Expression Analysis of DgD14, DgBRC1 and DgLsL in the Process of Chrysanthemum Lateral Bud Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Apical Bud Removal and Exogenous Hormone Treatment
2.3. Sampling
2.4. Expression Analysis
3. Results
3.1. Effects of Apical Bud Removal and Exogenous Hormones on the Growth of Lateral Buds
3.2. Effects of Apical Bud Removal on Gene Expression Characteristics
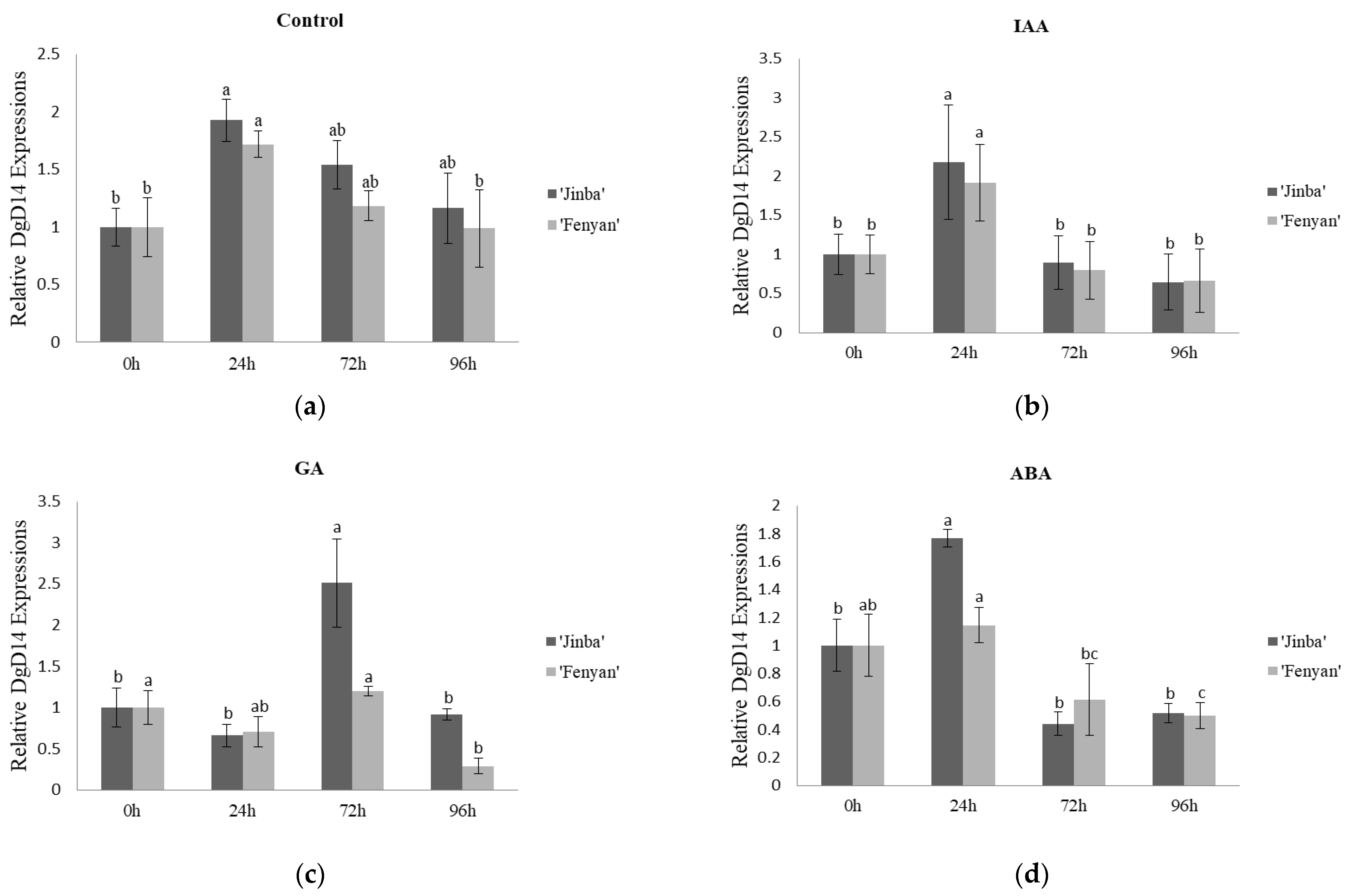
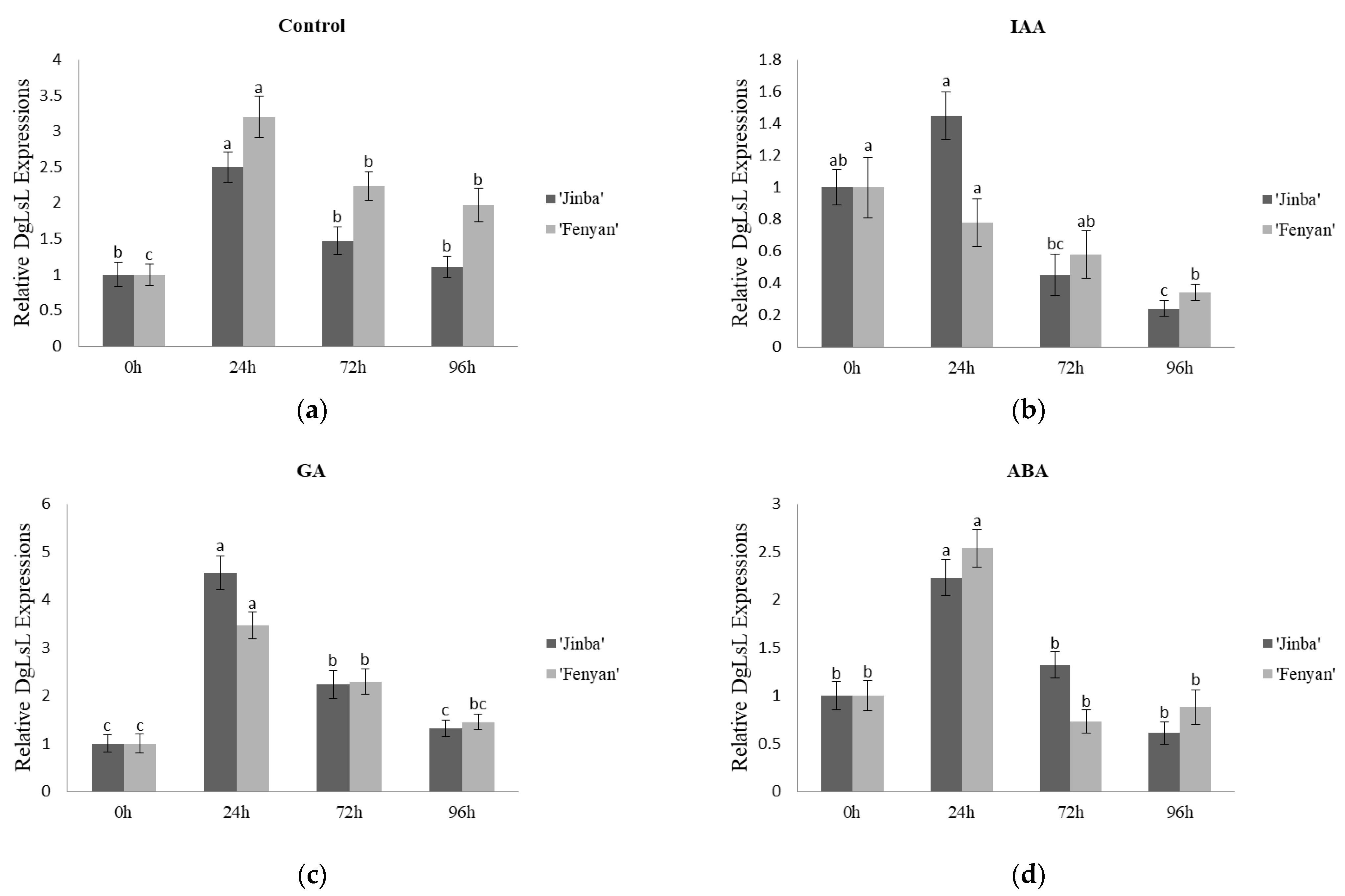
3.3. Effects of Exogenous Hormones on Gene Expression Characteristics
3.3.1. Effects on the Expression Characteristics of DgD14
3.3.2. Effects on the Expression Characteristics of DgBRC1
3.3.3. Effects on the Expression Characteristics of DgLsL
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABA | Abscisic acid |
| CKs | Cytokinins |
| GA | Gibberellic acid |
| IAA | Indole-3-acetic acid |
| SLs | Strigolactones |
References
- Zhu, W.Y.; Jiang, J.F.; Chen, S.M.; Wang, L.; Xu, L.L.; Wang, H.B.; Li, P.L.; Guan, Z.Y.; Chen, F.D. Intergeneric hybrid between Chrysanthemum × morifolium and Artemisia japonica achieved via embryo rescue shows salt tolerance. Euphytica 2013, 191, 109–119. [Google Scholar] [CrossRef]
- Leyser, O. The control of shoot branching: An example of plant information processing. Plant Cell Environ. 2009, 32, 694–703. [Google Scholar] [CrossRef]
- Wilkins, H.; Anderson, N.O. Flower Breeding and Genetics; Springer: Dordrecht, The Netherlands, 2007; pp. 389–437. [Google Scholar]
- Dong, L.; Ishak, A.; Yu, J.; Zhao, R.; Zhao, L. Identification and functional analysis of three MAX2 orthologs in Chrysanthemum. J. Integr. Plant Biol. 2013, 55, 434–442. [Google Scholar] [CrossRef]
- Kubo, T.; Tsuro, M.; Tsukimori, A.; Shizukawa, Y.; Takemoto, T. Morphological and physiological changes in transgenic Chrysanthemum morifolium Ramat. ‘Ogura-nishiki’ with rolC. J. Jpn. Soc. Hortic. Sci. 2006, 75, 312–317. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, X.; Xi, L.; Zhao, R.; Ma, N.; Zhao, L. Roles of DgBRCl in regulation of lateral branching in chrysanthemum (Dendranthema×grandiflora cv. Jinba). PLoS ONE 2013, 8, 617–717. [Google Scholar]
- Mehrnia, M.; Balazadeh, S.; Zanor, M.I.; Mueller-Roeber, B. EBE, an AP2/ERF transcription factor highly expressed in proliferating cells, affects shoot architecture in Arabidopsis. Plant Physiol. 2013, 162, 842–857. [Google Scholar] [CrossRef]
- Mizoi, J.; Shinozaki, K.; Yamaguchi, S.K. AP2/ERF family transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta BBA Gene Regul. Mech. 2012, 1819, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Jiao, Y. Regulation of axillary meristem initiation by transcription factors and plant hormones. Front. Plant Sci. 2016, 7, 183. [Google Scholar] [CrossRef]
- Wang, Y.H.; Li, J.Y. Branching in rice. Curr. Opin. Plant Biol. 2011, 14, 94–99. [Google Scholar] [CrossRef]
- Beveridge, C.A.; Symons, G.M.; Murfet, I.C. The rms1 mutant of pea has elevated indole-3-acetic acid levels and reduced root-sap zeatin riboside content but increased branching controlled by graft-transmissible signal(s). Plant Physiol. 1997, 115, 1251. [Google Scholar] [CrossRef]
- Xia, X.; Dong, H.; Yin, Y.; Song, X.; Gu, X.; Sang, K.; Zhou, J.; Shi, K.; Zhou, Y.; Foyer, C.H.; et al. Brassinosteroid signaling integrates multiple pathways to release apical dominance in tomato. Proc. Natl. Acad. Sci. USA 2021, 118, e2004384118. [Google Scholar] [CrossRef]
- Guan, J.C.; Koch, K.E.; Suzuki, M.; Wu, S.; Latshaw, S.; Petruff, T.; Goulet, C.; Klee, H.J.; McCarty, D.R. Diverse Roles of Strigolactone Signaling in Maize Architecture and the Uncoupling of a Branching-Specific Subnetwork. Plant Physiol. 2012, 160, 1303–1317. [Google Scholar] [CrossRef] [PubMed]
- Domagalska, M.A.; Leyser, O. Signal integration in the control of shoot branching. Nat. Rev. Mol. Cell Biol. 2011, 12, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Sorefan, K.; Booker, J.; Haurogne, K.; Goussot, M.; Bainbridge, K.; Foo, E.; Chatfield, S.; Ward, S.; Beveridge, C.; Rameau, C.; et al. MAX4 and RMS1 are orthologous dioxygenase-like genes that regulate shoot branching in Arabidopsis and pea. Genes Dev. 2003, 17, 1469–1474. [Google Scholar] [CrossRef]
- Dun, E.A.; Ferguson, B.J.; Beveridge, C.A. Apical Dominance and Shoot Branching. Divergent Opinions or Divergent Mechanisms? Plant Physiol. 2006, 142, 812–819. [Google Scholar] [CrossRef]
- Thimann, K.V.; Skoog, F. Studies in the growth hormone of plants.III. The inhibiting action of the growth substance on bud development. Proc. Natl. Acad. Sci. USA 1933, 19, 714–716. [Google Scholar] [CrossRef]
- Jones, B.; Gunneras, S.A.; Petersson, S.V.; Tarkowski, P.; Graham, N.; May, S.; Dolezal, K.; Sandberg, G.; Ljung, K. Cytokinin regulation of auxinsynthesis in Arabidopsis involves a homeostatic feedback loop regulated via auxin and cytokinin signal transduction. Plant Cell 2010, 22, 2956–2969. [Google Scholar] [CrossRef] [PubMed]
- Umehara, M.; Hanada, A.; Yoshida, S.; Akiyama, K.; Arite, T.; Takeda-Kamiya, N.; Magome, H.; Kamiya, Y.; Shirasu, K.; Yoneyama, K.; et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature 2008, 455, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Takei, K.; Kojima, M.; Sakakibara, H.; Mori, H. Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. Plant J. 2006, 45, 1028–1036. [Google Scholar] [CrossRef]
- Müller, D.; Leyser, O. Auxin, cytokinin and the control of shoot branching. Ann. Bot. 2011, 107, 1203–1212. [Google Scholar] [CrossRef]
- Kyozuka, J. Control of shoot and root meristem function by cytokinin. Curr. Opin. Plant Biol. 2007, 10, 442–446. [Google Scholar] [CrossRef]
- McSteen, P. Hormonal regulation of branching in grasses. Plant Physiol. 2009, 149, 46–55. [Google Scholar] [CrossRef]
- Khodakovskaya, M.; Vankova, R.; Malbeck, J.; Li, A.Z.; Li, Y.; McAvoy, R. Enhancement of flowering and branching phenotype in chrysanthemum by expression of ipt under the control of a 0.821 kb fragment of the LEACOI gene promoter. Plant Cell Rep. 2009, 28, 1351–1362. [Google Scholar] [CrossRef]
- Schmitz, G.; Theres, K. Genetic control of branching in Arabidopsis and tomato. Curr. Opin. Plant Biol. 1999, 2, 51–55. [Google Scholar] [CrossRef]
- Kohlen, W.; Charnikhova, T.; Liu, Q.; Bours, R.; Domagalska, M.A.; Beguerie, S.; Verstappen, F.; Leyser, O.; Bouwmeester, H.; Ruyter-Spira, C. Strigolactones are transported through the xylem and play a key role in shoot architectural response to phosphate deficiency in nonarbuscular mycorrhizal host Arabidopsis. Plant. Physiol. 2011, 155, 974–987. [Google Scholar] [CrossRef] [PubMed]
- Alder, A.; Jamil, M.; Marzorati, M. The path from β-carotene to carlactone, a strigolactone-like plant hormone. Science 2012, 335, 1348–1351. [Google Scholar] [CrossRef]
- Zhou, F.; Lin, Q.; Zhu, L. D14-SCF(D3)-dependent degradation of D53 regulates strigolactone signaling. Nature 2013, 504, 406–410, Corrigendum in 2016, 532, 402. [Google Scholar] [CrossRef]
- Yao, R.; Ming, Z.; Yan, L.; Li, S.; Wang, F.; Ma, S.; Yu, C.; Yang, M.; Chen, L.; Chen, L.; et al. DWARF14 is a non-canonical hormone receptor for strigolactone. J. Nature. 2016, 536, 469–473. [Google Scholar] [CrossRef]
- Wen, C.; Xi, L.; Gao, B.; Wang, K.Y.; Lv, S.H.; Kou, Y.P.; Ma, N.; Zhao, L.J. Roles of DgD14 in regulation of shoot branching in chrysanthemum (Dendranthema grandiflorum ‘Jinba’). Plant Physiol. Biochem. 2015, 96, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Imai, A.; Takahashi, S.; Nakayama, K.; Satoh, H. The promoter of the carotenoid cleavage dioxygenase 4a-5 gene of Chrysanthemum morifolium (CmCCD4a-5) drives petal-specific transcription of a conjugated gene in the developing flower. J. Plant Physiol. 2013, 170, 1295–1299. [Google Scholar] [CrossRef] [PubMed]
- Deruiter, H.A. Development of chrysanthemum cuttings: The influence of age and position of the ancillary buds. Ann. Bot. 1996, 77, 99–104. [Google Scholar] [CrossRef][Green Version]
- Schumacher, K.; Schmitt, T.; Rossberg, M.; Schmitz, G.; Theres, K. The lateral suppressor (Ls) gene of tomato encodes a new member of the VHIID protein family. Proc. Natl. Acad. Sci. USA 1999, 96, 290–295. [Google Scholar] [CrossRef]
- Tucker, D.J. Endogenous growth regulators in relation to side shoot development in the tomato. New Phytol. 1976, 77, 561–568. [Google Scholar] [CrossRef]
- Yang, D.H.; Yun, P.Y.; Park, S.Y.; Plaha, P.; Lee, D.S.; Lee, I.S.; Hwang, Y.S.; Kim, Y.A.; Lee, J.S.; Han, B.H. Cloning, characterization and expression of a Lateral suppressor–like gene from chrysanthemum (Dendranthema grandiflorum Kitamura). Plant Physiol. Biochem. 2005, 43, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Bessonov, N.; Morozova, N.; Volpert, V. Modeling of Branching Patterns in Plants. Bull. Math. Biol. 2008, 70, 868–893. [Google Scholar] [CrossRef]
- Jiang, B.B.; Miao, H.B.; Chen, S.M.; Zhang, S.M.; Chen, F.D.; Fang, W.M. The Lateral Suppressor-Like Gene, DgLsL, Alternated the Axillary Branching in Transgenic Chrysanthemum (Chrysanthemum × morifolium) by Modulating IAA and GA Content. Plant Mol. Biol. Rep. 2010, 28, 144. [Google Scholar] [CrossRef]
- Xu, J.; Ding, C.; Ding, Y.; Tang, S.; Zha, M.; Luo, B.; Wang, S. A Proteomic Approach to Analyze Differential Regulation of Proteins During Bud Outgrowth Under Apical Dominance Based on the Auxin Transport Canalization Model in Rice (Oryza sativa L.). J. Plant Growth Regul. 2015, 34, 122–136. [Google Scholar] [CrossRef]
- Brewer, P.B.; Dun, E.A.; Ferguson, B.J.; Rameau, C.; Beveridge, C.A. Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiol. 2009, 150, 482–493. [Google Scholar] [CrossRef]
- Johnson, X.; Brcich, T.; Dun, E.A.; Goussot, M.; Haurogné, K.; Beveridge, C.A.; Rameau, C. Branching genes are conserved across species. Genes controlling a novel signal in pea are co-regulated by other long-distance signals. Plant Physiol. 2006, 142, 1014–1026. [Google Scholar] [CrossRef]
- Liu, W.; Wu, C.; Fu, Y.; Hu, G.; Si, H.; Zhu, L.; Luan, W.; He, Z.; Sun, Z. Identification and characterization of HTD2: A novel gene negatively regulating tiller bud outgrowth in rice. Planta 2009, 230, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Li, P.; Song, A.; Wang, H.; Wang, Y.; Ren, L.; Qi, X.; Chen, F.; Jiang, J.; Chen, S. Isolation and characterization of six AP2/ERF transcription factor genes in Chrysanthemum nankingense. Int. J. Mol. Sci. 2015, 16, 2052–2065. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.; Wen, C.; Fang, S.; Chen, X.; Nie, J.; Chu, J.; Yuan, C.; Yan, C.; Ma, N.; Zhao, L. Impacts of strigolactone on shoot branching under phosphate starvation in chrysanthemum (Dendranthema grandiflorum cv. Jinba). Front. Plant Sci. 2015, 6, 694. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, C.A.; Ross, J.J.; Murfet, I.C. Branching in pea: Action of genes Rms3 and Rms4. Plant Physiol. 1996, 110, 859–865. [Google Scholar] [CrossRef] [PubMed]

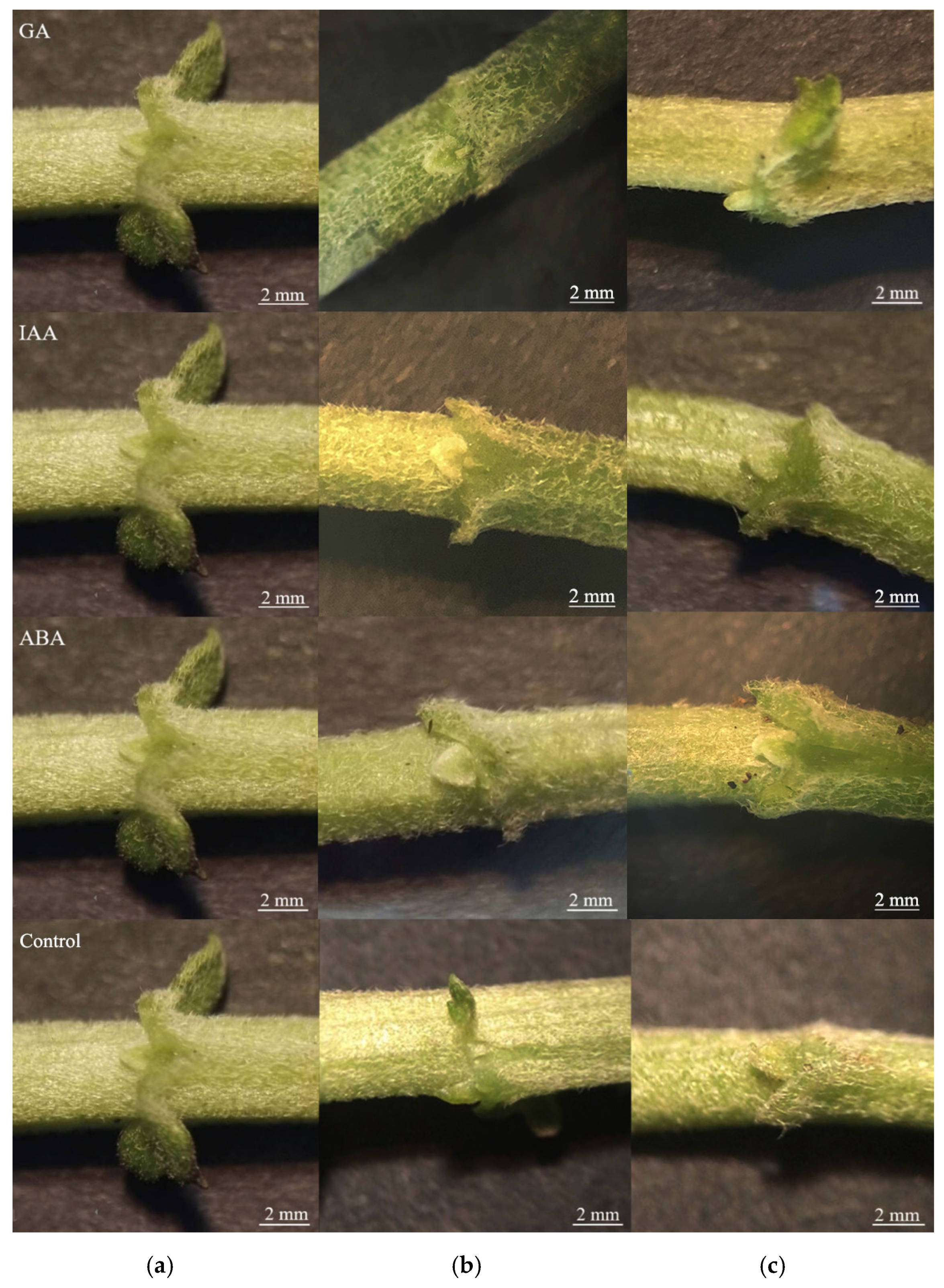


| Gene ID | Forward Primers (5′-3′) | Reverse Primers (5′-3′) |
|---|---|---|
| DgD14 | TACGAGGCATGGGTGTGTGGATC | GCACGGCGCCTTCACTAACCCT |
| DgBRC1 | CCCTTTTGGAGAGCATCAAG | AGACGTCGCGGATGAAGTAT |
| DgLsL | TTTACGCTTTACGGTGGTGGTGAG | GTGGCGGCGGAATCTGTATCTTC |
| β-Actin | TGGCATTGTGTTGGATTCTGG | CCATCCCAATCATAGACGGCT |
| Hormone | 0 Days after Treatment (mm) | 3 Days after Treatment (mm) | 6 Days after Treatment (mm) |
|---|---|---|---|
| Control | 1.26 ± 0.12 a | 1.96 ± 0.15 b | 2.33 ± 0.23 b |
| GA | 1.23 ± 0.11 a | 2.05 ± 0.18 a | 2.40 ± 0.28 a |
| ABA | 1.24 ± 0.13 a | 1.93 ± 0.12 c | 2.25 ± 0.23 c |
| IAA | 1.24 ± 0.12 a | 1.91 ± 0.15 c | 2.22 ± 0.18 c |
| Hormone | 0 Days after Treatment (mm) | 3 Days after Treatment (mm) | 6 Days after Treatment (mm) |
|---|---|---|---|
| Control | 1.22 ± 0.08 a | 1.99 ± 0.21 b | 2.26 ± 0.14 b |
| GA | 1.23 ± 0.08 a | 2.11 ± 0.23 a | 2.45 ± 0.33 a |
| ABA | 1.23 ± 0.08 a | 1.89 ± 0.17 d | 2.20 ± 0.20 c |
| IAA | 1.22 ± 0.07 a | 1.94 ± 0.15 c | 2.19 ± 0.16 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, C.; Wang, X.-J.; Ran, A.-N.; Song, J.-J.; Li, X.; Ma, Q.-Q.; Pan, Y.-Z.; Liu, Q.-L.; Jiang, B.-B. Expression Analysis of DgD14, DgBRC1 and DgLsL in the Process of Chrysanthemum Lateral Bud Formation. Agriculture 2021, 11, 1221. https://doi.org/10.3390/agriculture11121221
Luo C, Wang X-J, Ran A-N, Song J-J, Li X, Ma Q-Q, Pan Y-Z, Liu Q-L, Jiang B-B. Expression Analysis of DgD14, DgBRC1 and DgLsL in the Process of Chrysanthemum Lateral Bud Formation. Agriculture. 2021; 11(12):1221. https://doi.org/10.3390/agriculture11121221
Chicago/Turabian StyleLuo, Cheng, Xin-Jie Wang, Ai-Ning Ran, Jing-Jing Song, Xin Li, Qi-Qi Ma, Yuan-Zhi Pan, Qing-Lin Liu, and Bei-Bei Jiang. 2021. "Expression Analysis of DgD14, DgBRC1 and DgLsL in the Process of Chrysanthemum Lateral Bud Formation" Agriculture 11, no. 12: 1221. https://doi.org/10.3390/agriculture11121221
APA StyleLuo, C., Wang, X.-J., Ran, A.-N., Song, J.-J., Li, X., Ma, Q.-Q., Pan, Y.-Z., Liu, Q.-L., & Jiang, B.-B. (2021). Expression Analysis of DgD14, DgBRC1 and DgLsL in the Process of Chrysanthemum Lateral Bud Formation. Agriculture, 11(12), 1221. https://doi.org/10.3390/agriculture11121221






