Volatile Organic Compounds from Rice Rhizosphere Bacteria Inhibit Growth of the Pathogen Rhizoctonia solani
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Strains, Culture Medium, and Culture Conditions
2.2. Inhibition of R. Solani by VOCs
2.3. Collection of VOCs
2.4. Analysis of VOCs
2.5. In Vitro Verification Using Pure Compounds
2.6. Evaluation of the Inhibitory Activity of Pure Compounds on the Lesion Development by R. solani on Detached Rice Leaves
2.7. Statistical Analyses
3. Results
3.1. Inhibition of VOCs on R. solani
3.2. Composition of VOCs
3.3. Classification and Abundance of VOCs Emitted from Different Bacterial Strains
3.4. The Relative Importance of Different VOCs
3.5. The Inhibition of R. solani by Pure Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spence, C.; Alff, E.; Johnson, C.; Ramos, C.; Donofrio, N.; Sundaresan, V.; Bais, H. Natural rice rhizospheric microbes suppress rice blast infections. BMC Plant Biol. 2014, 14, 1–17. [Google Scholar] [CrossRef]
- Shrestha, B.K.; Karki, H.S.; Groth, D.E.; Jungkhun, N.; Ham, J.H. Biological Control Activities of Rice-Associated Bacillus sp. Strains against Sheath Blight and Bacterial Panicle Blight of Rice. PLoS ONE 2016, 11, e0146764. [Google Scholar] [CrossRef]
- Boukaew, S.; Klinmanee, C.; Prasertsan, P. Potential for the integration of biological and chemical control of sheath blight disease caused by Rhizoctonia solani on rice. World J. Microb. Biotechnol. 2013, 29, 1885–1893. [Google Scholar] [CrossRef]
- Mew, T.W.; Cottyn, B.; Pamplona, R.; Barrios, H.; Li, X.M.; Chen, Z.Y.; Lu, F.; Nilpanit, N.; Arunyanart, P.; Kim, P.V.; et al. Applying rice seed-associated antagonistic bacteria to manage rice sheath blight in developing countries. Plant Dis. 2004, 88, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Jiang, Z.Y.; Zhu, Q.; Zhong, G.H. Discovery of beta-Carboline Oxadiazole Derivatives as Fungicidal Agents against Rice Sheath Blight. J. Agric. Food Chem. 2018, 66, 9598–9607. [Google Scholar] [CrossRef] [PubMed]
- Bag, M.; Yadav, M.; Mukherjee, A. Bioefficacy of Strobilurin Based Fungicides against Rice Sheath Blight Disease. Transcriptomics 2016, 4, 2. [Google Scholar]
- Postma, J.; Schilder, M.T. Enhancement of soil suppressiveness against Rhizoctonia solani in sugar beet by organic amendments. Appl. Soil Ecol. 2015, 94, 72–79. [Google Scholar] [CrossRef]
- Agtmaal, M.V.; Straathof, A.L.; Termorshuizen, A.; Lievens, B.; Hoffland, E.; Boer, W.D. Volatile-mediated suppression of plant pathogens is related to soil properties and microbial community composition. Soil Biol. Biochem. 2018, 117, 164–174. [Google Scholar] [CrossRef]
- Ahsan, T.; Chen, J.; Zhao, X.; Irfan, M.; Wu, Y. Extraction and identification of bioactive compounds (eicosane and dibutyl phthalate) produced by Streptomyces strain KX852460 for the biological control of Rhizoctonia solani AG-3 strain KX852461 to control target spot disease in tobacco leaf. AMB Express. 2017, 7, 54. [Google Scholar] [CrossRef]
- Kanjanamaneesathian, M.; Kusonwiriyawong, C.; Pengnoo, A.; Nilratana, L. Screening of potential bacterial antagonists for control of sheath blight in rice and development of suitable bacterial formulations for effective application. Australas. Plant Path. 1998, 27, 198–206. [Google Scholar] [CrossRef]
- Ahmad, A.-G.M.; Attia, A.-Z.G.; Mohamed, M.S.; Elsayed, H.E. Fermentation, formulation and evaluation of PGPR Bacillus subtilis isolate as a bioagent for reducing occurrence of peanut soil-borne diseases. J. Integr. Agric. 2019, 18, 2080–2092. [Google Scholar] [CrossRef]
- Goldford, J.E.; Lu, N.X.; Bajic, D.; Estrela, S.; Tikhonov, M.; Sanchez-Gorostiaga, A.; Segre, D.; Mehta, P.; Sanchez, A. Emergent simplicity in microbial community assembly. Science 2018, 361, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Liu, Y.X.; Zhang, N.; Hu, B.; Jin, T.; Xu, H.R.; Qin, Y.; Yan, P.X.; Zhang, X.N.; Guo, X.X.; et al. NRT1.1B is associated with root microbiota composition and nitrogen use in field-grown rice. Nat. Biotechnol. 2019, 37, 676. [Google Scholar] [CrossRef] [PubMed]
- Carrion, V.J.; Cordovez, V.; Tyc, O.; Etalo, D.W.; de Bruijn, I.; de Jager, V.C.L.; Medema, M.H.; Eberl, L.; Raaijmakers, J.M. Involvement of Burkholderiaceae and sulfurous volatiles in disease-suppressive soils. ISME J. 2018, 12, 2307–2321. [Google Scholar] [CrossRef] [PubMed]
- Audrain, B.; Farag, M.A.; Ryu, C.M.; Ghigo, J.M. Role of bacterial volatile compounds in bacterial biology. FEMS Microbiol. Rev. 2015, 39, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Choudoir, M.; Rossabi, S.; Gebert, M.; Helmig, D.; Fierer, N. A Phylogenetic and Functional Perspective on Volatile Organic Compound Production by Actinobacteria. Msystems 2019, 4, e00295-18. [Google Scholar] [CrossRef]
- Cernava, T.; Aschenbrenner, I.A.; Grube, M.; Liebminger, S.; Berg, G. A novel assay for the detection of bioactive volatiles evaluated by screening of lichen-associated bacteria. Front. Microbiol. 2015, 6, 398. [Google Scholar] [CrossRef]
- Rojas-Solís, D.; Zetter-Salmón, E.; Contreras-Pérez, M.; Rocha-Granados, M.d.C.; Macías-Rodríguez, L.; Santoyo, G. Pseudomonas stutzeri E25 and Stenotrophomonas maltophilia CR71 endophytes produce antifungal volatile organic compounds and exhibit additive plant growth-promoting effects. Biocatal. Agric. Biotechnol. 2018, 13, 46–52. [Google Scholar] [CrossRef]
- Cordovez, V.; Carrion, V.J.; Etalo, D.W.; Mumm, R.; Zhu, H.; van Wezel, G.P.; Raaijmakers, J.M. Diversity and functions of volatile organic compounds produced by Streptomyces from a disease-suppressive soil. Front. Microbiol. 2015, 6, 1081. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I.; Racioppi, R.; Scrano, L.; Iacobellis, N.S.; Bufo, S.A. In vitro antifungal activity of Burkholderia gladioli pv. agaricicola against some phytopathogenic fungi. Int. J. Mol. Sci. 2012, 13, 16291–16302. [Google Scholar] [CrossRef]
- Ossowicki, A.; Jafra, S.; Garbeva, P. The antimicrobial volatile power of the rhizospheric isolate Pseudomonas donghuensis P482. PLoS ONE 2017, 12, e0174362. [Google Scholar] [CrossRef]
- Raza, W.; Ling, N.; Yang, L.D.; Huang, Q.W.; Shen, Q.R. Response of tomato wilt pathogen Ralstonia solanacearum to the volatile organic compounds produced by a biocontrol strain Bacillus amyloliquefaciens SQR-9. Sci. Rep.-UK 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Mookherjee, A.; Bera, P.; Mitra, A.; Maiti, M.K. Characterization and Synergistic Effect of Antifungal Volatile Organic Compounds Emitted by the Geotrichum candidum PF005, an Endophytic Fungus from the Eggplant. Microb. Ecol. 2018, 75, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Elkahoui, S.; Djebali, N.; Yaich, N.; Azaiez, S.; Hammami, M.; Essid, R.; Limam, F. Antifungal activity of volatile compounds-producing Pseudomonas P2 strain against Rhizoctonia solani. World J. Microb. Biot. 2015, 31, 175–185. [Google Scholar] [CrossRef]
- Li, X.Y.; Mao, Z.C.; Wu, Y.X.; Ho, H.H.; He, Y.Q. Comprehensive volatile organic compounds profiling of Bacillus species with biocontrol properties by head space solid phase microextraction with gas chromatography-mass spectrometry. Biocontrol. Sci. Technol. 2015, 25, 132–143. [Google Scholar] [CrossRef]
- Hernandez-Leon, R.; Rojas-Solis, D.; Contreras-Perez, M.; Orozco-Mosqueda, M.D.; Macias-Rodriguez, L.I.; Reyes-de la Cruz, H.; Valencia-Cantero, E.; Santoyo, G. Characterization of the antifungal and plant growth-promoting effects of diffusible and volatile organic compounds produced by Pseudomonas fluorescens strains. Biol. Control 2015, 81, 83–92. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Bufo, S.A.; Racioppi, R.; Camele, I. Biochemical characterization of volatile secondary matabolites produced by Burkholderia gladioli pv. agaricicola. Int. J. Drug Discov. 2013, 5, 181–184. [Google Scholar]
- Wang, Z.; Wang, C.; Li, F.; Li, Z.; Chen, M.; Wang, Y.; Qiao, X.; Zhang, H. Fumigant activity of volatiles from Streptomyces alboflavus TD-1 against Fusarium moniliforme Sheldon. J. Microbiol. 2013, 51, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Fernando, W.G.D.; Ramarathnam, R.; Krishnamoorthy, A.S.; Savchuk, S.C. Identification and use of potential bacterial organic antifungal volatiles in biocontrol. Soil Biol. Biochem. 2005, 37, 955–964. [Google Scholar] [CrossRef]
- Yuan, J.; Raza, W.; Shen, Q.; Huang, Q. Antifungal activity of Bacillus amyloliquefaciens NJN-6 volatile compounds against Fusarium oxysporum f. sp. cubense. Appl. Environ. Microbiol. 2012, 78, 5942–5944. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhang, B.; Liu, H.; Han, J.; Zhang, Y. Identification of endophytic Bacillus velezensis ZSY-1 strain and antifungal activity of its volatile compounds against Alternaria solani and Botrytis cinerea. Biol. Control 2017, 105, 27–39. [Google Scholar] [CrossRef]
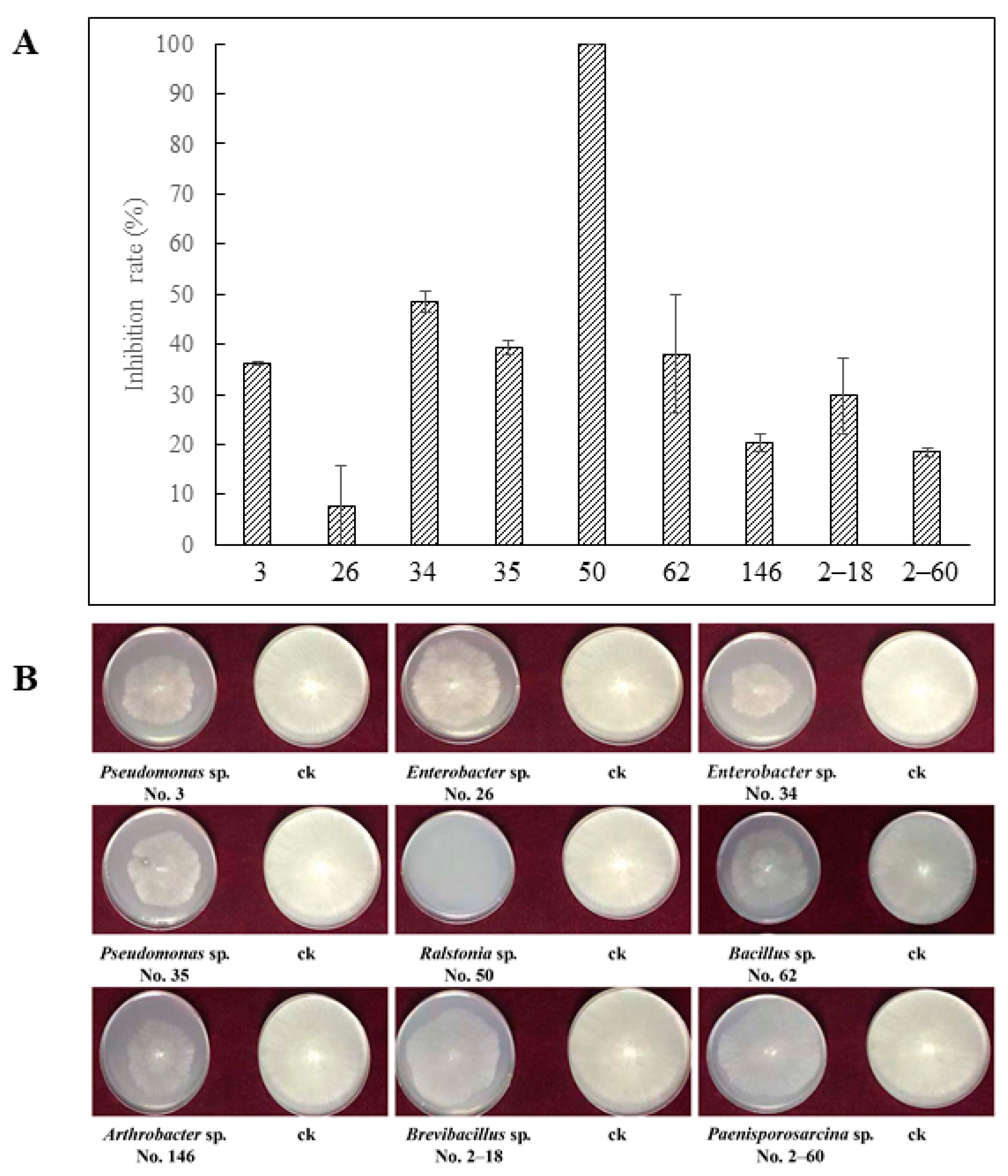


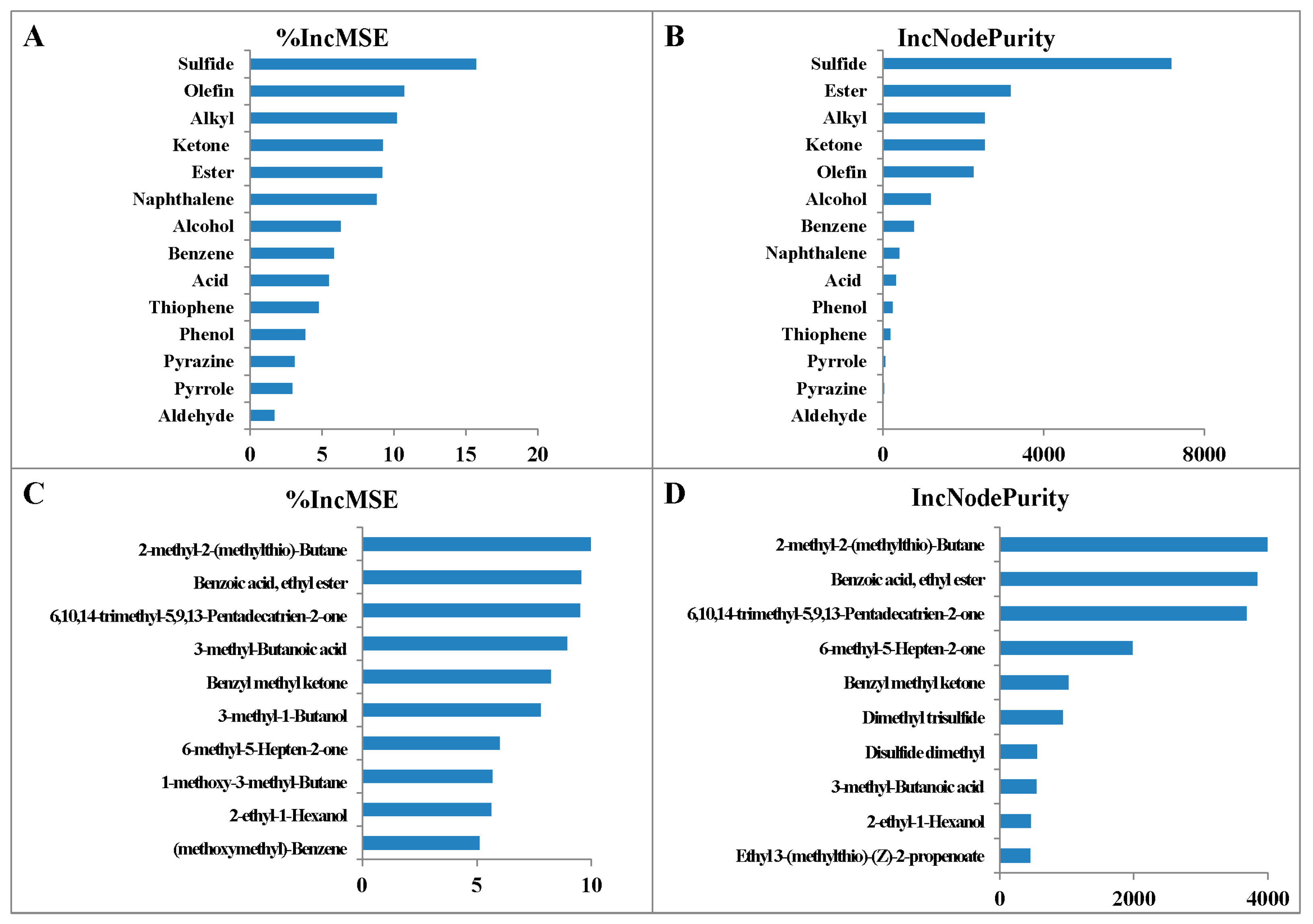


| VOCs | 3 | 26 | 34 | 35 | 50 | 62 | 146 | 2–18 | 2–60 |
|---|---|---|---|---|---|---|---|---|---|
| (×108 Ab × s) | |||||||||
| (E)-1-Methyl-2-(prop-1-en-1-yl)disulfane | 0.14 | ||||||||
| beta.-Phenylethyl butyrate | 0.15 | ||||||||
| 11-Dodecen-2-one | 2.34 | ||||||||
| 2-methyl-1-Butanol | 1.93 | ||||||||
| 3-methyl-1-Butanol | 3.12 | 4.76 | 1.16 | 0.11 | 0.51 | 0.18 | |||
| 1-Butanol, 3-methyl-, acetate | 0.17 | 0.12 | |||||||
| 1-Octadecene | 0.22 | ||||||||
| 1-Octanol | 0.47 | ||||||||
| 7-methyl-1-Octene | 0.30 | ||||||||
| 1-Pentadecene | 0.26 | ||||||||
| 2-Chloropropionic acid, hexadecyl ester | 0.93 | ||||||||
| 5-heptyldihydro-2(3H)-Furanone | 0.13 | 0.42 | |||||||
| 2,4,6-Cycloheptatrien-1-one | 1.23 | ||||||||
| 2,4-Dithiapentane | 0.43 | ||||||||
| 6-methyl-2,4-Heptanedione | 0.26 | ||||||||
| 3,7,11-trimethyl-2,6,10-Dodecatrien-1-ol | 1.82 | ||||||||
| 2-Decanone | 0.12 | 0.12 | 0.55 | 0.63 | 0.49 | 0.30 | |||
| 2-Dodecanone | 0.25 | 1.45 | |||||||
| 2-ethyl-1-Hexanol | 0.20 | 0.18 | 0.68 | 0.14 | 0.30 | 0.68 | 0.97 | ||
| 2-Heptanone | 0.18 | 0.25 | 1.99 | 1.67 | 0.27 | 0.38 | |||
| 3-methyl-2-Heptanone | 0.24 | ||||||||
| 5-methyl-2-Heptanone | 3.84 | 7.12 | 0.50 | ||||||
| 6-methyl-2-Heptanone | 1.12 | 2.75 | 1.36 | 1.48 | |||||
| 2-Hexadecanone | 0.12 | ||||||||
| 2-Hexanone | 0.12 | 0.41 | 0.18 | ||||||
| 3,4-dimethyl-2-Hexanone | 0.13 | ||||||||
| 5-methyl-2-Hexanone | 3.86 | 0.52 | 0.92 | ||||||
| 2-Nonanone | 0.48 | 0.40 | 0.71 | 0.87 | 0.40 | ||||
| 2-Octanone | 0.26 | 0.45 | |||||||
| 2-Pentadecanone | 0.14 | ||||||||
| 2-Pentanone | 0.13 | 0.55 | |||||||
| 3-methyl-2-Pentanone | 3.28 | ||||||||
| 2-Tetradecanone | 0.19 | 0.89 | 1.88 | ||||||
| 2-Tridecanone | 0.26 | ||||||||
| 2-Undecanone | 0.62 | 0.55 | 1.18 | 0.63 | |||||
| 3-Dodecanone | 0.20 | ||||||||
| 7-phenyl-3-Heptene | 0.19 | ||||||||
| 5-methyl-3-Hexanone | 3.58 | 0.90 | 0.57 | ||||||
| 3-Pentadecanone | 0.65 | ||||||||
| 3-Pentanone | 0.44 | ||||||||
| 3-Tridecanone | 0.52 | 0.12 | |||||||
| 5-methyl-4-Hexen-3-one | 0.90 | ||||||||
| 6,10,14-trimethyl-5,9,13-Pentadecatrien-2-one | 3.20 | ||||||||
| 6-methyl-5-Hepten-2-one | 0.16 | 0.15 | |||||||
| 6-tert-Butyl-2,4-dimethylphenol | 0.52 | 0.95 | |||||||
| 7-Methyloctane-2,4-dione, enol form | 0.24 | ||||||||
| Acetic acid, 2-phenylethyl ester | 0.31 | ||||||||
| Acetic acid, chloro-, hexadecyl ester | 0.29 | ||||||||
| Acetic acid, non-3-enyl ester, cis- | 0.22 | ||||||||
| (2-methoxyethyl)-Benzene | 1.43 | ||||||||
| (methoxymethyl)-Benzene | 1.35 | ||||||||
| Benzeneacetic acid, ethyl ester | 0.41 | ||||||||
| Benzoic acid, ethyl ester | 1.74 | ||||||||
| Benzyl alcohol | 1.59 | 0.44 | |||||||
| Benzyl methyl ketone | 0.14 | 0.79 | 0.13 | 0.52 | 0.11 | ||||
| 1-methoxy-3-methyl-Butane | 9.96 | ||||||||
| 2-methyl-2-(methylthio)-Butane | 0.63 | ||||||||
| Butanethioic acid, S-methyl ester | 0.74 | ||||||||
| Butanoic acid, 1-ethenylhexyl ester | 0.12 | ||||||||
| 3-methyl-Butanoic acid | 0.45 | 1.87 | 0.16 | ||||||
| Butanoic acid, 3-methyl-, ethyl ester | 0.12 | 0.42 | |||||||
| cis-Bicyclo [3.3.0]oct-2-ene | 0.83 | ||||||||
| Cycloheptene | 0.43 | ||||||||
| 1-methyl-Cyclohexene | 0.24 | ||||||||
| 3-ethenyl-Cyclopentene | 0.17 | ||||||||
| Dicyclopentadiene | 0.16 | 0.24 | 0.14 | 0.20 | 0.29 | 0.18 | |||
| Dimethyl trisulfide | 0.32 | 0.20 | 0.19 | 1.98 | 1.67 | 1.96 | 0.87 | ||
| Disulfide dimethyl | 18.7 | 1.18 | 1.33 | 2.23 | 16.1 | 3.13 | 9.98 | 3.21 | |
| Dodecanoic acid, ethyl ester | 0.14 | ||||||||
| 1-(2-aminophenyl)-Ethanone | 0.13 | 0.16 | |||||||
| Ethyl 13-methyl-tetradecanoate | 0.23 | ||||||||
| Ethyl 3-(methylthio)-(E)-2-propenoate | 0.23 | ||||||||
| Ethyl 3-(methylthio)-(Z)-2-propenoate | 0.26 | ||||||||
| Ethyl tridecanoate | 0.38 | ||||||||
| Methyl Isobutyl Ketone | 1.29 | 0.42 | 0.42 | ||||||
| Methyl isovalerate | 0.58 | ||||||||
| Methyl thiolacetate | 2.62 | ||||||||
| decahydro-Naphthalene | 0.44 | 0.84 | |||||||
| Octadecanal | 0.12 | ||||||||
| Phenylethyl Alcohol | 1.69 | 1.63 | 0.15 | 0.57 | |||||
| Propanoic acid, 2-phenylethyl ester | 0.15 | ||||||||
| Pyrazine, 2-ethyl-5-methyl- | 0.24 | ||||||||
| trimethyl-Pyrazine | 0.50 | ||||||||
| Pyrrole | 0.41 | ||||||||
| S-Methyl 3-methylbutanethioate | 0.17 | 3.70 | 0.34 | 0.15 | 3.72 | ||||
| TATP | 0.54 | ||||||||
| Tetradecanoic acid, ethyl ester | 0.64 | ||||||||
| 2-methoxy-5-methyl-Thiophene | 0.35 | ||||||||
| Undecanoic acid, ethyl ester | 0.24 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, E.; Liu, X.; Si, Z.; Li, X.; Bi, J.; Dong, W.; Chen, M.; Wang, S.; Zhang, J.; Song, A.; et al. Volatile Organic Compounds from Rice Rhizosphere Bacteria Inhibit Growth of the Pathogen Rhizoctonia solani. Agriculture 2021, 11, 368. https://doi.org/10.3390/agriculture11040368
Wang E, Liu X, Si Z, Li X, Bi J, Dong W, Chen M, Wang S, Zhang J, Song A, et al. Volatile Organic Compounds from Rice Rhizosphere Bacteria Inhibit Growth of the Pathogen Rhizoctonia solani. Agriculture. 2021; 11(4):368. https://doi.org/10.3390/agriculture11040368
Chicago/Turabian StyleWang, Enzhao, Xiongduo Liu, Zhiyuan Si, Xu Li, Jingjing Bi, Weiling Dong, Mingshun Chen, Sai Wang, Jiayin Zhang, Alin Song, and et al. 2021. "Volatile Organic Compounds from Rice Rhizosphere Bacteria Inhibit Growth of the Pathogen Rhizoctonia solani" Agriculture 11, no. 4: 368. https://doi.org/10.3390/agriculture11040368
APA StyleWang, E., Liu, X., Si, Z., Li, X., Bi, J., Dong, W., Chen, M., Wang, S., Zhang, J., Song, A., & Fan, F. (2021). Volatile Organic Compounds from Rice Rhizosphere Bacteria Inhibit Growth of the Pathogen Rhizoctonia solani. Agriculture, 11(4), 368. https://doi.org/10.3390/agriculture11040368






