The Effect of Zinc Oxide Nanoparticles for Enhancing Rice (Oryza sativa L.) Yield and Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Zn Origination and Characteristic Traits
2.2. Experimental Location and Design Process
2.3. Measurements and Analysis
2.3.1. Dry Matter Accumulation and Leaf Area Index
2.3.2. SPAD Value and Photosynthesis Potential
2.3.3. Grain Yield and Yield Components
2.3.4. Rice Quality
2.3.5. Determination of Zn Content
2.4. Data Analysis
3. Results
3.1. Rice Yield and Its Components
3.2. Dry Matter Accumulation
3.3. Leaf Area Index, Decreasing Rate of Leaf Area at the Grain-Filling Stage
3.4. Photosynthetic Potential
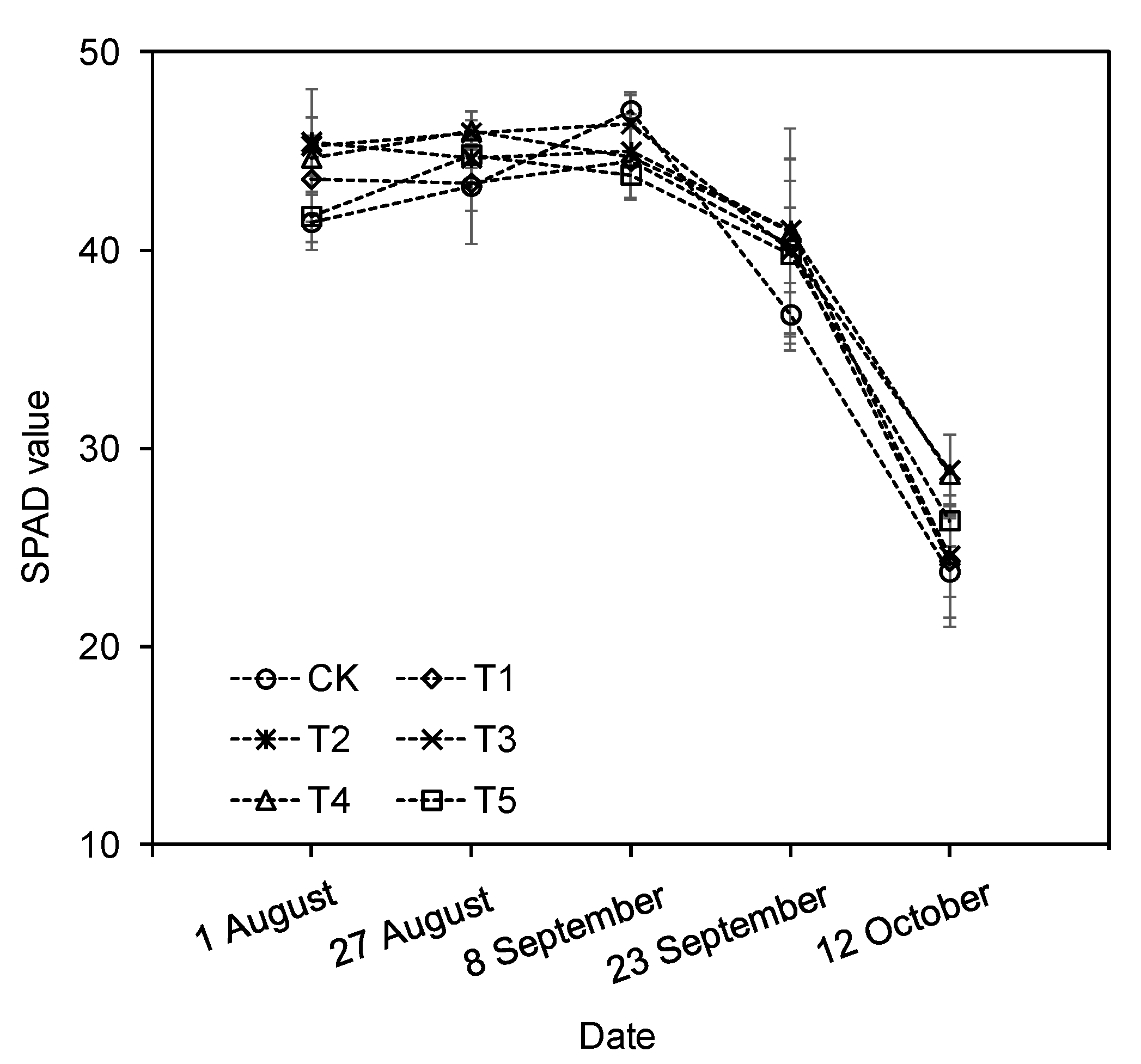
3.5. SPAD Value
3.6. Grain Quality of the Rice
3.7. Zn Content of Rice Grain
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Gibson, R.S. Zinc: The missing link in combating micronutrient malnutrition in developing countries. Proc. Nutr. Soc. 2006, 65, 51–60. [Google Scholar] [PubMed] [Green Version]
- Hotz, C. Dietary indicators for assessing the adequacy of population zinc intakes. Food Nutr. Bull. 2007, 28, 430–453. [Google Scholar]
- Yang, X.E.; Chen, W.R.; Feng, Y. Improving human micronutrient nutrition through biofortification in the soil-plant system: China as a case study. Environ. Geochem. Health 2007, 29, 413–428. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortifying crops with essential mineral elements. Trends Plant Sci. 2005, 10, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Frossard, E.; Bucher, M.; Mahler, F.; Mozafar, A.; Hurrell, R.F. Potential for increasing the content and bioavailability of Fe, Zn and Ca in plants for human nutrition. J. Sci. Food Agric. 2000, 80, 861–879. [Google Scholar]
- Yan, S.; Wu, F.; Zhou, S.; Yang, J.; Tang, X.; Ye, W. Zinc oxide nanoparticles alleviate the arsenic toxicity and decrease the accumulation of arsenic in rice (Oryza sativa L.). BMC Plant Biol. 2021, 21, 150. [Google Scholar]
- Phattarakul, N.; Rerkasem, B.; Li, L.J.; Wu, L.H.; Zou, C.Q.; Ram, H.; Sohu, V.S.; Kang, B.S.; Surek, H.; Kalayci, M.; et al. Biofortification of rice grain with zinc through zinc fertilization in different countries. Plant Soil 2012, 361, 131–141. [Google Scholar]
- Cakmak, I.; Kalayci, M.; Kaya, Y.; Torun, A.A.; Aydin, N.; Wang, Y.; Arisoy, Z.; Erdem, H.; Gokmen, O.; Ozturk, L.; et al. Biofortification and localization of zinc in wheat grain. J. Agric. Food Chem. 2010, 58, 9092–9102. [Google Scholar] [PubMed]
- Zeng, Y.W.; Wang, L.X.; Du, J.; Wang, S.M.; Yang, Y.C.; Li, Q.W.; Sun, Z.H.; Pu, X.Y.; Du, W. Correlation of mineral elements between milled and brown rice and soils in Yunnan studied by ICP-AES. Spectrosc. Spect. Anal. 2009, 29, 1413–1417. [Google Scholar]
- Hussain, A.; Ali, S.; Rizwan, M.; Rehman, M.Z.U.; Javed, M.R.; Imran, M.; Chatha, S.A.; Nazir, R. Zinc oxide nanoparticles alter the wheat physiological response and reduce the cadmium uptake by plants. Environ. Pollut. 2018, 242, 1518–1526. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Ali, B.; Adrees, M.; Arshad, M.; Hussain, A.; Rahman, M.Z.U.; Waris, A.A. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 2019, 214, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Rehman, M.Z.U.; Adrees, M.; Arshad, M.; Qayyum, M.F.; Ali, L.; Hussain, A.; Chatha, S.A.S.; Imran, M. Alleviation of cadmium accumulation in maize (Zea mays L.) by foliar spray of zinc oxide nanoparticles and biochar to contaminated soil. Environ. Pollut. 2019, 248, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Wang, L.; Xin, Z.H.; Zhao, L.Y.; An, X.X.; Hu, Q.H. Effect of foliar application of zinc, selenium, and iron fertilizers on nutrients concentration and yield of rice grain in China. J. Agric. Food Chem. 2008, 56, 2079–2084. [Google Scholar] [CrossRef]
- Prakash, P.; Hemalatha, M.; Joseph, M. Zinc accounting for lowland rice (Oryza sativa L.) under different methods of zinc application with green leaf manuring. Adv. Crop Sci. Tech. 2018, 6, 1000374. [Google Scholar]
- Wu, F.; Fang, Q.; Yan, S.W.; Pan, L.; Tang, X.J.; Ye, W.L. Effects of zinc oxide nanoparticles on arsenic stress in rice (Oryza sativa L.): Germination, early growth, and arsenic uptake. Environ. Sci. Pollut. Res. 2020, 27, 26974–26981. [Google Scholar] [CrossRef]
- Naik, S.K.; Das, D.K. Relative performance of chelated zinc and zinc sulphate for lowland rice (Oryza sativa L.). Nutr. Cycl. Agroecosyst. 2008, 81, 219–227. [Google Scholar] [CrossRef]
- Yang, G.Y.; Yuan, H.Y.; Ji, H.T.; Liu, H.J.; Zhang, Y.F.; Wang, G.D.; Chen, L.G.; Guo, Z. Effect of ZnO nanoparticles on the productivity, Zn biofortification, and nutritional quality of rice in a life cycle study. Plant Physiol. Bioch. 2021, 163, 87–94. [Google Scholar] [CrossRef]
- Elemike, E.E.; Uzoh, I.M.; Onwudiwe, D.C.; Babalola, O.O. The role of nanotechnology in the fortification of plant nutrients and improvement of crop production. Appl. Sci. 2019, 9, 499. [Google Scholar] [CrossRef] [Green Version]
- Lowry, G.V.; Avellan, A.; Gilbertson, L.M. Opportunities and challenges for nanotechnology in the agri-tech revolution. Nat. Nanotechnol. 2019, 14, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Kah, M.; Tufenkji, N.; White, J.C. Nano-enabled strategies to enhance crop nutrition and protection. Nat. Nanotechnol. 2019, 14, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; Andrews, J.; Fugice, J.; Singh, U.; Bindraban, P.S.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Facile coating of urea with low-dose ZnO nanoparticles promotes wheat performance and enhances Zn uptake under drought stress. Front. Plant Sci. 2020, 11, 168. [Google Scholar] [CrossRef] [Green Version]
- Subbaiah, L.V.; Prasad, T.N.V.K.V.; Krishna, T.G.; Sudhakar, P.; Reddy, P.R.; Pradeep, T. Novel effects of nanoparticulate delivery of zinc on growth, productivity, and zinc biofortification in maize (Zea mays L.). J. Agric. Food Chem. 2016, 64, 3778–3788. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; White, J.C.; Elmer, W.H.; Gardea-Torresdy, J. Nanoparticle and ionic Zn promote nutrient loading of sorghum grain under low NPK fertilization. J. Agric. Food Chem. 2017, 65, 8552–8559. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Noureen, S.; Anwar, S.; Ali, B.; Naveed, M.; Allah, E.F.A.; Alqarawi, A.A.; Ahamad, P. Combined use of biochar and zinc oxide nanoparticle foliar spray improved the plant growth and decreased the cadmium accumulation in rice (Oryza sativa L.) plant. Environ. Sci. Pollut. Res. 2019, 26, 11288–11299. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; Singh, U.; Bindraban, P.S.; Adisa, I.O.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Addition-omission of zinc, copper, and boron nano and bulk oxide particles demonstrate element and size-specific response of soybean to micronutrients exposure. Sci. Total Environ. 2019, 665, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Kumar, A. Quantification of metal uptake in Spinacia oleracea irrigated with water containing a mixture of CuO and ZnO nanoparticles. Chemosphere 2020, 243, 125239. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.J.; Cong, S.M.; Zhang, H.C. Comparison of the grain quality and starch physicochemical properties between Japonica rice cultivars with different contents of amylose, as affected by nitrogen fertilization. Agriculture 2021, 11, 616. [Google Scholar] [CrossRef]
- Liu, Z. Regularities of content and distribution of zinc in soils of China. Sci. Agric. Sin. 1994, 27, 30–37. [Google Scholar]
- Dou, Z.; Li, Y.Y.; Guo, H.L.; Chen, L.R.; Jiang, J.L.; Zhou, Y.C.; Xu, Q.; Xing, Z.P.; Gao, H.; Zhang, H.C. Effects of mechanically transplanting methods and planting densities on yield and quality of Nanjing 2728 under rice-crayfish continuous production system. Agronomy 2021, 11, 488. [Google Scholar] [CrossRef]
- Zhang, H.C.; Gong, J.L. Research status and development discussion on high-yield agronomy of mechanized planting rice in China. Sci. Agric. Sin. 2014, 47, 1273–1289. [Google Scholar]
- Kheyri, N.; Norouzi, H.A.; Mobasser, H.R.; Torabi, B. Effect of silicon and zinc nanoparticles on growth, yield, and biochemical characteristics of rice. Agron. J. 2019, 111, 3084–3090. [Google Scholar] [CrossRef]
- Tyagi, R.; Sharma, A.; Srivastava, P.C.; Shankhdhar, D.; Shankhdhar, S.C. Modulation of phytic acid and phytic acid-zinc molar ratio by different modes of zinc application in rice. Indian J. Plant Physiol. 2018, 23, 529–535. [Google Scholar] [CrossRef]
- Yin, H.J.; Guo, X.P.; Stomph, T.; Li, L.J.; Zhang, F.S.; Zou, C.Q. Zinc concentration in rice (Oryza sativa L.) grains and allocation in plants as affected by different zinc fertilization strategies. Commun. Soil Sci. Plan. 2016, 47, 761–768. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, H.D.; Lv, Z.Y.; Cui, L.L.; Mao, H.; Kopittke, P.M. Using synchrotron-based approaches to examine the foliar application of ZnSO4 and ZnO nanoparticles for field-growth winter wheat. J. Agric. Food Chem. 2018, 66, 2572–2579. [Google Scholar] [CrossRef]
- Rui, M.M.; Ma, C.X.; Hao, Y.; Guo, J.; Rui, Y.K.; Tang, X.L.; Zhao, Q.; Fan, X.; Zhang, Z.T.; Hou, T.Q.; et al. Iron oxide nanoparticles as a potential iron fertilizer for peanut (Arachis hypogaea). Front. Plant Sci. 2016, 7, 815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samart, S.; Chutipaijit, S. Growth of pigmented rice (Oryza sativa L. cv. Riceberry) exposed to ZnO nanoparticles. Mater. Today Proc. 2019, 17, 1987–1997. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Lombi, E.; Wang, P.; Schjoerring, J.K.; Husted, S. Nanomaterials as fertilizers for improving plant mineral nutrition and environmental outcomes. Environ. Sci. Nano 2019, 6, 3513. [Google Scholar] [CrossRef]
- Sun, H.D.; Du, W.; Peng, Q.Q.; Lv, Z.Y.; Mao, H.; Kopittke, P.M. Development of ZnO nanoparticles as an efficient Zn fertilizer: Using synchrotron-based techniques and laser ablation to examine elemental distribution in wheat grain. J. Agric. Food Chem. 2020, 68, 5068–5075. [Google Scholar] [CrossRef]
- Garcia-Gomez, C.; Obrador, A.; Gonzalez, D.; Babin, M.; Fernandez, M.D. Comparative effect of ZnO NPs, ZnO bulk and ZnSO4 in the antioxidant defences of two plant species growing in two agricultural soils under greenhouse conditions. Sci. Total Environ. 2017, 589, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Josko, I.; Oleszczuk, P.; Futa, B. The effect of inorganic nanoparticles (ZnO, Cr2O3, CuO and Ni) and their bulk counterparts on enzyme activities in different soils. Geoderma 2014, 232–234, 528–537. [Google Scholar] [CrossRef]
- Wang, X.P.; Li, Q.Q.; Pei, Z.M.; Wang, S.C. Effects of zinc oxide nanoparticle on the growth, photosynthetic traits, and antioxidative enzymes in tomato plants. Biol. Plant. 2018, 62, 801–808. [Google Scholar] [CrossRef]
- Siddiqui, Z.A.; Parveen, A.; Ahmad, L.; Hashem, A. Effects of graphene oxide and zinc oxide nanoparticles on growth, chlorophyll, carotenoids, proline contents and diseases of carrot. Sci. Hortic. 2019, 249, 374–382. [Google Scholar] [CrossRef]
| Treatments | Panicle (×106 hm−2) | Spikelets Per Panicle | 1000-Grain Weight (g) | Filled Grain Rate (%) | Harvest Yield (t hm−2) |
|---|---|---|---|---|---|
| CK | 3.73 ± 0.15 b | 108.09 ± 1.39 d | 25.68 ± 0.24 c | 88.22 ± 0.57 d | 10.07 ± 0.15 c |
| T1 | 3.99 ± 0.20 a,b | 113.29 ± 1.14 c | 26.67 ± 0.08 b | 88.47 ± 0.15 d | 10.40 ± 0.25 bc |
| T2 | 4.03 ± 0.15 a,b | 116.40 ± 0.73 b | 27.38 ± 0.02 a | 89.30 ± 0.17 c | 10.57 ± 0.15 a,b |
| T3 | 3.97 ± 0.15 a,b | 119.64 ± 0.80 a | 27.13 ± 0.02 a | 89.61 ± 0.11 b,c | 10.62 ± 0.30 a,b |
| T4 | 4.22 ± 0.25 a | 113.92 ± 3.03 b,c | 27.26 ± 0.14 a | 90.30 ± 0.07 a | 10.83 ± 0.33 a |
| T5 | 3.91 ± 0.16 a,b | 116.20 ± 1.36 b | 26.68 ± 0.23 b | 90.01 ± 0.34 a,b | 10.55 ± 0.21 a,b |
| Treatments | Dry Matter Weight (t hm−2) | Harvest Index | ||
|---|---|---|---|---|
| Jointing | Heading | Maturity | ||
| CK | 3.79 ± 0.25 b | 10.58 ±1.11 b | 16.90 ± 1.08 b | 0.37 ± 0.08 a |
| T1 | 4.32 ± 0.40 a | 12.63 ± 1.41 a | 18.57 ± 1.25 a | 0.36 ± 0.05 a |
| T2 | 4.30 ± 0.38 a | 12.74 ± 0.80 a | 19.37 ± 1.16 a | 0.35 ± 0.09 a |
| T3 | 4.28 ± 0.27 a | 12.87 ± 1.85 a | 19.66 ± 1.50 a | 0.35 ± 0.04 a |
| T4 | 4.53 ± 0.37 a | 13.00 ± 1.55 a | 19.77 ± 1.56 a | 0.35 ± 0.09 a |
| T5 | 4.45 ± 0.52 a | 13.01 ± 0.95 a | 19.73 ± 1.22 a | 0.35 ± 0.08 a |
| Treatments | Sowing-Jointing | Jointing-Heading | Heading-Maturity | |||
|---|---|---|---|---|---|---|
| Accumulation (t hm−2) | Ratio (%) | Accumulation (t hm−2) | Ratio (%) | Accumulation (t hm−2) | Ratio (%) | |
| CK | 3.79 ± 0.25 b | 22.76 ± 6.58 a | 6.79 ± 0.78 b | 40.07 ± 9.54 a | 6.32 ± 1.28 a | 37.17 ± 8.43 a |
| T1 | 4.32 ± 0.40 a | 23.24 ± 0.72 a | 8.31 ± 1.41 a | 44.86 ± 6.48 a | 5.94 ± 1.25 a | 31.90 ± 6.81 a |
| T2 | 4.30 ± 0.38 a | 22.30 ± 2.10 a | 8.44 ± 0.80 a | 43.71 ± 2.96 a | 6.63 ± 1.16 a | 33.99 ± 3.46 a |
| T3 | 4.28 ± 0.27 a | 21.93 ± 3.00 a | 8.59 ± 1.85 a | 43.30 ± 7.28 a | 6.79 ± 1.50 a | 34.77 ± 4.35 a |
| T4 | 4.53 ± 0.37 a | 23.10 ± 3.83 a | 8.47 ± 1.55 a | 42.92 ± 7.21 a | 6.77 ± 1.56 a | 33.99 ± 9.25 a |
| T5 | 4.45 ± 0.52 a | 22.51 ± 1.66 a | 8.56 ± 0.95 a | 43.51 ± 6.22 a | 6.72 ± 1.22 a | 33.98 ± 4.59 a |
| Treatments | Leaf Area Index | Attenuation Rate of Leaf Area Index at Grain-Filling Stage (LAI d−1) | ||
|---|---|---|---|---|
| Jointing | Heading | Maturity | ||
| CK | 4.29 ± 0.10 c | 6.57 ± 0.33 a,b | 3.80 ± 0.08 c | 0.0603 ± 0.0102 a |
| T1 | 4.38 ± 0.19 b,c | 6.55 ± 0.25 a,b | 4.09 ± 0.13 b | 0.0536 ± 0.0067 b |
| T2 | 4.42 ± 0.12 b,c | 6.54 ± 0.19 b | 4.14 ± 0.07 b | 0.0522 ± 0.0076 b |
| T3 | 4.63 ± 0.23 a,b | 6.82 ± 0.13 a,b | 4.28 ± 0.08 ab | 0.0552 ± 0.0021 b |
| T4 | 4.86 ± 0.20 a | 6.90 ± 0.13 a,b | 4.51 ± 0.16 a | 0.0520 ± 0.0062 b |
| T5 | 4.85 ± 0.15 a | 6.97 ± 0.14 a | 4.51 ± 0.09 a | 0.0535 ± 0.0023 b |
| Treatments | Photosynthetic Potential (×104 m2 d hm−2) | ||
|---|---|---|---|
| Sowing-Jointing | Jointing-Heading | Heading-Maturity | |
| CK | 141.75 ± 4.28 a | 172.11 ± 3.02 b | 270.07 ± 5.79 b |
| T1 | 138.84 ± 1.63 a | 182.38 ± 2.38 a | 271.45 ± 4.12 b |
| T2 | 143.61 ± 4.43 a | 180.80 ± 4.54 a | 274.74 ± 0.87 a,b |
| T3 | 142.26 ± 2.41 a | 182.87 ± 3.51 a | 281.21 ± 2.90 a |
| T4 | 144.76 ± 3.65 a | 177.91 ± 3.46 a,b | 274.06 ± 4.93 a,b |
| T5 | 143.12 ± 3.93 a | 178.35 ± 6.65 a,b | 273.41 ± 3.63 b |
| Treatments | Brown Rice Rate (%) | Milled Rice Rate (%) | Head Rice Rate (%) | Chalkiness Size (%) | Chalkiness Grain Rate (%) | Chalkiness Degree (%) | Amylose Content (%) | Protein Content (%) |
|---|---|---|---|---|---|---|---|---|
| CK | 85.07 ± 0.22 a | 75.45 ± 0.22 a | 64.07 ± 0.81 a,b | 56.81 ± 1.23 a | 15.42 ± 1.03 a | 8.76 ± 0.29 a | 17.47 ± 0.40 a | 7.20 ± 0.20 a |
| T1 | 85.74 ± 0.03 a | 75.67 ± 0.37 a | 67.39 ± 1.73 a | 54.20 ± 0.34 a | 14.56 ± 0.98 a,b | 7.89 ± 0.22 a,b | 17.63 ± 0.12 a | 7.37 ± 0.15 a |
| T2 | 85.51 ± 0.06 a | 76.10 ± 0.15 a | 63.31 ± 0.38 b | 49.47 ± 1.92 a | 11.01 ± 0.96 b | 5.45 ± 0.14 b | 17.62 ± 0.10 a | 7.47 ± 0.15 a |
| T3 | 85.84 ± 0.08 a | 76.22 ± 0.21 a | 65.85 ± 0.29 a,b | 47.63 ± 3.00 a | 10.71 ± 1.08 b | 5.10 ± 0.22 b | 17.70 ± 0.06 a | 7.47 ± 0.15 a |
| T4 | 85.94 ± 0.33 a | 76.11 ± 0.33 a | 62.86 ± 0.18 b | 53.27 ± 0.90 a | 13.73 ± 0.32 a,b | 7.31 ± 0.21 a,b | 17.93 ± 0.10 a | 7.63 ± 0.31 a |
| T5 | 86.06 ± 0.13 a | 75.85 ± 0.25 a | 63.71 ± 0.23 b | 49.53 ± 4.91 a | 11.97 ± 1.04 b | 5.93 ± 0.19 b | 17.63 ± 0.25 a | 7.40 ± 0.15 a |
| Treatments | Rice Grain (mg kg−1) | Glume (mg kg−1) | Brown Rice (mg kg−1) | Polished Rice (mg kg−1) | Aleurone Layer (mg kg−1) |
|---|---|---|---|---|---|
| CK | 16.86 ± 0.40 f | 34.25 ± 0.80 c | 13.81 ± 0.45 d | 11.39 ± 0.49 d | 32.81 ± 1.07 f |
| T1 | 19.67 ± 0.08 e | 33.52 ± 1.16 c | 17.37 ± 0.17 c | 13.72 ± 0.12 c | 44.78 ± 0.24 e |
| T2 | 20.95 ± 0.07 d | 41.01 ± 1.52 a | 17.55 ± 0.31 c | 13.90 ± 0.23 c | 47.07 ± 0.96 d |
| T3 | 21.79 ± 0.05 c | 38.24 ± 2.32 a,b | 19.07 ± 0.34 b | 15.33 ± 0.29 b | 48.75 ± 0.39 c |
| T4 | 22.47 ± 0.05 b | 34.67 ± 3.02 b,c | 20.48 ± 0.50 a | 16.07 ± 0.65 a | 54.63 ± 0.90 a |
| T5 | 22.82 ± 0.12 a | 40.74 ± 2.13 a | 19.92 ± 0.45 a | 15.79 ± 0.36 a,b | 50.53 ± 1.03 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Wang, R.; Chen, Z.; Cui, P.; Lu, H.; Yang, Y.; Zhang, H. The Effect of Zinc Oxide Nanoparticles for Enhancing Rice (Oryza sativa L.) Yield and Quality. Agriculture 2021, 11, 1247. https://doi.org/10.3390/agriculture11121247
Zhang H, Wang R, Chen Z, Cui P, Lu H, Yang Y, Zhang H. The Effect of Zinc Oxide Nanoparticles for Enhancing Rice (Oryza sativa L.) Yield and Quality. Agriculture. 2021; 11(12):1247. https://doi.org/10.3390/agriculture11121247
Chicago/Turabian StyleZhang, Haipeng, Rui Wang, Zhiqing Chen, Peiyuan Cui, Hao Lu, Yanju Yang, and Hongcheng Zhang. 2021. "The Effect of Zinc Oxide Nanoparticles for Enhancing Rice (Oryza sativa L.) Yield and Quality" Agriculture 11, no. 12: 1247. https://doi.org/10.3390/agriculture11121247
APA StyleZhang, H., Wang, R., Chen, Z., Cui, P., Lu, H., Yang, Y., & Zhang, H. (2021). The Effect of Zinc Oxide Nanoparticles for Enhancing Rice (Oryza sativa L.) Yield and Quality. Agriculture, 11(12), 1247. https://doi.org/10.3390/agriculture11121247







