Abstract
For the ammonia generated in Korea, the contribution rate of livestock manure is high, and a large amount of ammonia (NH3) is emitted into the atmosphere during the soil application process. Volatilization of NH3 is affected by soil characteristics as well as manure characteristics, but the current inventory does not sufficiently reflect this. This study was conducted to confirm the change of the NH3 emitted from liquid fertilizer (LF) due to soil pH and to evaluate the impacts of biochar (BC) on the suppression of NH3 volatilization. Estimating the NH3 emission flux using the chamber for 24 soils after LF treatment, it showed a tendency to increase exponentially as the pH in soil increased from 4 to 7. In addition, the parallel treatment of BC and LF increased the soil pH, thereby increasing the NH3 emission flux. The rise of soil pH due to LF treatment is a temporary phenomenon that appears in the early stage, but since NH3 volatilization is also highest at the beginning of LF application, the effect of soil characteristics on emission factor and its inventory should be considered when calculating the amount of NH3 emissions. Therefore, follow-up studies such as subdividing and enhancing the NH3 emission factor by soil characteristics and developing a reduction coefficient to certify the amount of emission reduction are needed.
1. Introduction
Particulate matter (PM) has a differing effect depending on its particle size, the exposure time, and the health status of the receptor. The smaller the particle, the more easily it is absorbed into human organs and the deeper it can reach down the respiratory tract, causing a fatally adverse effect on health [1,2]. In Korea, due to the common perception of the dangers and urgency of resolving PM crises, measures to improve atmosphere quality have been implemented and, as a result, amounts of both PM-10 (<10 μm) and PM-2.5 (<2.5 μm) are generally decreasing [3].
Coarse-sized particles (>2.5 μm) containing primary aerosols discharged directly from pollutants in a particulate form are mainly produced by mechanical processes on the surface. In contrast, PM-2.5 is caused by condensation and aggregation of substances in the atmosphere. Therefore, it is difficult to manage and control PM-2.5 [4]. The major constituents of PM-2.5 are ammonium (NH4+), SOX, NOX, and organic carbon. Its small size and large surface area make it easy to adsorb various heavy metals and organic pollutants [5]. Among the precursors of PM-2.5, ammonia (NH3) is one of the few base gases present on the earth that reacts readily with acid gases to form particulate inorganic ammonium salts [6].
According to the Clean Air Policy Support System (CAPSS) of the National Institute of Environmental Research (NIER) in Korea, ammonia emissions in 2017 amounted to 308,298 tons, up 2.3% compared to 2016, of which 79.3% was emitted from agriculture. The increase was attributed to an increase in livestock (pigs and chickens) [7]. In domestic inventory, livestock manure management accounts for 73.5% of total emissions. The livestock species’ contribution rate in manure management was in the order of pigs, chickens, cows, and dairy [8]. The current emissions are calculated as the product of the emission factor (kg head−1) and activity level (number of head) for each livestock type, the emission factor was developed by the NIER in 2008 and categorized by age: less than 1 year, 1–2 years, more than 2 years for cows and piglets, growing pigs, finishing pigs, and sows for pigs [9]. For example, cattle over 2 years has an emission factor of 16.8 kg NH3 head−1, which consists of in the barn (8.8 kg-NH3 head−1), composting (2.0 kg-NH3 head−1), and soil spreading (6.0 kg-NH3 head−1). In the case of pigs, sow has an emission factor of 21.42 NH3 head−1, which consists of in the pig house (11.01 kg-NH3 head−1), composting facility (0.25 kg-NH3 head−1), livestock manure facility (0.07 kg-NH3 head−1), and soil spreading (10.10 kg-NH3 head−1) [9]. Looking at the domestic emission factors calculated through empirical experiments in the field scale, it seems that the stage in which a large amount of NH3 is generated and discharged in the manure management sector is the stage of breeding facilities and the soil application stage. This trend can also be found in the NH3 inventory of the US and EU-28 [8]. In other words, for accurate calculation of emissions, it is necessary to understand the NH3 emission processes and the contribution of breeding facilities and soil spreading to NH3, which have a high contribution to emissions. Of the two stages, this study focuses on the soil spreading stage.
Sommer and Hutchings [10] summarized the factors that affect the volatilization of NH3 discharged from compost and liquid manure sprayed on agricultural land in four primary categories. The first is the concentration of NH3 on the surface of the compost and liquid manure (the pH of the manure and the NH4+ concentration in the manure, the atmospheric temperature, the volatilization rate, etc.). The second is the transition of NH3 from the surface to the atmosphere (height of the crop, surface roughness, wind speed, amount of light, etc.). The third is the area to which the compost and liquid manure is applied (total amount sprayed, the method of spraying, and the contamination of crop by compost and liquid manure, etc.). The fourth is the time exposed to the atmosphere (spraying time, tillage, infiltration rate, evaporation rate, etc.). In general, it is known that the volatilization of NH4+ to NH3 increases as the concentration of total ammoniacal nitrogen (TAN) in compost and liquid manure increases; the pH also increases [11]. It also increases with higher wind speed and ventilation rate [12]. When liquid manure is applied to soil, several soil characteristics such as the number of pores and infiltration rate, cation exchange capacity (CEC), temperature, soil moisture, affect the volatilization of NH3 [10]. To reduce the volatilization of NH3 after spraying, methods such as microbial agents or acidic substances being added to the liquid manure, injection into the soil rather than surface spraying, or a method of prespraying an adsorbent onto the soil, are being carried out in many studies [6,9,10,13,14]. Among the various types of absorbents, BC is known to have excellent adsorption capacity due to its porous structure, so it is used in various fields for the purpose of adsorbing and removing pollutants from the wastewater and stabilizing pollutants in the soil. Still, the alkaline properties of BC also inevitably affect the environmental medium’s pH [15]. Eventually, the change of the environmental medium’s pH once again affects the volatilization of NH3, suggesting that even if the same amount of liquid manure is applied to the soil, the emission amount and emission factor may vary according to the characteristics of the soil. However, as described above, the NH3 inventory does not sufficiently reflect the soil factors that affect the emission factor of the compost/liquid application process.
Therefore, the purpose of this study is to examine the effect of soil pH on NH3 emissions and to evaluate the effect of BC treatment on soil pH and NH3 volatilization after LF application, focusing on the pH that has an immediate impact on NH3 volatilization.
2. Materials and Methods
2.1. Sampling and Analysis of Soil and Liquid Fertilizer
Soils were chosen in the agricultural land of an affiliated farm located in Namyangju, Gyeonggi-do in Korea. Through the preliminary investigation, soils having various pH ranges were collected (total 24 points). At the time of field collection, a 10 mm sieve was used for primary filtration, and after air drying, a 2 mm sieve was used as a sample for soil chemistry analysis. Soil pH and electrical conductivity (EC) were measured using an electrode after adding distilled water at a ratio of 1:5 and stirring for an hour (Thermo Orion 920A, Thermo Fisher Scientific, Waltham, MA, USA). The organic matter content of the soil was quantified by heating at 400 °C for 16 h using a loss-on-ignition (LOI) method [16]. The 1 M CH3COONH4 extraction method was used to measure soil CEC [17]. The liquid fertilizer to be used in the experiment was obtained through a resource facility located in Hongcheon-gun, Gangwon-do in Korea. After sample collection, until immediately before analysis, it was stored in a freezer (−20°C) in order to minimize the effect of microorganisms. The pH of the liquid fertilizer was measured using an electrode (Thermo Orion 920A, Thermo Fisher Scientific, Waltham, MA, USA), and the total nitrogen (TN) and total ammonia nitrogen (TAN) in the liquid fertilizer were distilled using Kjeldahl (KjelDigester K-446, BÜCHI, Flawil, Switzerland). In addition, it was quantified using a color development method [17].
2.2. Estimation of NH3 Emission Flux Using a Dynamic Chamber-Capture System
To calculate the amount of NH3 after the application of liquid fertilizer to the soil, a dynamic chamber-capture system (DCS) manufactured and improved in a previous study was used (Figure 1). In brief, this system is able to control the flow rate by installing a rotameter at the front and rear ends based on a closed chamber located in a constant temperature water bath (20 °C) and treats the air entering the chamber using activated carbon and distilled water [12]. The soil passed through a 10 mm sieve was put into the chamber to a height of 5 cm, and then treated with the liquid fertilizer at a ratio of 200 kg-N ha−1 in consideration of the nitrogen concentration in the liquid fertilizer [volume of liquid fertilizer (L) = 200 kg ha−1 × chamber surface area (ha) ÷ nitrogen concentration in liquid fertilizer (mg L−1)] × 106 mg kg−1]. A ratio of 200 kg-N ha−1 is commonly used when conducting nitrogen experiment in domestic paddy soil [18,19,20]. The soil and liquid fertilizer were treated in the chamber, and the ammonia volatilized and discharged from the liquid fertilizer was evacuated through a vacuum pump located at the end of the system, and the discharged ammonia was arranged to pass through a 5% boric acid solution, and the whole amount was collected. The NH3 collected for 1 h after the liquid fertilizer treatment was colored with bromocresol green and methyl red mixed indicators, and then quantified using 0.01 N or 0.1 N sulfuric acid solution. The NH3 that was emitted and collected for a given time and quantified was calculated as an emission flux (mg m−2 h−1) using the chamber area and discharge time.

Figure 1.
Schematic of a dynamic chamber-capture system with a constant temperature water bath [12].
2.3. Effect of Biochar Treatment on Soil pH and NH3 Emission Flux
Spent coffee grounds, raw materials of the biochar used in the experiment were collected from a nearby coffee shop. After drying of the grounds, pyrolysis was conducted at 400 °C for one hour. The prepared coffee ground char (SCGC) was homogenized through a 0.5 mm sieve. Based on the results of the previous experiment, two soils were selected out of 24 soils, and treated with biochar at 1%, 2%, and 3% levels, respectively; then, water was added to the level of 50% of the water holding capacity for 1 week. After aging, the liquid fertilizer was applied, and the NH3 emission flux was measured using the same chamber system and method. The pH and EC of the soil after biochar and liquid fertilizer treatment were measured using the same method described in Section 2.1.
2.4. Statistical Analysis
All experiments were performed in three repetitions, and the results are expressed as average values. Significance between treatment groups was tested using the Tukey test after one-way ANOVA using a statistical analysis program (SAS 9.2, SAS Institute Inc., Cary, NC, USA).
3. Results and Discussion
3.1. Chemical Characteristics of Soils and Liquid Fertilizer
The basic chemical properties of the 24 site soils used in this experiment are shown in Table 1. The average pH of the soil is 5.92, showing a weak acidity trend and electrical conductivity (EC) of 0.114 ds m−1. The average loss-on-ignition (LOI) and CEC are 4.94% and 10.95 cmol kg−1, respectively. There are no specific issues that could be problematic in the study procedure, and the standard deviation of soil pH is wide, while other chemical properties show relatively low deviation. This indicates that suitable soils were selected to ascertain the meaningful effect of soil pH on NH3 volatilization. The pH of the LF used in the experiment is 8.9; the EC is 23.2 ds m−1; and the TN and TAN are 2004 and 1598 mg L−1, respectively.

Table 1.
Chemical properties of examined soils (n = 24).
3.2. Effect of Soil pH on NH3 Emission Flux
The NH3 emission trend is highest immediately after application of the LF and showed a tendency to decrease gradually over time [12]. Therefore, in this study, the emitted NH3 per unit area was calculated by collecting the NH3 emitted during the initial hour, with the highest emitted flow rate after LF was applied to the soil. As a result, as the soil pH increases, the NH3 emission flux tends to increase (Figure 2). Although a total of 24 soils were treated with the same fertilizer at the same ratio, the emission flux values show a wide range, from a minimum of 12.38 mg m−2 h−1 to a maximum of 137.35 mg m−2 h−1. In a study conducted under the same conditions using granite weathered soil (GWS) rather than agricultural soil, an emission flux was calculated as 115.34 mg m−2 h−1 [12]. At that time, the pH of GWS was 6.9 and the LOI was 0.8%. In this study, the highest pH among the investigated soils is 6.80, the highest LOI is 5.0%, and the emission flux is calculated as 105.52 mg m−2 h−1. It is judged that the large difference in organic matter does not have a significant effect on the NH3 emission flux. On the other hand, while soil pH increases from 4.44 to 6.47, the NH3 emission flux increases more than ten times, from 12.38 mg m−2 h−1 to 130.27 mg m−2 h−1. The volatilization of NH4+ into NH3 is based on Henry’s constant at the surface of liquid and gas and proceeds by diffusion, and volatilization is promoted as the pH of the solution increases [21]. Considering that the NH3 emission flux is 855.5 mg m−2 h−1 when only LF is put in the chamber without any solid medium [12], soil media appears to play a role in reducing NH3 volatilization. Since the ability to buffer the effect of increasing the pH of the soil–LF mixed medium improves as the pH of the soil is lowered, it is considered that the lower the soil pH, the more effectively the NH3 emission flux is decreased. In the end, the results indicate that the characteristics of fertilizer and compost/LF are important in the volatilization of nitrogen originating from fertilizer to NH3, but it is also greatly influenced by the characteristics of the agricultural soil that is applied. Not only soil pH, but also CEC and infiltration depth are known to be factors that significantly affect NH3 volatilization [22]; however, in this study, the effect of pH is very high and the CEC difference between soils is not significant. Due to the characteristics of the chamber experiment, it is difficult to confirm a significant effect because there is no difference in the penetration depth.
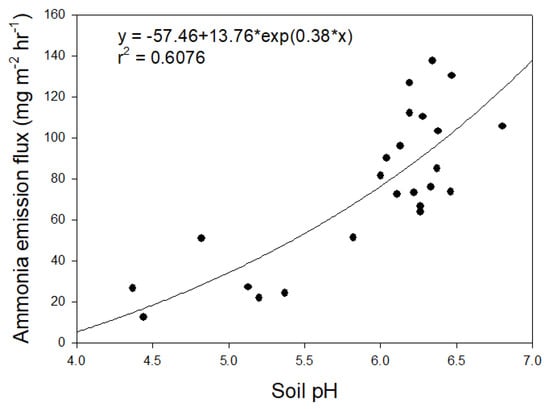
Figure 2.
Changes in ammonia emission rate according to soil pH after liquid fertilizer application using the dynamic chamber-capture system (n = 24).
3.3. Effect of Biochar on Soil pH and NH3 Emission Flux
Based on the experimental results previously obtained, two acidic soils (pH 4.80 and 5.34) out of 24 soils were selected. After the application of BC, the change in pH was confirmed (Figure 3). As a result, the pH of the soil increased due to BC treatment and a significant increase was found for 3% BC treatment compared to the control in both soils. Although it varies depending on the raw material, in general, BC increases in alkalinity when applied into the environment due to its characteristics of having structural groups, functional groups, structural alkalis, carbonates, inorganic alkalis, and dissolved organic alkalis [15]. The increase in soil pH due to an increase in alkalinity can be confirmed through several previous studies, and its effect is known to be superior in acidic soils compared to neutral and alkaline soils [23]. Therefore, despite the relatively short aging period of one week, the effect of increasing the pH by BC treatment could be confirmed in both acidic soils.
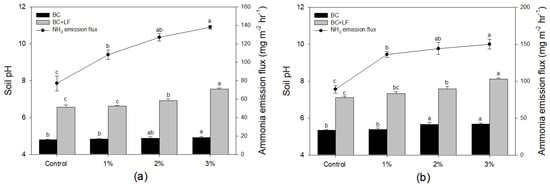
Figure 3.
Effect of biochar (BC) and liquid fertilizer application (LF) on soil pH and ammonia emission rate for two soil. Initial pH of soil (a) and soil (b) are 4.80 and 5.34, respectively. Different letters indicate significant differences according to Tukey’s test. Bar graph indicates soil pH (left y-axis) and dot graph indicates ammonia emission flux (right y-axis).
After the LF was applied, and the amount of NH3 emitted for an hour was calculated, the pH of the soil was also measured. As a result, the pH of the soil significantly increased in all soils and BC treatments (Figure 3). Looking at the extent of the increase in soil pH, in the case of soil (Figure 3a), the soil pH increases from 4.80 to 6.57 when the LF is applied, but when the BC is treated at the level of 3%, the soil pH during LF treatment is 4.92 to 7.54 compared to the control, indicating a greater pH increase. This trend can be confirmed in the same manner in soil (Figure 3b), suggesting that the effect of increasing soil pH by simultaneous treatment of LF and BC is immediate, and that they affect each other. In the early stages of treatment, the effect of increasing soil pH is much greater with LF treatment rather than that of BC. However, the rapid increase in soil pH due to LF treatment is due to the supply of a high concentration of NH3, which is a general phenomenon that occurs during LF treatment [10], and it then returns to its original state through the infiltration and nitrification process [24]. Nevertheless, the temporary synergistic effect of increasing pH when BC and LF are simultaneously treated will need to be observed with interest in the future. As the soil pH increased significantly due to the simultaneous treatment of BC and LF, an increase in the NH3 emission flux from the LF is expected. NH3 emission flux is increased in both soils (Figure 3). In the case of soil (a), the NH3 emission flux increased as the BC treatment increased, showing 77.22 mg m−2 h−1 in the control and 138.14 mg m−2 h−1 in the 3% treatment. In the case of soil (b), the NH3 emission flux increased as the BC treatment increased, with 89.01 mg m−2 h−1 in the control and 150.32 mg m−2 h−1 in the 3% treatment. Sha et al. [25] performed a meta-analysis based on 144 observations from 41 references, and the effect of reducing NH3 volatilization by BC treatment proceeds only under very limited conditions. It was reported that NH3 volatilization increased by BC application. On the other hand, Mandal et al. [26] announced the results of a reduction in volatilization of NH3 by up to 70% when urea, ammonium phosphate, and poultry fertilizer were added to five types of soil and demonstrated that the adsorption, immobilization, and nitrification of NH4+ are significant mechanisms of the reduction in NH3 volatilization by BC. Subsequently, Mandal et al. [27] reported an increase in wheat growth as NH3 volatilization decreased to around 40%, and nitrogen loss decreased when BC was treated up to 2% in pH 7.8 soil. In summary, NH3 volatilization and emission reduction in soil by BC proceeds under some restrictive conditions, and NH3 volatilization is determined by the complex action of the liming effect among the properties of BC and nitrogen fertilizers as well as soil pH [25].
4. Conclusions
The results of estimating the NH3 emission flux using the chamber for 24 soils after LF showed a tendency to increase exponentially as the soil pH increased from 4 to 7 ranges. In addition, the parallel treatment of BC and LF increased the soil pH, thereby increasing the NH3 emission flux. The increase of soil pH due to LF treatment is a temporary phenomenon that appears in the early stage, but since NH3 volatilization is also at its highest at the beginning of LF application, the effect of soil characteristics on emission factor and its inventory should be considered when calculating the amount of NH3 emission. Therefore, follow-up studies such as subdividing and enhancing the NH3 emission factor by soil characteristics and developing a reduction coefficient to certify the amount of emission reduction are needed.
Author Contributions
Conceptualization, M.-S.K.; methodology, H.-G.M.; validation, N.K.; writing—original draft preparation, M.-S.K.; writing—review and editing, J.-G.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Foundation of Korea (NRF) [2019R1I1A1A01043684] and partly supported by Korea University and OJeong Resilience Institute.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Straif, K.; Cohen, A.; Samet, J. Air Pollution and Cancer. IARC Scientific Publication n.161; International Agency for Research on Cancer, World Health Organization: Lyo, France, 2013. [Google Scholar]
- Cadelis, G.; Tourres, R.; Molinie, J. Short-term effects of the particulate pollutants contained in Saharan dust on the visits of children to the emergency department due to asthmatic conditions in Guadeloupe (French Archipelago of the Caribbean). PLoS ONE 2014, 9, e91136. [Google Scholar] [CrossRef]
- Yeo, M.J.; Kim, Y.P. Trends of the PM10 concentrations and high PM10 concentration cases in Korea. J. Korean Soc. Atmos. Environ. 2019, 35, 249–264. [Google Scholar] [CrossRef]
- Ko, H.J.; Lee, Y.S.; Kim, W.H.; Song, J.M.; Kang, C.H. Chemical composition characteristics of fine particulate matter at atmospheric boundary layer of background area in fall. J. Korean Chem. Soc. 2012, 58, 267–276. [Google Scholar] [CrossRef]
- He, Z.; Kim, Y.J.; Ogunjobi, K.O.; Kim, J.E.; Ryu, S.Y. Carbonaceous aerosol characteristics of PM2.5 particles in Northeastern Asia in summer 2002. Atmos. Environ. 2004, 38, 1795–1800. [Google Scholar] [CrossRef]
- Hodan, W.B.; Barnard, W.R. Evaluating the Contribution of PM2.5 Precursor Gases and Re-Entrained Road Emissions to Mobile Source PM2.5 Particulate Matter Emissions; MACTEC under Contract to the Federal Highway Administration: Raleigh, NC, USA, 2004. [Google Scholar]
- National Institute of Environmental Research. 2017 National Air Pollutants Emission; NIER: Incheon, Korea, 2020. [Google Scholar]
- Kim, M.S.; Koo, N.; Kim, J.G. A comparative study on ammonia emission inventory in livestock manure compost application through a foreign case study. Korean J. Environ. Biol. 2020, 38, 71–81. [Google Scholar] [CrossRef]
- National Institute of Environmental Research. Estimation of Ammonia Emission in Air and Inventory Construction (II); NIER: Incheon, Korea, 2008. [Google Scholar]
- Sommer, S.G.; Hutchings, N.J. Ammonia emission from field applied manure and its reduction. Eur. J. Agron. 2001, 15, 1–15. [Google Scholar] [CrossRef]
- Svensson, L. A new dynamic chamber technique for measuring ammonia emissions from land-spread manure and fertilizers. Acta Agric. Scand. Sect. B Plant Soil Sci. 1994, 44, 35–46. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, J.G. Effects of thickness of solid media, ventilation rate, and chamber volume on ammonia emission from liquid fertilizers using dynamic chamber-capture system (DCS). Agriculture 2020, 10, 226. [Google Scholar] [CrossRef]
- Janczak, D.; Malińska, K.; Czekala, W.; Cáceres, R.; Lewicki, A.; Dach, J. Biochar to reduce ammonia emissions in gaseous and liquid phase during composting of poultry manure with wheat straw. Waste Manag. 2017, 66, 36–45. [Google Scholar] [CrossRef]
- Sanz-Cobena, A.; Misselbrook, T.H.; Hernáiz, P.; Vallejo, A. Impact of rainfall to the effectiveness of pig slurry shallow injection method for NH3 mitigation in Mediterranean soil. Atmos. Environ. 2019, 216, 116913. [Google Scholar] [CrossRef]
- Fidel, R.B.; Laird, D.A.; Thompson, M.L. Characterization and quantification of biochar alkalinity. Chemosphere 2017, 167, 367–373. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon and organic matter. In Methods of Soil Analysis. Part 3—Chemical Methods; Spark, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnson, C.T., Sommer, M.E., Eds.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- National Institute of Agricultural Science and Technology. Method of Soil and Plant Analysis; Rural Development Administration: Suwon, Korea, 2008. [Google Scholar]
- Hwang, K.J.; Park, H.S.; Park, N.G.; Ko, M.S.; Kim, M.C.; Song, S.T. Effect of applying pig slurry fermented with probiotics on forage crops productivity and chemical changes in soil. J. Korean Grassl. Sci. 2006, 26, 293–300. [Google Scholar]
- Kim, M.C.; Song, S.T.; Hwang, K.J.; Lim, H.C. The effects of liquid pig manure application on the production of Hapanese millet (Echinochloa crusgalli), soil properties, and the chemical characteristics of leaching water. J. Korean Grassl. Sci. 2006, 26, 257–266. [Google Scholar]
- Park, N.G.; Ko, S.B.; Lee, C.E.; Hwang, K.J.; Kim, M.C.; Song, S.T. Effect of pig slurry application on the forage yield of sorghum × sudangrass hybrid and leaching of NO3-N in volcanic ash soil. J. Korean Grassl. Sci. 2003, 23, 151–158. [Google Scholar]
- Gross, A.; Bod, C.E.; Wood, C.W. Ammonia volatilization from freshwater fish ponds. J. Environ. Qual. 1999, 38, 793–797. [Google Scholar] [CrossRef]
- Sommer, S.G.; Jensen, L.S.; Clausen, S.B.; Søgaard, H.T. Ammonia volatilization from surface-applied livestock slurry as affected by slurry composition and slurry infiltration depth. J. Agric. Sci. 2006, 144, 229–235. [Google Scholar] [CrossRef]
- Ress, R.; Simonnot, M.O.; Morel, J.L. Short-term effects of biochar on soil heavy metal mobility are controlled by intra-particle diffusion and soil pH increase. Eur. J. Soil Sci. 2014, 65, 149–161. [Google Scholar] [CrossRef]
- Chantigny, M.H.; MacDonald, J.D.; Beaupré, C.; Rochette, P.; Angers, D.A.; Massé, D.; Parent, L.E. Ammonia volatilization following surface application of raw and treated liquid swine manure. Nutr. Cycl. Agroecosyst. 2009, 85, 275–286. [Google Scholar] [CrossRef]
- Sha, Z.; Li, Q.; Lv, T.; Misselbrook, T.; Liu, X. Response of ammonia volatilization to biochar addition: A meta-analysis. Sci. Total Environ. 2019, 655, 1387–1396. [Google Scholar] [CrossRef]
- Mandal, S.; Thangarajan, R.; Bolan, N.S.; Sarkar, B.; Khan, N.; Ok, Y.S.; Naidu, R. Biochar-induced concomitant decrease in ammonia volatilization and increase in nitrogen use efficiency by wheat. Chemosphere 2016, 142, 120–127. [Google Scholar] [CrossRef]
- Mandal, S.; Donner, E.; Smith, E.; Sarkar, B.; Lombi, E. Biochar with near-neutral pH reduces ammonia volatilization and improves plant growth in a soil-plant system: A closed chamber experiment. Sci. Total Environ. 2019, 697, 134114. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).