Biochar Grafted on CMC-Terpolymer by Green Microwave Route for Sustainable Agriculture
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Biochar Production
2.3. Sandy Soil
2.4. Preparation of CMC-g-(TerPols)/BC by Conventional Method
2.5. Preparation of CMC-g-(TerPols)/BC by Microwave Method
2.6. Swelling Behavior in Different Media and Equilibrium Water Absorbance of (CMC-TerPols)/BC Superabsorbent Composite
2.7. Water Retention (WR) and Water Holding Capacity (WHC) of Soil with IPNCB Composite
2.8. Degradation Rate (DR) of IPNCB Composite in Soil
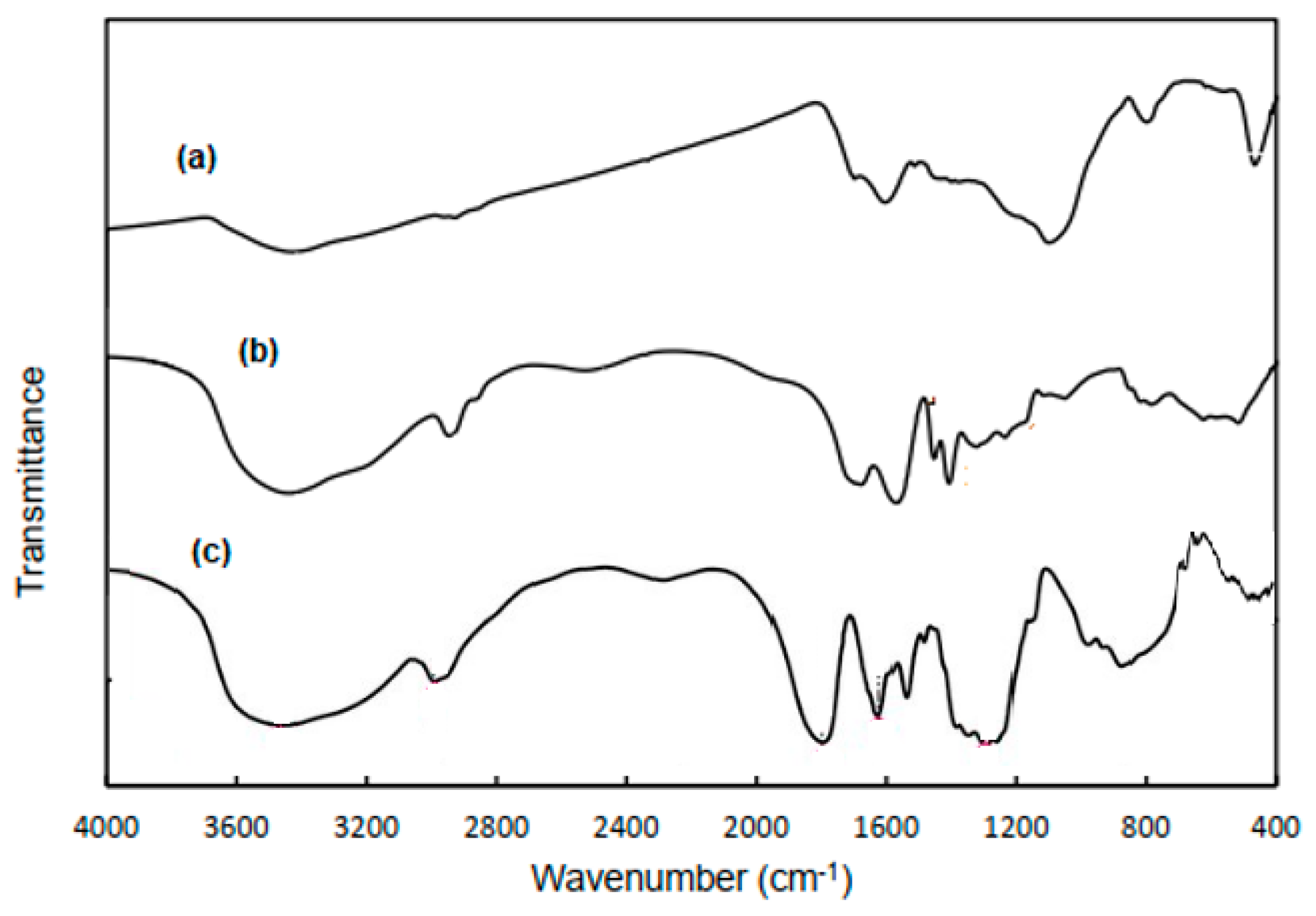
2.9. Characterization of IPNBC Superabsorbent Composite
3. Results and Discussion
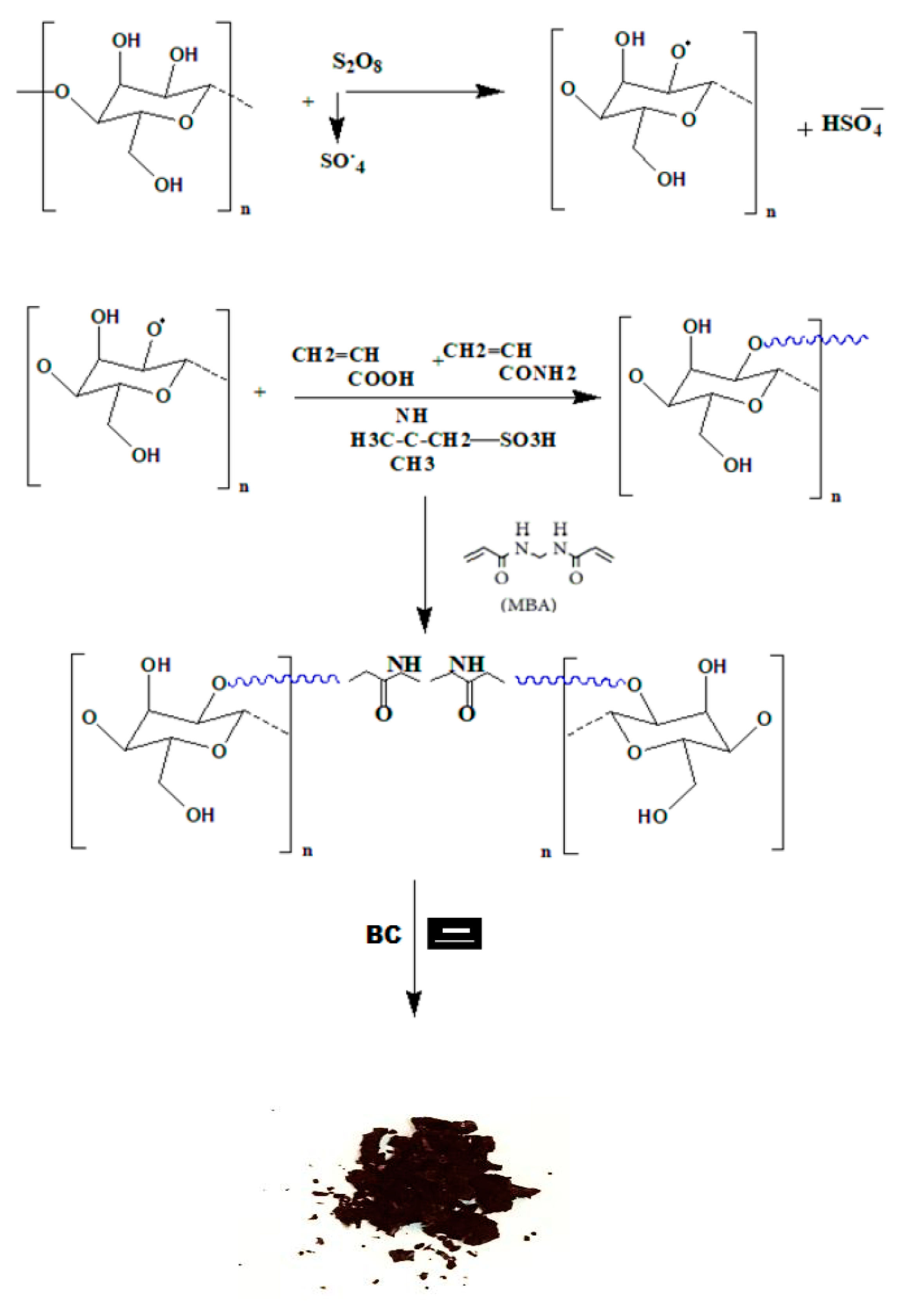
3.1. Synthesis of CMC-g-(TerPols)/BC Composite and Spectral Analysis
3.2. Optimization Parameters
3.2.1. Optimization Time
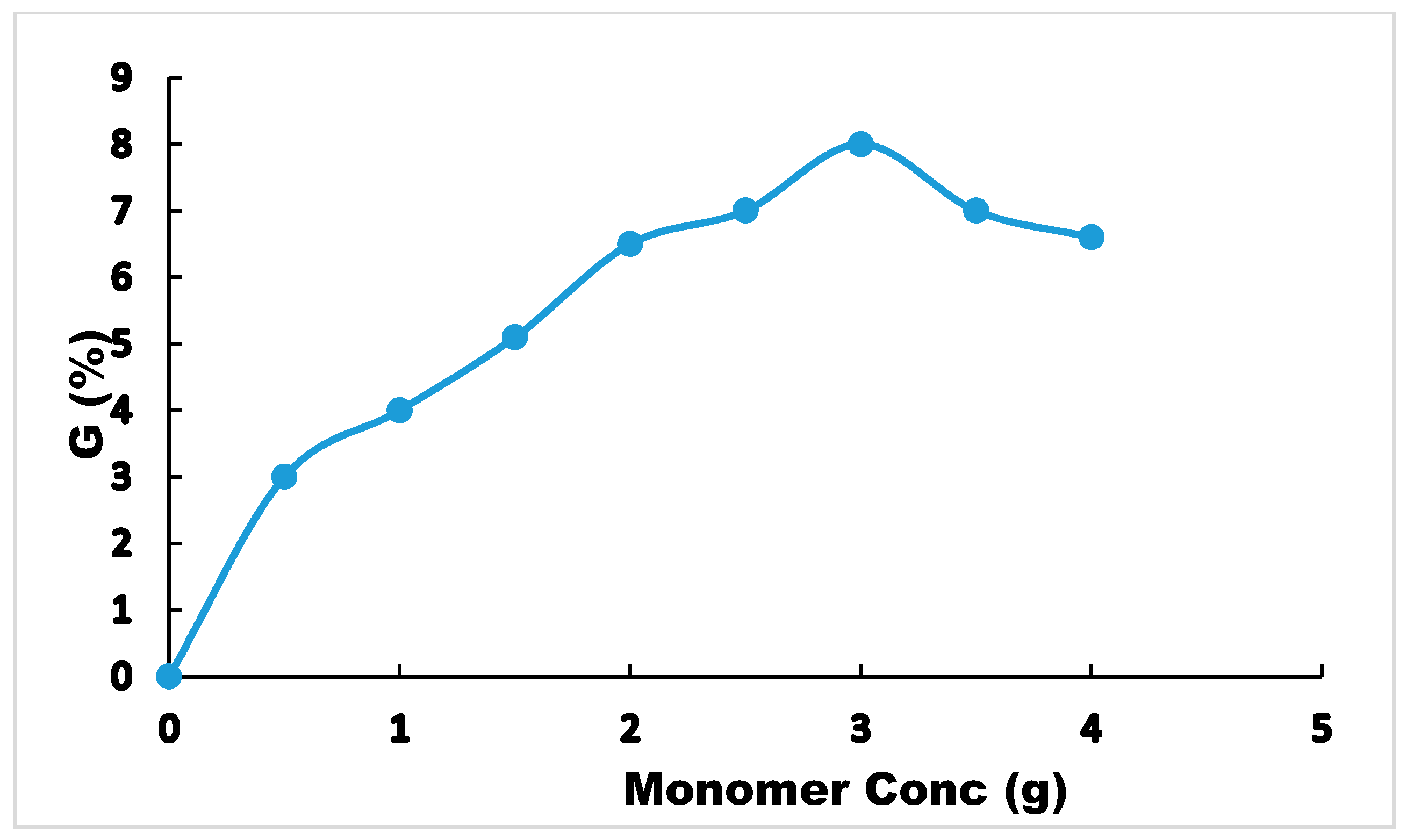
3.2.2. Optimum Initiator and Monomer Ratio
3.2.3. Optimum Crosslinker Ratio
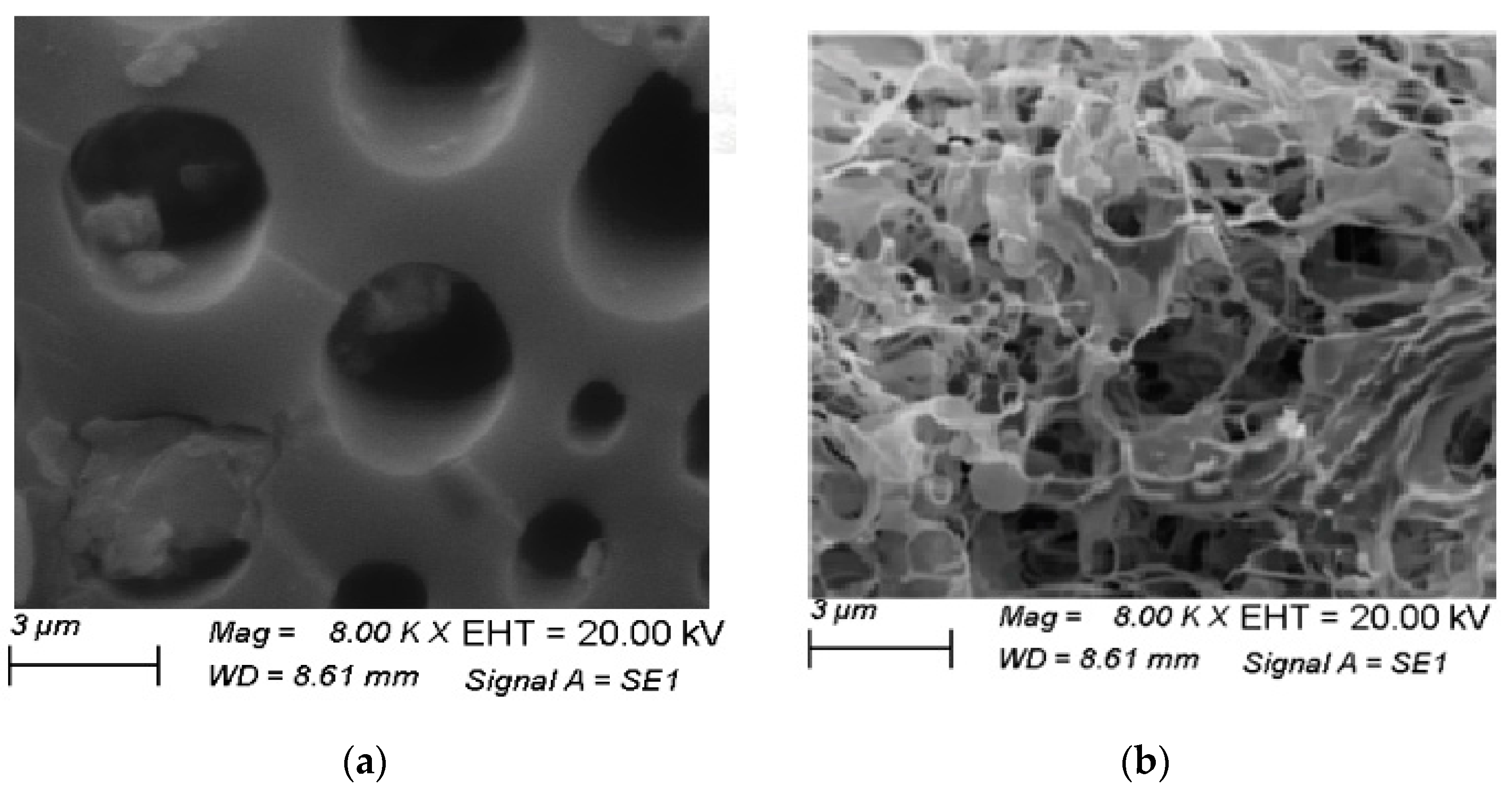
3.3. Morphological Analysis SEM
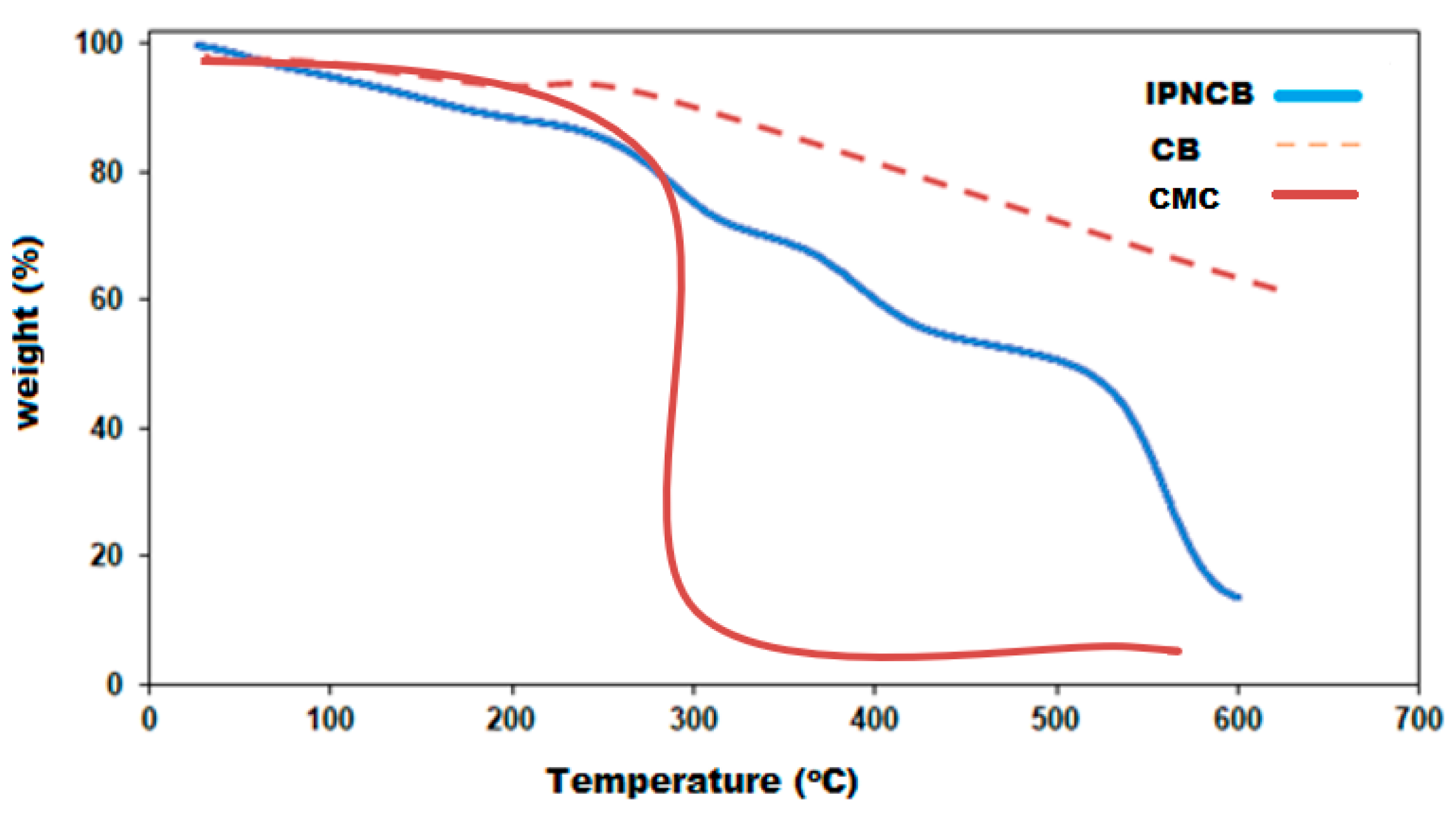
3.4. Thermogravimetric Analysis (TGA)
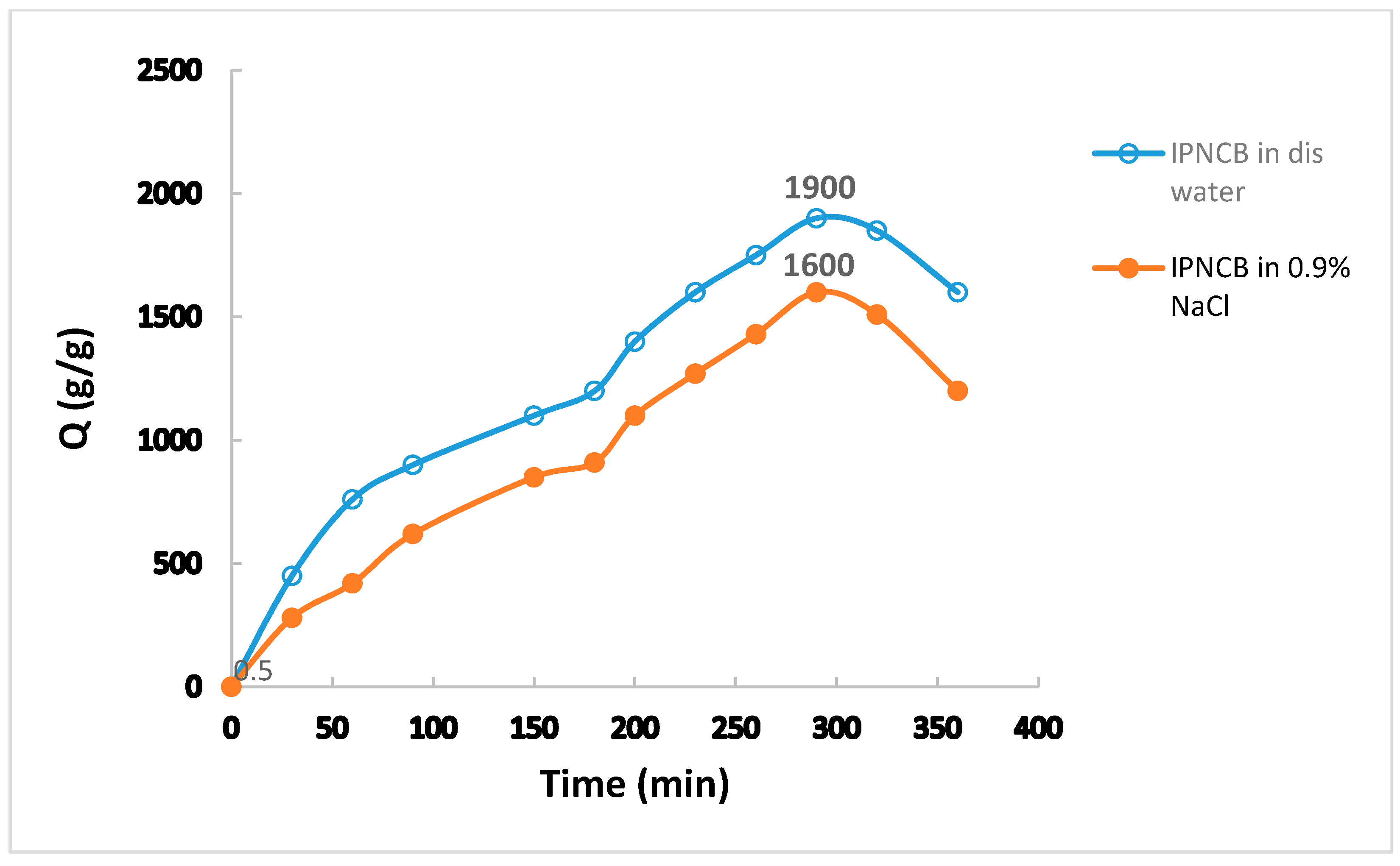
3.5. Equilibrium Water Absorbency and Swelling Behavior of IPNCB Composite in Different Media
3.6. Physical Properties of the Soil with IPNCB Composite
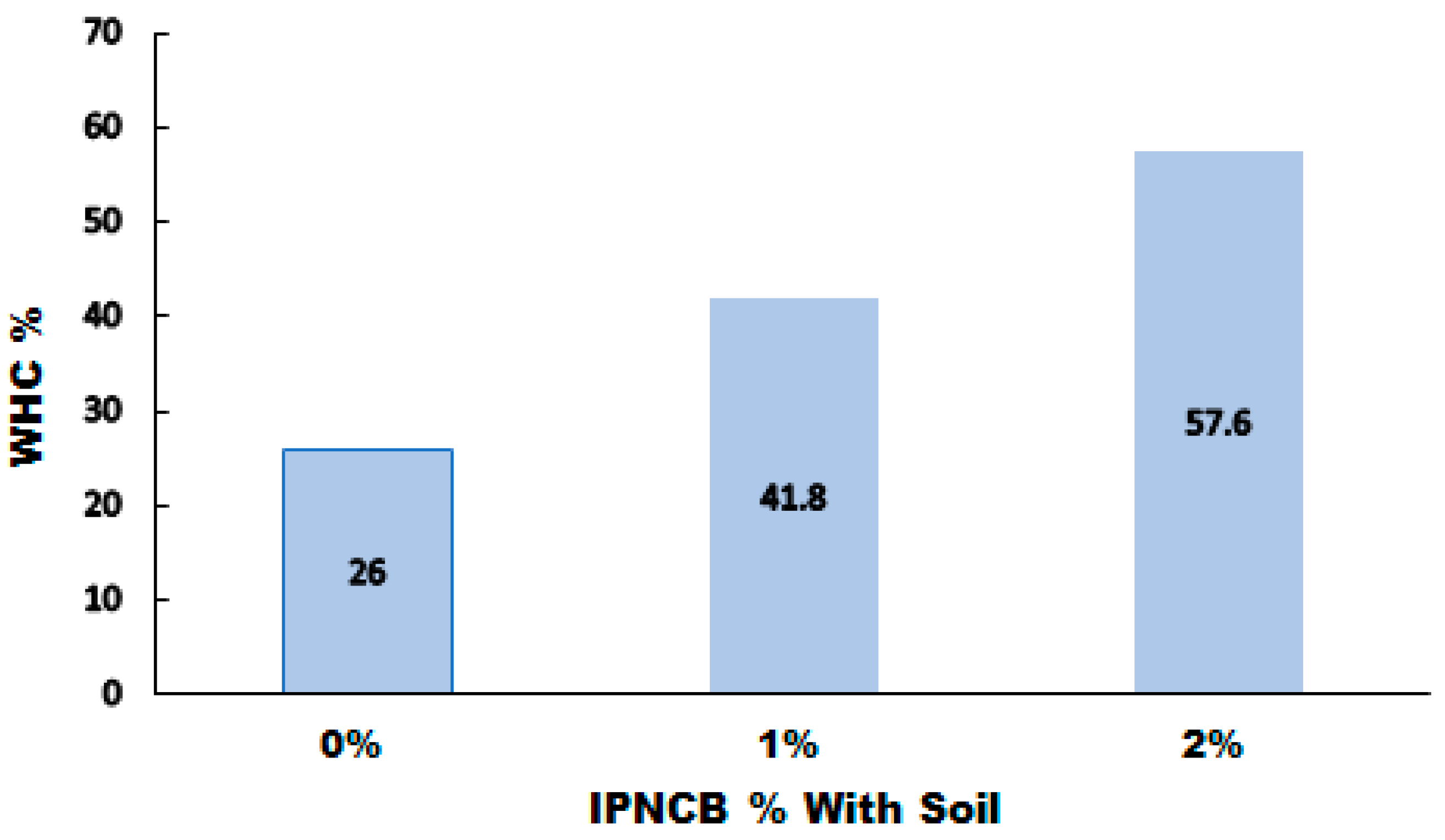
3.6.1. Water Holding Capacity (WHC)
3.6.2. Water Retention (WR)
3.7. Degradation Rate of IPNCB in Sandy Soil
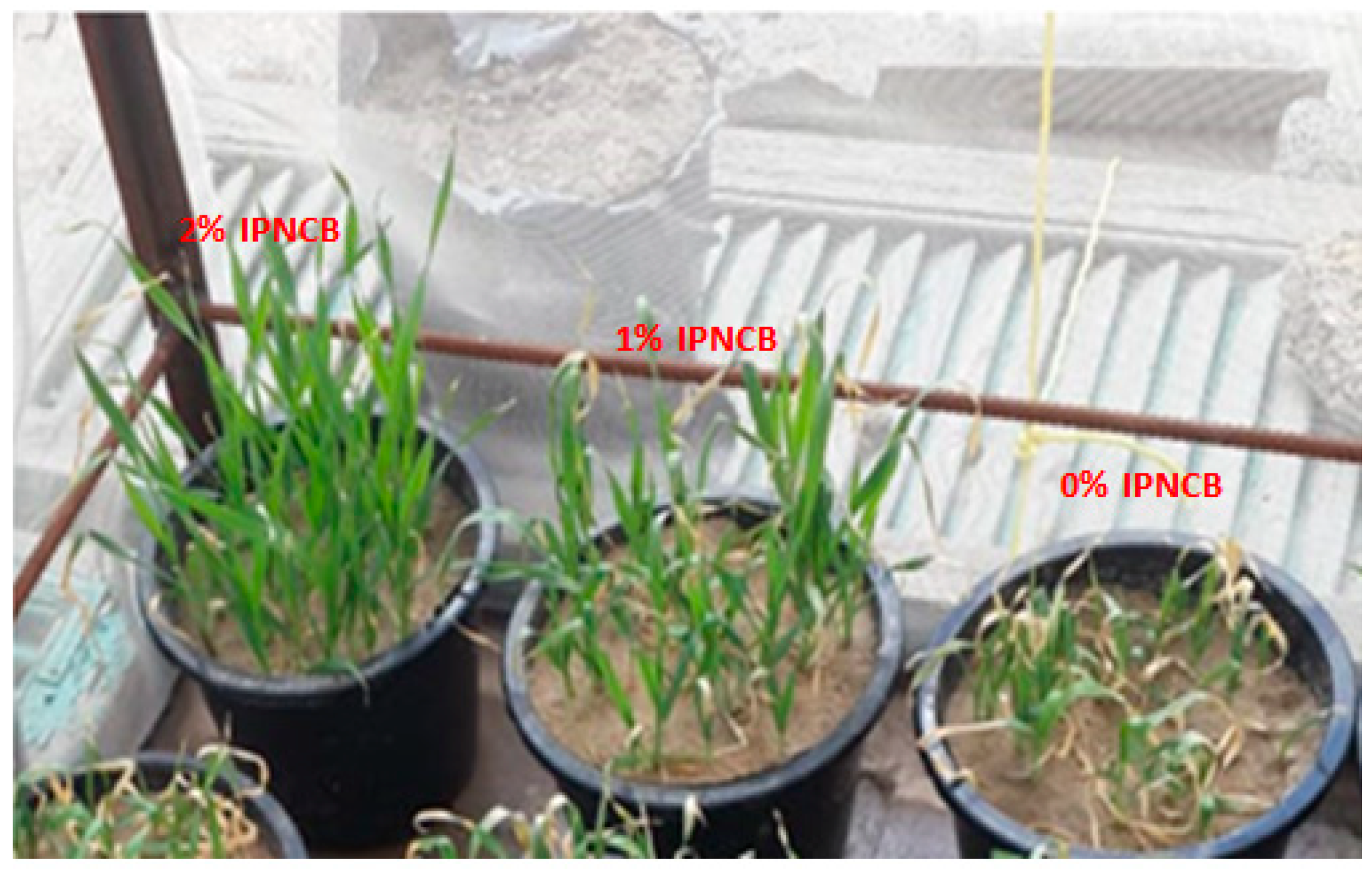
3.8. Effect of IPNCB Composite on Plant Growth
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Han, B.; Benner, S.G.; Flores, A.N. Evaluating impacts of climate change on future water scarcity in an intensively managed semi-arid region using a coupled model of biophysical processes and water rights. Hydrol. Earth Syst. Sci. Discuss. 2018, 2017, 1–53. [Google Scholar]
- Rashidzadeh, A.; Olad, A. Slow-released NPK fertilizer encapsulated by NaAlg-g-poly (AA-co-AAm)/MMT superabsorbent nanocomposite. Carbohydr. Polym. 2014, 114, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Raouf, M.E.; El-Saeed, S.M.; Zaki, E.G.; Al-Sabagh, A.M. Green chemistry approach for preparation of hydrogels for agriculture applications through modification of natural polymers and investigating their swelling properties. Egypt. J. Pet. 2018, 27, 1345–1355. [Google Scholar] [CrossRef]
- Hasija, V.; Sharma, K.; Kumar, V.; Sharma, S.; Sharma, V. Green synthesis of agar/Gum Arabic based superabsorbent as an alternative for irrigation in agriculture. Vacuum 2018, 157, 458–464. [Google Scholar] [CrossRef]
- El-Kafrawy, A.F.; El-Saeed, S.M.; Farag, R.K.; El-Saied, H.A.-A.; Abdel-Raouf, M.E.-S. Adsorbents based on natural polymers for removal of some heavy metals from aqueous solution. Egypt. J. Pet. 2017, 26, 23–32. [Google Scholar] [CrossRef]
- Abdul-Raheim, A.-R.M.; El-Saeed Shimaa, M.; Farag, R.K.; Abdel-Raouf Manar, E.; Reem, K.F.; Abdel-Raouf Manar, E. Low cost biosorbents based on modified starch iron oxide nanocomposites for selective removal of some heavy metals from aqueous solutions. Adv. Mater. Lett. 2016, 7, 402–409. [Google Scholar] [CrossRef]
- Farag, R.K.; El-Saeed, S.M.; Abdel-Raouf, M.E. Synthesis and investigation of hydrogel nanoparticles based on natural polymer for removal of lead and copper(II) ions. Desalination Water Treat. 2015, 57, 16150–16160. [Google Scholar] [CrossRef]
- Peppas, N.A.; Van Blarcom, D.S. Hydrogel-based biosensors and sensing devices for drug delivery. J. Control. Release 2016, 240, 142–150. [Google Scholar] [CrossRef]
- Chen, Y.; Tan, Z.; Wang, W.; Peng, Y.-Y.; Narain, R. Injectable, Self-Healing, and Multi-Responsive Hydrogels via Dynamic Covalent Bond Formation between Benzoxaborole and Hydroxyl Groups. Biomacromolecules 2019, 20, 1028–1035. [Google Scholar] [CrossRef]
- Yuan, T.; Zhang, L.; Li, K.; Fan, H.; Fan, Y.; Liang, J.; Zhang, X. Collagen hydrogel as an immunomodulatory scaffold in cartilage tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 102, 337–344. [Google Scholar] [CrossRef]
- Ahmad, M.; Zhang, B.; Wang, J.; Xu, J.; Manzoor, K.; Ahmad, S.; Ikram, S. New method for hydrogel synthesis from diphenylcarbazide chitosan for selective copper removal. Int. J. Biol. Macromol. 2019, 136, 189–198. [Google Scholar] [CrossRef]
- Du, H.; Shi, S.; Liu, W.; Teng, H.; Piao, M. Processing and modification of hydrogel and its application in emerging contaminant adsorption and in catalyst immobilization: A rev1. Environ. Sci. Pollut. Res. 2020, 27, 12967–12994. [Google Scholar] [CrossRef]
- Akl, Z.F.; El-Saeed, S.M.; Atta, A.M. In-situ synthesis of magnetite acrylamide amino-amidoxime nanocomposite adsorbent for highly efficient sorption of U(VI) ions. J. Ind. Eng. Chem. 2016, 34, 105–116. [Google Scholar] [CrossRef]
- El-Hoshoudy, A.; Zaki, E.; Elsaeed, S. Experimental and Monte Carlo simulation of palmitate-guar gum derivative as a novel flooding agent in the underground reservoir. J. Mol. Liq. 2020, 302, 112502. [Google Scholar] [CrossRef]
- El-hoshoudy, A.N.; Zaki, E.G.; Elsaeed, S.M. Biopolymers composites as oil improving candidates-article review. Pet. Coal 2019, 61, 1365–1377. [Google Scholar]
- Kluczka, J.; Gnus, M.; Kazek-Kęsik, A.; Dudek, G. Zirconium-chitosan hydrogel beads for removal of boron from aqueous solutions. Polymer 2018, 150, 109–118. [Google Scholar] [CrossRef]
- Demitri, C.; Scalera, F.; Madaghiele, M.; Sannino, A.; Maffezzoli, A. Potential of Cellulose-Based Superabsorbent Hydrogels as Water Reservoir in Agriculture. Int. J. Polym. Sci. 2013, 2013, 435073. [Google Scholar] [CrossRef]
- Yoon, S.-D. Cross-Linked Potato Starch-Based Blend Films Using Ascorbic Acid as a Plasticizer. J. Agric. Food Chem. 2013, 62, 1755–1764. [Google Scholar] [CrossRef]
- Chandrika, K.P.; Singh, A.; Rathore, A.; Kumar, A.; Poorna, C.K. Novel cross linked guar gum-g-poly(acrylate) porous superabsorbent hydrogels: Characterization and swelling behaviour in different environments. Carbohydr. Polym. 2016, 149, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Nnadi, F.; Brave, C. Environmentally friendly superabsorbent polymers for water conservation in agricultural lands. J. Soil Sci. Environ. Manag. 2011, 2, 206–211. [Google Scholar]
- Refat, M.S.; Ibrahim, H.K.; Sowellim, S.Z.A.; Soliman, M.H.; Saeed, E.M. Spectroscopic and Thermal Studies of Mn(II), Fe(III), Cr(III) and Zn(II) Complexes Derived from the Ligand Resulted by the Reaction between 4-Acetyl Pyridine and Thiosemicarbazide. J. Inorgan. Organomet. Polym. Mater. 2009, 19, 521–531. [Google Scholar] [CrossRef]
- Samak, D.H.; El-Sayed, Y.S.; Shaheen, H.M.; El-Far, A.H.; Abd El-Hack, M.E.; Noreldin, A.E.; El-Naggar, K.; Abdelnour, S.A.; Saied, E.M.; El-Seedi, H.R.; et al. Developmental Toxicity of Carbon Nanoparticles during Embryogenesis in Chicken. Environ. Sci. Pollut. Res. 2020, 27, 19058–19072. [Google Scholar] [CrossRef]
- Saied, E.M.; Banhart, S.; Bürkle, S.E.; Heuer, D.; Arenz, C. A Series of Ceramide Analogs Modified at the 1-Position with Potent Activity against the Intracellular Growth of Chlamydia Trachomatis. Future Med. Chem. 2015, 7, 1971–1980. [Google Scholar] [CrossRef]
- Xiao, R.; Awasthi, M.K.; Li, R.; Park, J.; Pensky, S.M.; Wang, Q.; Wang, J.J.; Zhang, Z. Recent developments in biochar utilization as an additive in organic solid waste composting: A review. Bioresour. Technol. 2017, 246, 203–213. [Google Scholar] [CrossRef]
- Tang, J.; Mu, B.; Zong, L.; Wang, A. From waste hot-pot oil as carbon precursor to development of recyclable attapulgite/carbon composites for wastewater treatment. J. Environ. Sci. 2019, 75, 346–358. [Google Scholar] [CrossRef]
- Sial, T.A.; Lan, Z.; Khan, M.N.; Zhao, Y.; Kumbhar, F.; Liu, J.; Zhang, A.; Hill, R.L.; Lahori, A.H.; Memon, M. Evaluation of orange peel waste and its biochar on greenhouse gas emissions and soil biochemical properties within a loess soil. Waste Manag. 2019, 87, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Yun, S.G.; Kweon, U.G.; Park, S.B.; Chung, E.S.; Kang, W.S. Seasonal difference in aerobic storage, ruminal degradation and chemical composition of wet brewers’ grain. RDA J. Agric. Sci. (Korea Repub.) 1996, 38, 609. [Google Scholar]
- Zhang, M.; Cheng, Z.; Zhao, T.; Liu, M.; Hu, M.; Li, J. Synthesis, Characterization, and Swelling Behaviors of Salt-Sensitive Maize Bran–Poly(acrylic acid) Superabsorbent Hydrogel. J. Agric. Food Chem. 2014, 62, 8867–8874. [Google Scholar] [CrossRef] [PubMed]
- Kuang, J.; Yuk, K.Y.; Huh, K.M. Polysaccharide-based superporous hydrogels with fast swelling and superabsorbent properties. Carbohydr. Polym. 2011, 83, 284–290. [Google Scholar] [CrossRef]
- Sharma, K.; Kaith, B.; Kumar, V.; Kalia, S.; Kumar, V.; Swart, H. Water retention and dye adsorption behavior of Gg-cl-poly(acrylic acid-aniline) based conductive hydrogels. Geoderma 2014, 232, 45–55. [Google Scholar] [CrossRef]
- Casaburi, A.; Rojo, Ú.M.; Cerrutti, P.; Vázquez, A.; Foresti, M.L. Carboxymethyl cellulose with tailored degree of substitution obtained from bacterial cellulose. Food Hydrocoll. 2018, 75, 147–156. [Google Scholar] [CrossRef]
- Peng, Z.; Chen, F. Synthesis and Properties of Lignin-Based Polyurethane Hydrogels. Int. J. Polym. Mater. 2011, 60, 674–683. [Google Scholar] [CrossRef]
- Sen, G.; Mishra, S.; Jha, U.; Pal, S. Microwave initiated synthesis of polyacrylamide grafted guar gum (GG-g-PAM)—Characterizations and application as matrix for controlled release of 5-amino salicylic acid. Int. J. Biol. Macromol. 2010, 47, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Anbarasan, R.; Jayaseharan, J.; Sudha, M.; Nirmala, P.V.; Gopalan, A. Peroxydisulphate initiated graft copolymerization of o-toluidine onto synthetic fibres–A kinetic approach. Macromol. Chem. Phys. 2000, 201, 1869–1876. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953. [Google Scholar]
- Wang, W.B.; Wang, A.Q. Preparation, Swelling and Water-Retention Properties of Crosslinked Superabsorbent Hydrogels Based on Guar Gum. Adv. Mater. Res. 2010, 96, 177–182. [Google Scholar] [CrossRef]
- Takaya, C.; Fletcher, L.; Singh, S.; Anyikude, K.; Ross, A. Phosphate and ammonium sorption capacity of biochar and hydrochar from different wastes. Chemosphere 2016, 145, 518–527. [Google Scholar] [CrossRef]
- Wang, T.; Liu, X.; Wang, D.; Gong, Z.; Si, B.; Zhai, Y. Persulfate assisted hydrothermal processing of spirulina for enhanced deoxidation carbonization. Bioresour. Technol. 2021, 322, 124543. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Fang, Q.; Chen, B.; Lin, Y.; Guan, Y. Aromatic and Hydrophobic Surfaces of Wood-derived Biochar Enhance Perchlorate Adsorption via Hydrogen Bonding to Oxygen-containing Organic Groups. Environ. Sci. Technol. 2014, 48, 279–288. [Google Scholar] [CrossRef]
- Zhao, Y.; Su, H.; Fang, L.; Tan, T. Superabsorbent hydrogels from poly (aspartic acid) with salt-, temperature-and pH-responsiveness properties. Polymer (Guildf.) 2005, 46, 5368–5376. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Q.; Wang, A. pH-responsive carboxymethylcellulose-g-poly (sodium acrylate)/polyvinylpyrrolidone semi-IPN hydrogels with enhanced responsive and swelling properties. Macromol. Res. 2011, 19, 57–65. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Kumar, B.; Rhim, J.-W. Green and facile synthesis of carboxymethylcellulose/ZnO nanocomposite hydrogels crosslinked with Zn2+ ions. Int. J. Biol. Macromol. 2020, 162, 229–235. [Google Scholar] [CrossRef]
- Sanyang, M.; Ghani, W.A.W.A.K.; Idris, A.; Bin Ahmad, M. Hydrogel biochar composite for arsenic removal from wastewater. Desalin. Water Treat. 2016, 57, 3674–3688. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M.; Ni, B.; Xie, L. κ-Carrageenan–sodium alginate beads and superabsorbent coated nitrogen fertilizer with slow-release, water-retention, and anticompaction properties. Ind. Eng. Chem. Res. 2012, 51, 1413–1422. [Google Scholar] [CrossRef]
- Lü, S.; Feng, C.; Gao, C.; Wang, X.; Xu, X.; Bai, X.; Gao, N.; Liu, M. Multifunctional environmental smart fertilizer based on l-aspartic acid for sustained nutrient release. J. Agric. Food Chem. 2016, 64, 4965–4974. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Wang, Y.; He, J.; Yang, Y.; Yu, X.; Yue, G. Preparation and properties of a degradable interpenetrating polymer networks based on starch with water retention, amelioration of soil, and slow release of nitrogen and phosphorus fertilizer. J. Appl. Polym. Sci. 2012, 128, 407–415. [Google Scholar] [CrossRef]
- Wang, X.; Lü, S.; Gao, C.; Xu, X.; Wei, Y.; Bai, X.; Feng, C.; Gao, N.; Liu, M.; Wu, L. Biomass-based multifunctional fertilizer system featuring controlled-release nutrient, water-retention and amelioration of soil. RSC Adv. 2014, 4, 18382–18390. [Google Scholar] [CrossRef]




| Sample | C | H | N | O | S |
|---|---|---|---|---|---|
| BC | 67.43 | 4.91 | 1.12 | 25.21 | 0.33 |
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| Soil Chemical Properties | |||
| EC (dS/m) | 0.52 | Cl− (meq/L) | 1.50 |
| pH | 7.88 | SO4−2 (meq/L) | 2.35 |
| Na+ (cmol+ kg−1) | 1.25 | CaCO3 (%) | 1.08 |
| Ca++ (cmol+ kg−1) | 2.75 | SAR | 6.04 |
| Mg+ (cmol+ kg−1) | 0.55 | OM (%) | 0.40 |
| K+ (cmol+ kg−1) | 0.38 | Available N (mg/kg) | 28.9 |
| CO3 = (cmol+ kg−1) | 0.0 | Available P (mg/kg) | 6.85 |
| HCO3− (cmol+ kg−1) | 5.5 | Available K (mg/kg) | 65.5 |
| Soil Physical Properties | |||
| Bd (Mg/m3) | 1.58 | FC (%) | 18.2 |
| Ks (m/day) | 2.70 | PWP (%) | 8.6 |
| SP (%) | 34.0 | AWC (%) | 9.6 |
| Particle Size Distribution (%) | |||
| Sand | 84.8 | Clay | 9.6 |
| Silt | 5.6 | Texture | Sandy |
| Samples | CMC (g) | Ac (mL) | Am (g) | AMPS (g) | BC (%) | KPS (g) | MBA (g) |
|---|---|---|---|---|---|---|---|
| IPNCB | 5 | 3 | 3 | 3 | 1, 2 | 0.7 | 0.5 |
| Crosslinker (MBA) Concentration (g) | Equilibrium Swelling (EW) of IPNCB |
|---|---|
| 0.02 | 1075 |
| 0.04 | 1200 |
| 0.06 | 1520 |
| 0.1 | 1730 |
| 0.5 | 1900 |
| CB (%) | Equilibrium Swelling (EW) |
|---|---|
| 0 | 1200 |
| 1 | 1700 |
| 2 | 1900 |
| CB (%) | EW (g/g) |
|---|---|
| D-water 2 | 1900 |
| S-water 2 | 1600 |
| Acidic 2 | 1190 |
| Neutral 2 | 1455 |
| Alkaline 2 | 1260 |
| Sample | Hight of Plant (cm) | Length of Roots (cm) | Germination Rate (%) |
|---|---|---|---|
| 2% IPNCB | 14.3 | 7.1 | 90.12 ± 2.44 |
| 1% IPNCB | 11.5 | 6.7 | 88.43 ± 2.44 |
| 0% IPNCB | 7.3 | 5.1 | 70.33 ± 2.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsaeed, S.M.; Zaki, E.G.; Ibrahim, T.M.; Ibrahim Talha, N.; Saad, H.A.; Gobouri, A.A.; Elkelish, A.; Mohamed el-kousy, S. Biochar Grafted on CMC-Terpolymer by Green Microwave Route for Sustainable Agriculture. Agriculture 2021, 11, 350. https://doi.org/10.3390/agriculture11040350
Elsaeed SM, Zaki EG, Ibrahim TM, Ibrahim Talha N, Saad HA, Gobouri AA, Elkelish A, Mohamed el-kousy S. Biochar Grafted on CMC-Terpolymer by Green Microwave Route for Sustainable Agriculture. Agriculture. 2021; 11(4):350. https://doi.org/10.3390/agriculture11040350
Chicago/Turabian StyleElsaeed, Shimaa M., E. G. Zaki, Tarek M. Ibrahim, Nasser Ibrahim Talha, Hosam A. Saad, Adil A. Gobouri, Amr Elkelish, and Salah Mohamed el-kousy. 2021. "Biochar Grafted on CMC-Terpolymer by Green Microwave Route for Sustainable Agriculture" Agriculture 11, no. 4: 350. https://doi.org/10.3390/agriculture11040350
APA StyleElsaeed, S. M., Zaki, E. G., Ibrahim, T. M., Ibrahim Talha, N., Saad, H. A., Gobouri, A. A., Elkelish, A., & Mohamed el-kousy, S. (2021). Biochar Grafted on CMC-Terpolymer by Green Microwave Route for Sustainable Agriculture. Agriculture, 11(4), 350. https://doi.org/10.3390/agriculture11040350









