abortion in Loquat
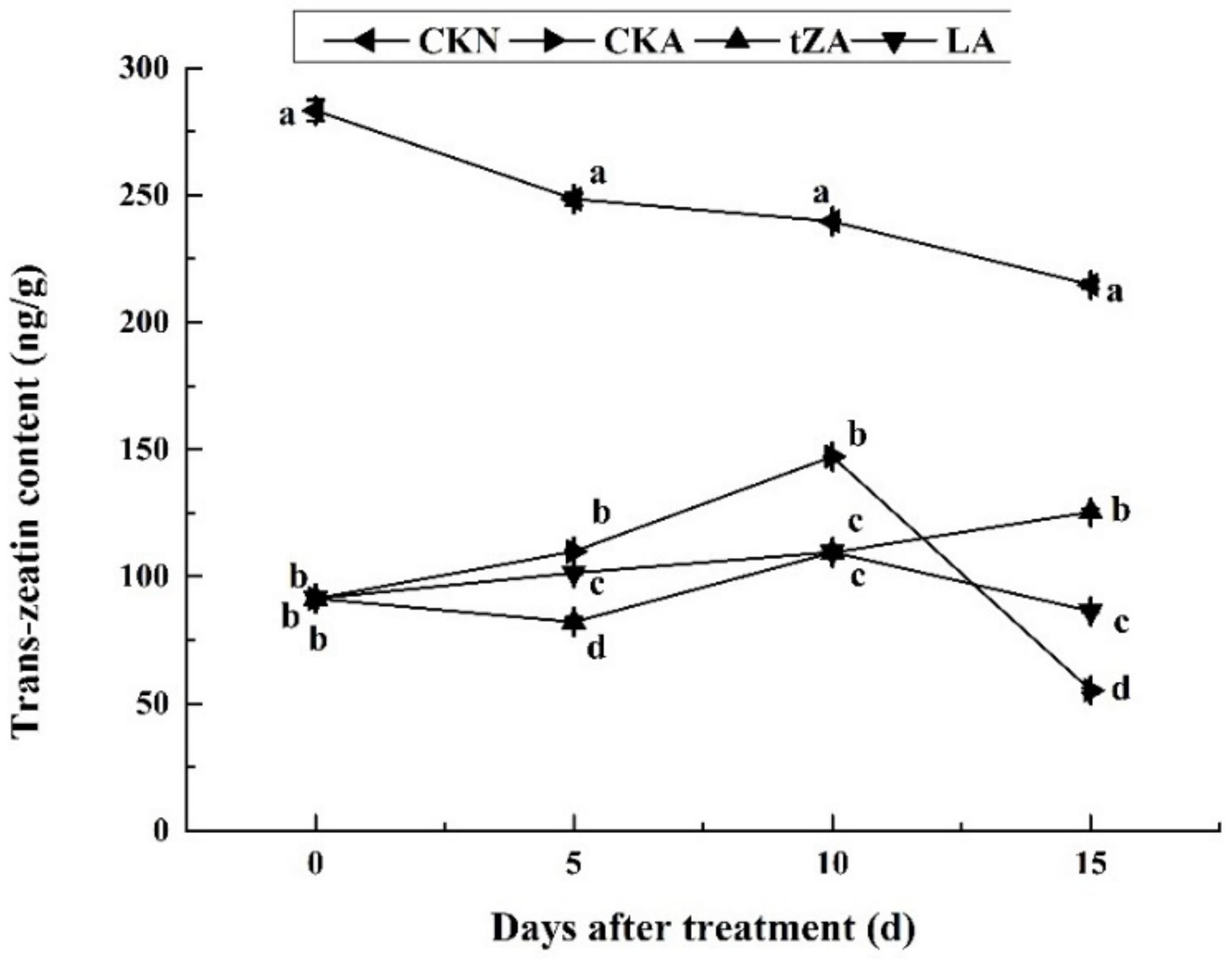
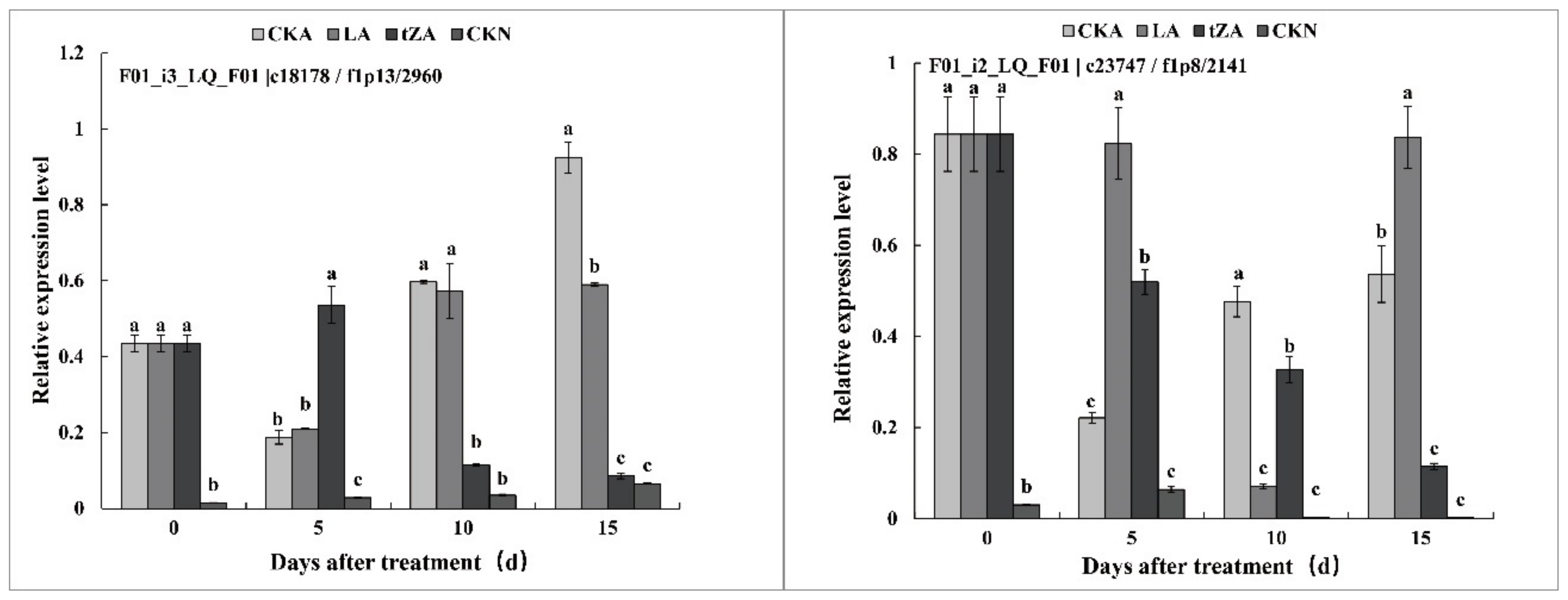
3.3. The Effects of Exogenous Trans-Zeatin and Lovastatin on the Content of Endogenous Trans-Zeatin and Expression Pattern of Related Genes during Seed Abortion of Loquat
3.4. Effects of Exogenous Trans-Zeatin and Lovastatin on Antioxidant Enzymes Activity and Expression Pattern of Related Genes during Seed Abortion of Loquat
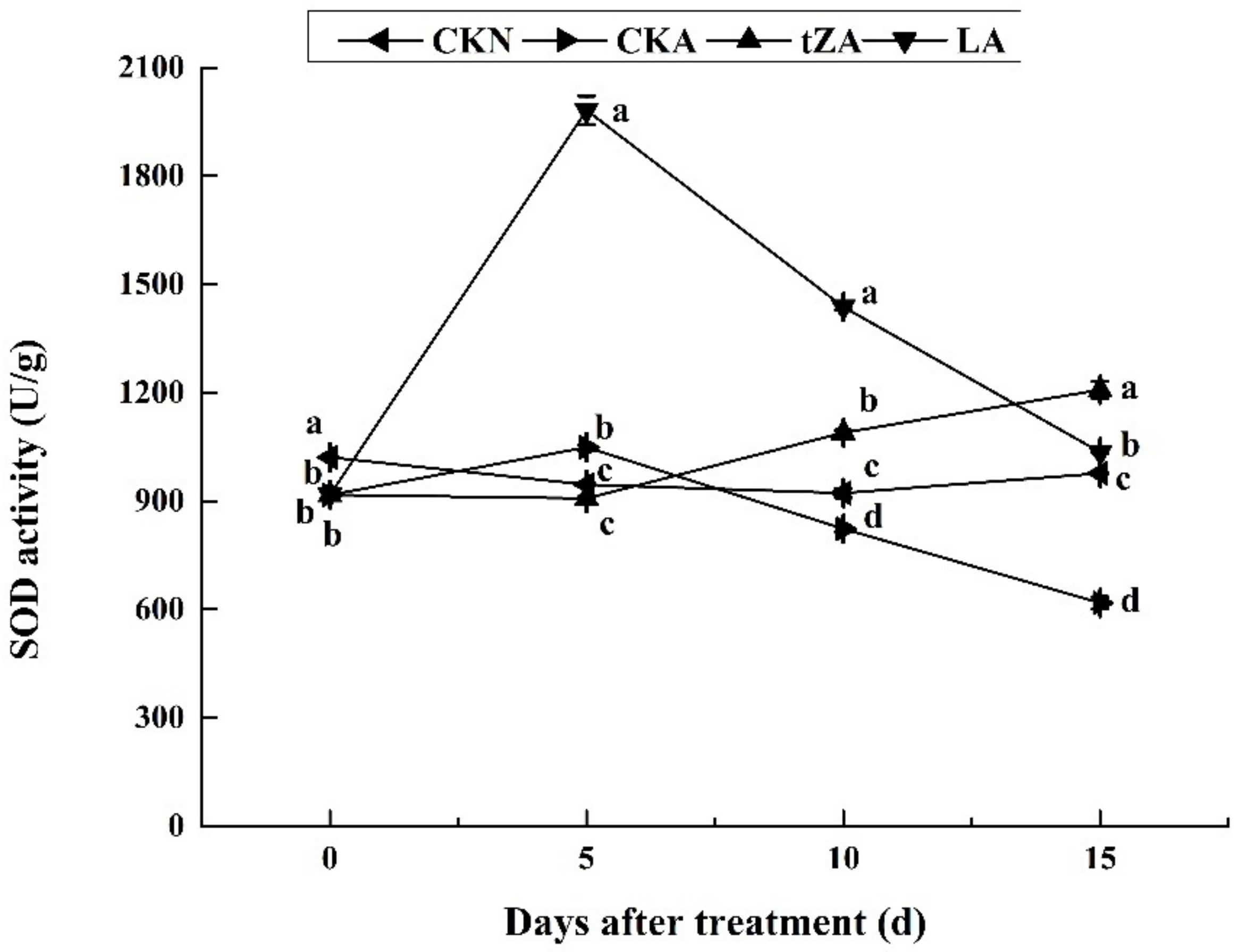
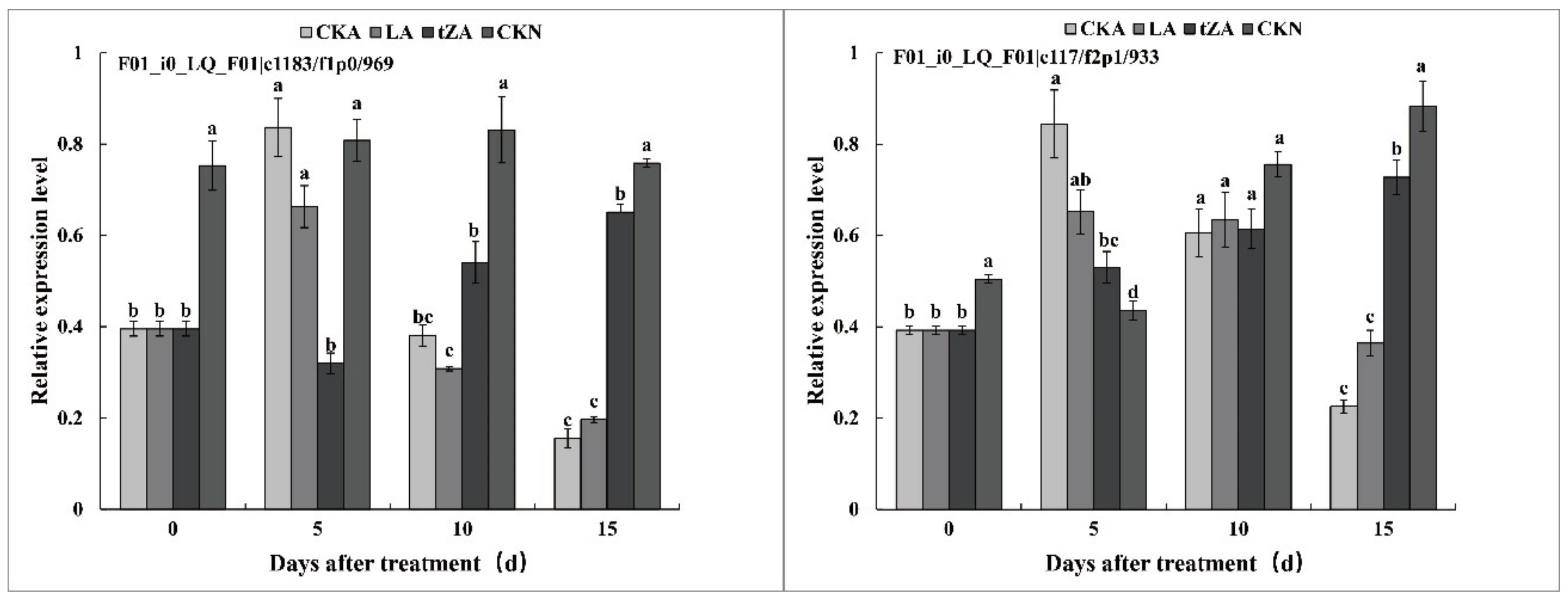
3.4.1. Effects of Exogenous Trans-Zeatin and Lovastatin on SOD Activity and Expression Pattern of Related Genes during Seed Abortion of Loquat
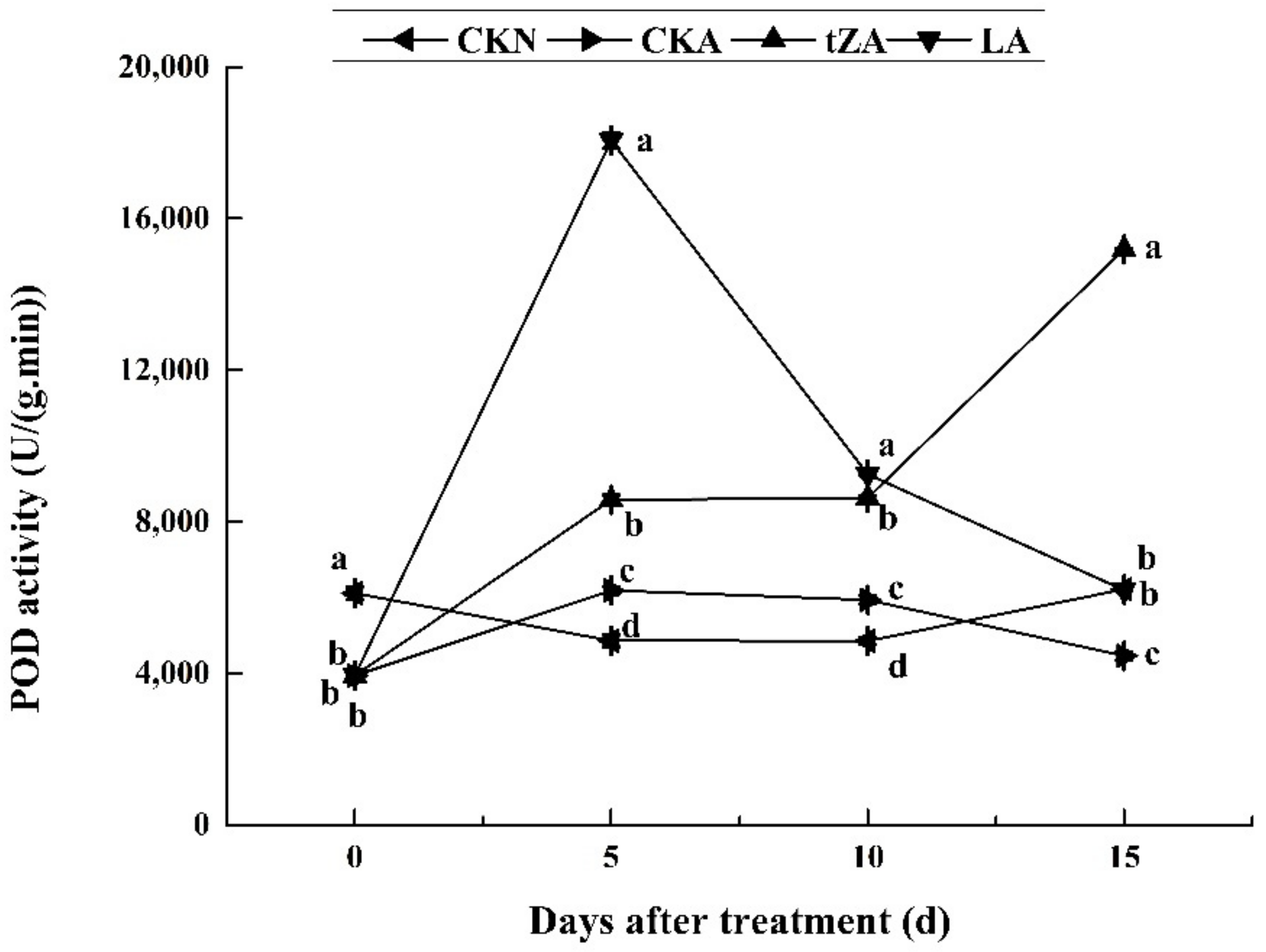
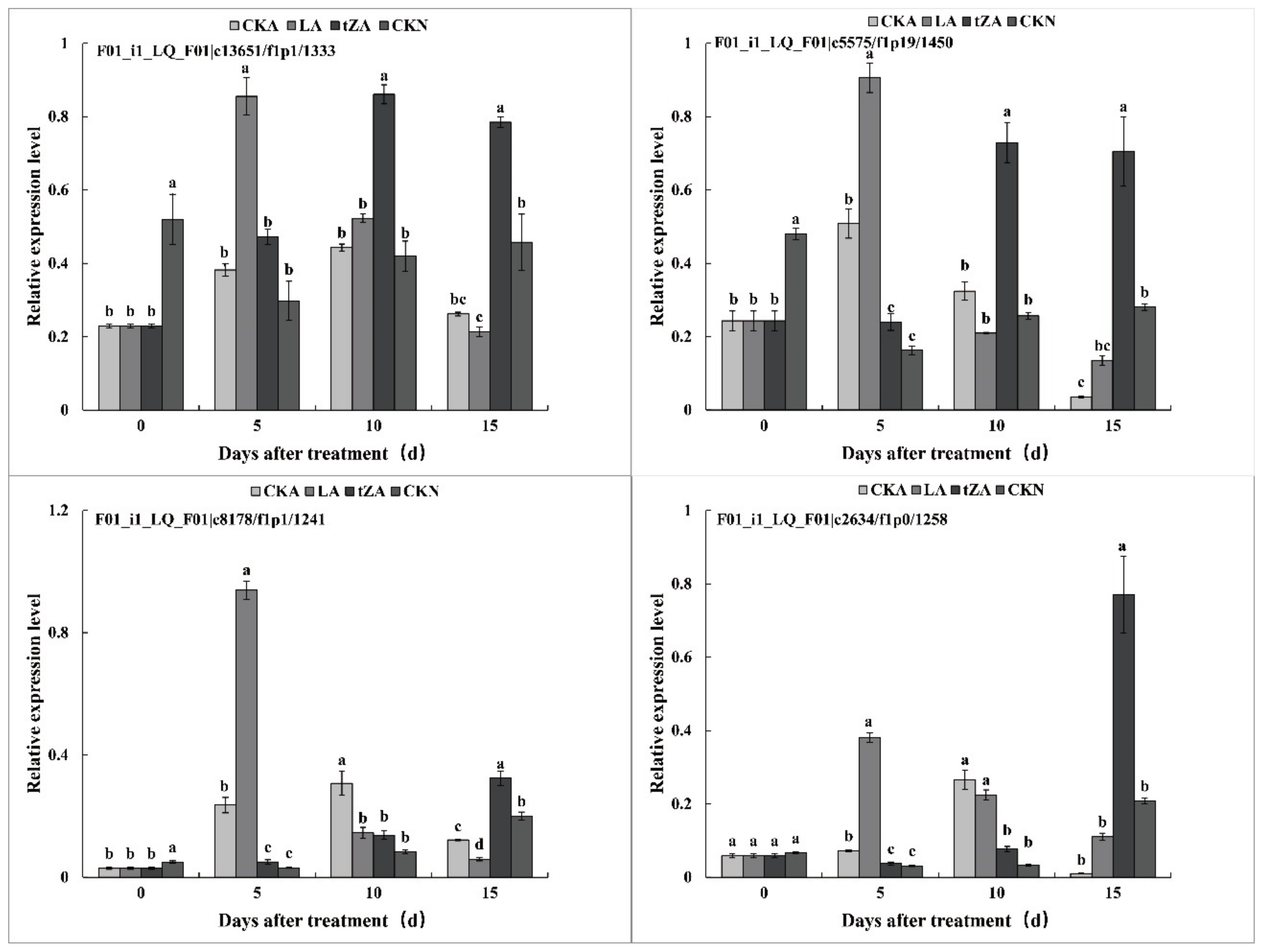
3.4.2. Effects of Exogenous Trans-Zeatin and Lovastatin on POD Activity and Expression Pattern of Related Genes during Seed Abortion of Loquat
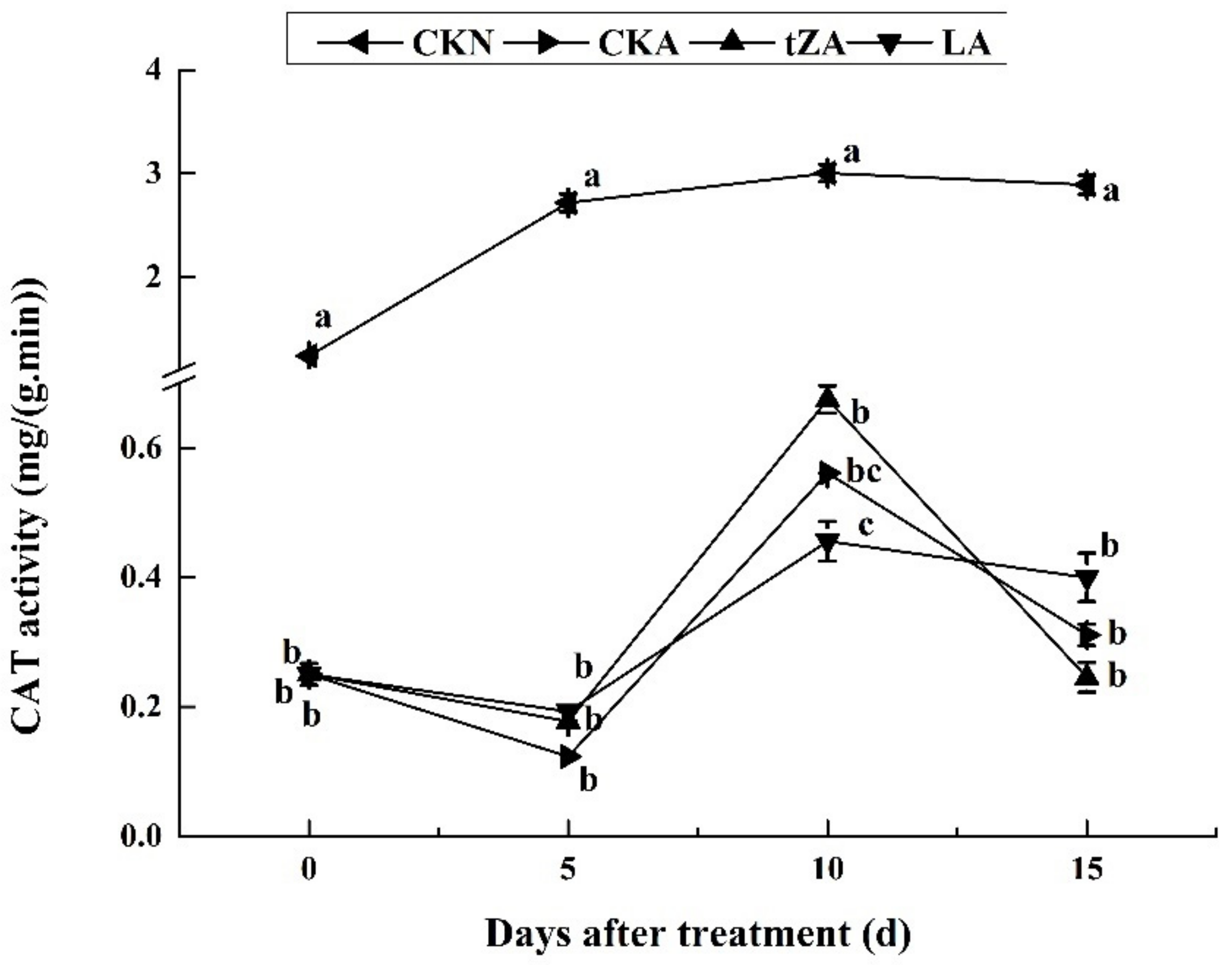
3.4.3. Effects of Exogenous Trans-Zeatin and Lovastatin on CAT Activity and Expression Pattern of Related Gene during Seed Abortion of Loquat
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Zhang, S.; Cai, L.; Fang, D. The germplasm resources of the genus Eriobotrya with special reference on the origin of E. japonica Lindl. Acta Hortic. Sin. 1990, 17, 5–12. [Google Scholar]
- Deng, Q.; Dong, Y.; Wang, Y.; Luo, N.; Li, J.; Yang, Q.; Fu, Y. Studies on seed degeneration and embryo abortion of loquat (Eriobotrya japonica Lindl.). South China Fruits 2007, 36, 46–48. [Google Scholar]
- Tao, L.; Xie, H.; Yang, W.; Wang, Y.; Pan, C.; Tu, M.; Li, J.; Zhang, Y. Study on Diversity of Leaf Morphological Traits of Plants Regenerated from ‘Miniature Seeds’ in Loquat. Southwest China J. Agric. Sci. 2016, 29, 1958–1961. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, J.; Tao, L.; Yang, Q.; Deng, R.; Fan, J.; Du, K.; Cui, W.; Yang, Z. Haploid can be obtained from small seeds of loquat. In Proceedings of the 2012 Academic Seminar on Chromosome Ploidy Operation and Genetic Improvement of Horticultural Plants, Chongqing, China, 13 April 2012; p. 2. [Google Scholar]
- Tao, L.; Wang, Y.; Yan, J.; Peng, S.; Deng, Q.; Yang, Z.; Du, K.; Cui, W. Investigation on Hardiness and Preliminary Screening for Cold Resistant Plants Regenerated from “Miniature Seeds”in Loquat. In Proceedings of the 5th International Symposium on Loquat, Shimian, China, 1 May 2011; p. 5. [Google Scholar]
- Zhou, L.; Wei, Q.; Gao, F. The Effect of Cytokinins on Fruit and Seed Development. Plant Physiol. Commun. 2006, 42, 549–553. [Google Scholar]
- Dai, Y.; Zhang, S.; Yang, S. Effect of CTK on Assimilate Unloading and Metabolism of Embryo in Developing Seeds of Soybean. Acta Agron. Sin. 1998, 24, 613–617. [Google Scholar]
- Truernit, E.; Schmid, J.; Epple, P.; Illig, J.; Sauer, N. The sink-specific and stress-regulated Arabidopsis STP4 gene: Enhanced expression of a gene encoding a monosaccharide transporter by wounding, elicitors, and pathogen challenge. Plant Cell 1996, 8, 2169–2182. [Google Scholar] [PubMed]
- Harms, K.; Wohner, R.V.; Schulz, B.; Frommer, W.B. Isolation and characterization of P-type H(+)-ATPase genes from potato. Plant Mol. Biol. 1994, 26, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Jameson, P.E.; Song, J. Cytokinin: A key driver of seed yield. J. Exp. Bot. 2016, 67, 593–606. [Google Scholar] [CrossRef]
- Han, Y.; Yang, H.; Jiao, Y. Regulation of inflorescence architecture by cytokinins. Front. Plant Sci. 2014, 5, 669. [Google Scholar] [CrossRef]
- Yang, J.; Peng, S.; Visperas, R.M.; Sanico, A.L.; Zhu, Q.; Gu, S. Grain filling pattern and cytokinin content in the grains and roots of rice plants. Plant Growth Regul. 2000, 30, 261–270. [Google Scholar] [CrossRef]
- Lur, H.S.; Setter, T.L. Role of Auxin in Maize Endosperm Development (Timing of Nuclear DNA Endoreduplication, Zein Expression, and Cytokinin). Plant Physiol. 1993, 103, 273–280. [Google Scholar] [CrossRef]
- Morris, R.; Blevins, D.; Dietrich, J.; Durley, R.; Gray, S.G.J.; Hommes, N.; Kaminek, M.; Mathews, L.J. Cytokinins in Plant Pathogenic Bacteria and Developing Cereal Grains. Aust. J. Plant Physiol. 1993, 5, 621–637. [Google Scholar] [CrossRef]
- Yang, J. Correlation of Cytokinin Levels in the Endosperms and Roots with Cell Number and Cell Division Activity during Endosperm Development in Rice. Ann. Bot. 2002, 90, 369–377. [Google Scholar] [CrossRef]
- Morris, R.O. Hormonal Regulation of Seed Development. In Cellular and Molecular Biology of Plant Seed Development; Springer: Dordrecht, The Netherlands, 1997; pp. 117–148. [Google Scholar]
- Song, J.; Jiang, L.; Jameson, P.E. Expression patterns of Brassica napus genes implicate IPT, CKX, sucrose transporter, cell wall invertase, and amino acid permease gene family members in leaf, flower, silique, and seed development. J. Exp. Bot. 2015, 66, 5067–5082. [Google Scholar] [CrossRef]
- Nagel, L.; Brewster, R.; Riedell, W.E.; Reese, R.N. Cytokinin Regulation of Flower and Pod Set in Soybeans (Glycine max (L.) Merr.). Ann. Bot. 2001, 88, 27–31. [Google Scholar] [CrossRef]
- Atkins, C.A.; Pigeaire, A. Application of cytokinins to flowers to increase pod set in Lupinus angustifolius L. Aust. J. Agric. Res. 1993, 8, 1799–1819. [Google Scholar] [CrossRef]
- El-Dengawy, E.F.A. Promotion of seed germination and subsequent seedling growth of loquat (Eriobotrya japonica, Lindl) by moist-chilling and GA3 applications. Sci. Hortic. 2005, 105, 331–342. [Google Scholar] [CrossRef]
- Wang, G.Y.; Wang, Q. Effects of Different Temperatures and Different GA_3 Concentrations Treatments on the Loquat Seed Germination and Seedling Growth. Tianjin Agric. Sci. 2015, 21, 110–113. [Google Scholar]
- Hu, Z.; Lin, Y. Effect of GA_3+CPPU induction time on loquat pit development. Fujian J. Agric. Sci. 2010, 25, 707–710. [Google Scholar]
- Takei, K.; Yamaya, T.; Sakakibara, H. Arabidopsis CYP735A1 and CYP735A2 Encode Cytokinin Hydroxylases That Catalyze the Biosynthesis of trans-Zeatin. J. Biol. Chem. 2004, 279, 41866–41872. [Google Scholar] [CrossRef]
- Chemists, A. Cereal Chem 1975|A Note on Sugar Determination by the Anthrone Method. Publications 1975, 52, 857–860. [Google Scholar]
- Hansen, J.; Mller, I. Percolation of starch and soluble carbohydrates from plant tissue for quantitative determination with anthrone. Anal. Biochem. 1975, 68, 87–94. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of proteins utilizing the principle of protein dye binding. Nature 1976, 227, 248–254. [Google Scholar] [CrossRef]
- Giannopolites, C.N.; Ries, S.K. Superoxide dismutase occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Lee, T.M.; Lin, Y.H. Peroxidase activity in ethylene-, ABA-, or MeJA-treated rice (Oryza sativa L.) roots. Bot. Bull. Acad. Sin. 1996, 37, 201–207. [Google Scholar]
- Machly, A.C.; Chance, P. Assay of catalase and peroxidases. Method Enzym. 1955, 2, 764–775. [Google Scholar]
- Fu, X.; Kong, W.; Peng, G.; Zhou, J.; Azam, M.; Xu, C.; Grierson, D.; Chen, K. Plastid structure and carotenogenic gene expression in red- and white-fleshed loquat (Eriobotrya japonica) fruits. J. Exp. Bot. 2012, 63, 341–354. [Google Scholar] [CrossRef]
- Deng, Q.X. Studies on Embryological Mechanism of Seed Degeneration and Genetic Diversity of Seedlings from Middle-Degenerated seeds inloquat (Eriobotrya japonica Lindl). Ph.D. Thesis, Sichuan Agricultural University, Ya’an, China, 2009. [Google Scholar]
- Ganeshaiah, K.N.; Shaanker, R.U. Seed abortion in wind-dispersed pods of Dalbergia sissoo: Maternal regulation or sibling rivalry? Oecologia 1988, 77, 135–139. [Google Scholar] [CrossRef]
- Mohan, R.B.; Uma, S.R.; Ganeshaiah, K.N. Intra-fruit seed abortion in a wind dispersed tree. Dalbergia sissoo Roxb: Proximate mechanisms. Sex. Plant Reprod. 1996, 9, 273–278. [Google Scholar] [CrossRef]
- Arathi, H.S.; Ganeshaiah, K.N.; Shaanker, R.U.; Hegde, S.G. Factors Affecting Embryo Abortion in Syzygium cuminii (L.) Skeels (Myrtaceae). Int. J. Plant Sci. 1996, 157, 49–52. [Google Scholar] [CrossRef]
- Mohana, G.S.; Shaanker, R.U.; Ganeshaiah, K.N.; Dayanandan, S. Genetic relatedness among developing seeds and intra fruit seed abortion in Dalbergia sissoo (Fabaceae). Am. J. Bot. 2001, 88, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Köhne, J.S.; Kremer-Köhne, S.; Schutte, J.M. Effect of CPPU sprays on yield and fruit size in avocadocv hass. S. Afr. Avocado Growers’ Assoc. Yearb. 1993, 16, 31–32. [Google Scholar]
- Steyn, E.M.A.; Robbertse, P.J.; Smith, D.G. Cuke development in consistently low producing trees of the ‘fuerte’ avocado with special reference to seed abortion. S. Afr. Avocado Growers’ Assoc. Yearb. 1993, 16, 5–8. [Google Scholar]
- Tomer, E.; Gazit, S.; Eisenstein, D. Seedless fruit in ‘Fuerte’ and ‘Ettinger’ avocado. J. Am. Soc. Hortic. Sci. 1980, 105, 341–346. [Google Scholar] [CrossRef]
- Hare, R.C. Reducing conelet abortion in longleaf pine with chemicals. Can. J. For. Res. 1981, 11, 448–450. [Google Scholar] [CrossRef]
- Itai, A.; Tanabe, K.; Tamura, F.; Susaki, S.; Yonemori, K.; Sugiura, A. Synthetic cytokinins control persimmon fruit shape, size and quality. J. Hortic. Sci. 1995, 70, 867–873. [Google Scholar] [CrossRef]
- Ogawa, Y.; Nishikawa, S.; Inoue, N.; Aoki, S. Promotive Effects of Different Cytokinins on the Fruit Growth in Cucumis sativus L. Eng. Gakkai Zasshi. 1990, 59, 597–601. [Google Scholar] [CrossRef]
- Antognozzi, E.; Famiani, F.; Proietti, P.; Tombesi, A.; Frenguelli, G.; Frenguelli, G. Effect of cppu (cytokinin) treatments on fruit anatomical structure and quality m actinidia deliciosa. Acta Hortic. 1997, 444, 459–465. [Google Scholar] [CrossRef]
- Srivastava, L.M. Chapter 8—Cytokinins. In Plant Growth and Development; Srivastava, L.M., Ed.; Academic Press: San Diego, CA, USA, 2002; pp. 191–204. [Google Scholar]
- Davey, J.E.; Staden, J. Cytokinin Activity in Lupinus albus. III. Distribution in Fruits. Physiol. Plant 1978, 43, 87–93. [Google Scholar] [CrossRef]
- Kudoyarova, G.R.; Vysotskaya, L.B.; Cherkozyanova, A.; Dodd, I.C. Effect of partial rootzone drying on the concentration of zeatin-type cytokinins in tomato (Solanum lycopersicum L.) xylem sap and leaves. J. Exp. Bot. 2006, 58, 161–168. [Google Scholar] [CrossRef]
- Ghanem, M.E.; Albacete, A.; Martínez-Andújar, C.; Acosta, M.; Romero-Aranda, R.; Dodd, I.C.; Lutts, S.; Pérez-Alfocea, F. Hormonal changes during salinity-induced leaf senescence in tomato (Solanum lycopersicum L.). J. Exp. Bot. 2008, 59, 3039–3050. [Google Scholar] [CrossRef]
- Merewitz, E.; Gianfagna, T.; Huang, B. Photosynthesis, water use, and root viability under water stress as affected by expression of SAG12-ipt controlling cytokinin synthesis in Agrostis stolonifera. J. Exp. Bot. 2011, 62, 383. [Google Scholar] [CrossRef]
- Nishiyama, R.; Watanabe, Y.; Fujita, Y.; Le, D.T.; Kojima, M.; Werner, T.; Vankova, R.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Kakimoto, T.; et al. Analysis of Cytokinin Mutants and Regulation of Cytokinin Metabolic Genes Reveals Important Regulatory Roles of Cytokinins in Drought, Salt and Abscisic Acid Responses, and Abscisic Acid Biosynthesis. Plant Cell 2012, 23, 2169–2183. [Google Scholar] [CrossRef]
- Hutton, M.J.; Van, S.J.; Davey, J.E. Cytokinins in Germinating Seeds of Phaseolus vulgaris L. I. Changes in Endogenous Levels Within the Cotyledons. Ann. Bot. 1982, 49, 685–691. [Google Scholar] [CrossRef]
- Gilad, T.; Ilan, I.; Reinhold, L. The effect of kinetin and of the embryo axis on the level of reducing sugars in sunflower cotyledons. Isr. J. Bot. 1970, 19, 447–450. [Google Scholar]
- Gepstein, S.; Ilan, I. Evidence for the involvement of cytokinins in the regulation of proteolytic activity in cotyledons of germinating beans. Plant Cell Physiol. 1980, 21, 57–63. [Google Scholar] [CrossRef]
- Hutton, M.J.; Van, S.J. Cytokinins in Germinating Seeds of Phaseolus vulgaris L. II. Transport and Metabolism of 8[14C]t-Zeatin Applied to the Radicle. Ann. Bot. 1982, 49, 701–706. [Google Scholar] [CrossRef]
- Galuszka, P.; Frébort, I.; Sebela, M.; Sauer, P.; Jacobsen, S.; Pec, P. Cytokinin oxidase or dehydrogenase? Mechanism of cytokinin degradation in cereals. Eur. J. Biochem. 2001, 268, 450–461. [Google Scholar] [CrossRef]
- Galuszka, P.; Popelková, H.; Werner, T.; Frébortová, J.; Pospíšilová, H.; Mik, V.; Köllmer, I.; Schmülling, T.; Frébort, I. Biochemical Characterization of Cytokinin Oxidases/Dehydrogenases from Arabidopsis thaliana Expressed in Nicotiana tabacum L. J. Plant Growth Regul. 2007, 26, 255–267. [Google Scholar] [CrossRef]
- Schmülling, T.; Werner, T.; Riefler, M.; Krupková, E.; Bartrina y Manns, I. Structure and function of cytokinin oxidase/dehydrogenase genes of maize, rice, Arabidopsis and other species. J. Plant Res. 2003, 116, 241–252. [Google Scholar] [CrossRef]
- Cai, L.; Zhang, L.; Fu, Q.; Xu, Z. Identification and expression analysis of cytokinin metabolic genesIPTs, CYP735A andCKXs in the biofuel plantJatropha curcas. PeerJ 2018, 6, e4812. [Google Scholar] [CrossRef]
- Chatfield, J.M.; Armstrong, D.J. Regulation of Cytokinin Oxidase Activity in Callus Tissues of Phaseolus vulgaris L. cv Great Northern. Plant Physiol. 1986, 80, 493–499. [Google Scholar] [CrossRef]
- Motyka, V.; Kamínek, M. Regulation of Cytokinin Catabolism in Tobacco Callus Cultures. In Progress in Plant Cellular and Molecular Biology; Springer: Berlin/Heidelberg, Germany, 1990; pp. 492–497. [Google Scholar]
- Motyka, V.; Faiss, M.; Strand, M.; Kaminek, M.; Schmulling, T. Changes in Cytokinin Content and Cytokinin Oxidase Activity in Response to Derepression of ipt Gene Transcription in Transgenic Tobacco Calli and Plants. Plant Physiol. 1996, 112, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Reese, R.N.; Dybing, C.D.; White, C.A.; Page, S.M.; Larson, J.E. Expression of vegetative storage protein (VSP-β) in soybean raceme tissues in response to flower set. J. Exp. Bot. 1995, 46, 957–964. [Google Scholar] [CrossRef]
- Mothes, K.; Engelbrecht, L. Kinetin-induced directed transport of substances in excised leaves in the dark. Phytochemistry 1961, 1, 58–62. [Google Scholar] [CrossRef]
- Han, J.; Mao, P.; Niu, Z.; Sun, R. Changes of Physiology and Biochemistry During Seed Development of Siberian Wildrye. Acta Agrestia Sin. 2000, 8, 237–244. [Google Scholar] [CrossRef]
- Mao, P.; Han, J.; Wang, P.; Rong, Y. Changes of Physiology and Biochemistry During Seed Development of Smooth Bromegrass. Chin. J. Grassl. 2001, 23, 26–31. [Google Scholar] [CrossRef]
- Shi, P. Study on panicle initiation and eight physiological in dicators changes during seeds development of Puccinellia tenuiflora(Griseb.)Scribn.et Merr.cv. Tongde. Master’s Thesis, Qinghai University, Xining, Qinghai, China, 2012. [Google Scholar]
- Bailly, C. Active oxygen species and antioxidants in seed biology. Seed Sci. Res. 2004, 14, 93–107. [Google Scholar] [CrossRef]
- Rashid, M.; Hampton, J.G.; Shaw, M.L.; Rolston, M.P.; Khan, K.M.; Saville, D.J. Oxidative damage in forage rape (Brassica napus L.) seeds following heat stress during seed development. J. Agron. Crop Sci. 2020, 206, 101–117. [Google Scholar] [CrossRef]
- Bhatia, V.S.; Yadav, S.; Jumrani, K.; Guruprasad, K.N. Field Deterioration of Soybean Seed: Role of Oxidative Stresses and Antioxidant Defense Mechanism. J. Plant Biol. 2010, 37, 179–190. [Google Scholar]
- Wang, L.; Ma, H.; Song, L.; Shu, Y.; Gu, W. Comparative proteomics analysis reveals the mechanism of pre-harvest seed deterioration of soybean under high temperature and humidity stress. J. Proteom. 2012, 75, 2109–2127. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.H.; Panda, S.K. Induction of Oxidative Stress in Roots of Oryza sativa L. in Response to Salt Stress. Biol. Plant 2002, 45, 625–627. [Google Scholar] [CrossRef]
- Clarke, S.F.; Guy, P.L.; Burritt, D.J.; Jameson, P.E. Changes in the activities of antioxidant enzymes in response to virus infection and hormone treatment. Physiol. Plant 2002, 114, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Gidrol, X.; Lin, W.S.; Dégousée, N.; Yip, S.F.; Kush, A. Accumulation of reactive oxygen species and oxidation of cytokinin in germinating soybean seeds. Eur. J. Biochem. 1994, 224, 21–28. [Google Scholar] [CrossRef]
- Laureys, F.; Dewitte, W.; Witters, E.; Van Montagu, M.; Inzé, D.; Van Onckelen, H. Zeatin is indispensable for the G2-M transition in tobacco BY-2 cells. FEBS Lett. 1998, 426, 29–32. [Google Scholar] [CrossRef]

| Treatment | 0 Days after Treatment (mg) | 5 Days after Treatment (mg) | 10 Days after Treatment (mg) | 15 Days after Treatment (mg) |
|---|---|---|---|---|
| CK | 8.6 ± 0.9 aA | 35.4 ± 1.3 bAB | 17.2 ± 3.9 bB | 17.9 ± 1.9 bB |
| tZ | 8.6 ± 0.9 aA | 46.5 ± 4.9 aA | 106.4 ± 7.9 aA | 80.6 ± 6.4 aA |
| L | 8.6 ± 0.9 aA | 21.5 ± 1.8 cB | 12.9 ± 1.3 bB | 18.9 ± 2.6 bB |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Wang, Y.-Q.; Deng, Q.-X.; Yang, Z.-W.; Pan, C.-P.; Chi, Z.-H.; Wen, L.; Yang, Y.-M. Effects of Exogenous Trans-Zeatin and Lovastatin on Abortion of Small Seeds in ‘Dawuxing’ Loquat (Eriobotrya Japonica Lindl.). Agriculture 2021, 11, 409. https://doi.org/10.3390/agriculture11050409
Zhang H, Wang Y-Q, Deng Q-X, Yang Z-W, Pan C-P, Chi Z-H, Wen L, Yang Y-M. Effects of Exogenous Trans-Zeatin and Lovastatin on Abortion of Small Seeds in ‘Dawuxing’ Loquat (Eriobotrya Japonica Lindl.). Agriculture. 2021; 11(5):409. https://doi.org/10.3390/agriculture11050409
Chicago/Turabian StyleZhang, Hui, Yong-Qing Wang, Qun-Xian Deng, Zhi-Wu Yang, Cui-Ping Pan, Zhuo-Heng Chi, Lu Wen, and Yun-Miao Yang. 2021. "Effects of Exogenous Trans-Zeatin and Lovastatin on Abortion of Small Seeds in ‘Dawuxing’ Loquat (Eriobotrya Japonica Lindl.)" Agriculture 11, no. 5: 409. https://doi.org/10.3390/agriculture11050409
APA StyleZhang, H., Wang, Y.-Q., Deng, Q.-X., Yang, Z.-W., Pan, C.-P., Chi, Z.-H., Wen, L., & Yang, Y.-M. (2021). Effects of Exogenous Trans-Zeatin and Lovastatin on Abortion of Small Seeds in ‘Dawuxing’ Loquat (Eriobotrya Japonica Lindl.). Agriculture, 11(5), 409. https://doi.org/10.3390/agriculture11050409






