Identification and Regionalization of Cold Resistance of Wine Grape Germplasms (V. vinifera)
Abstract
:1. Introduction
2. Materials and Methods
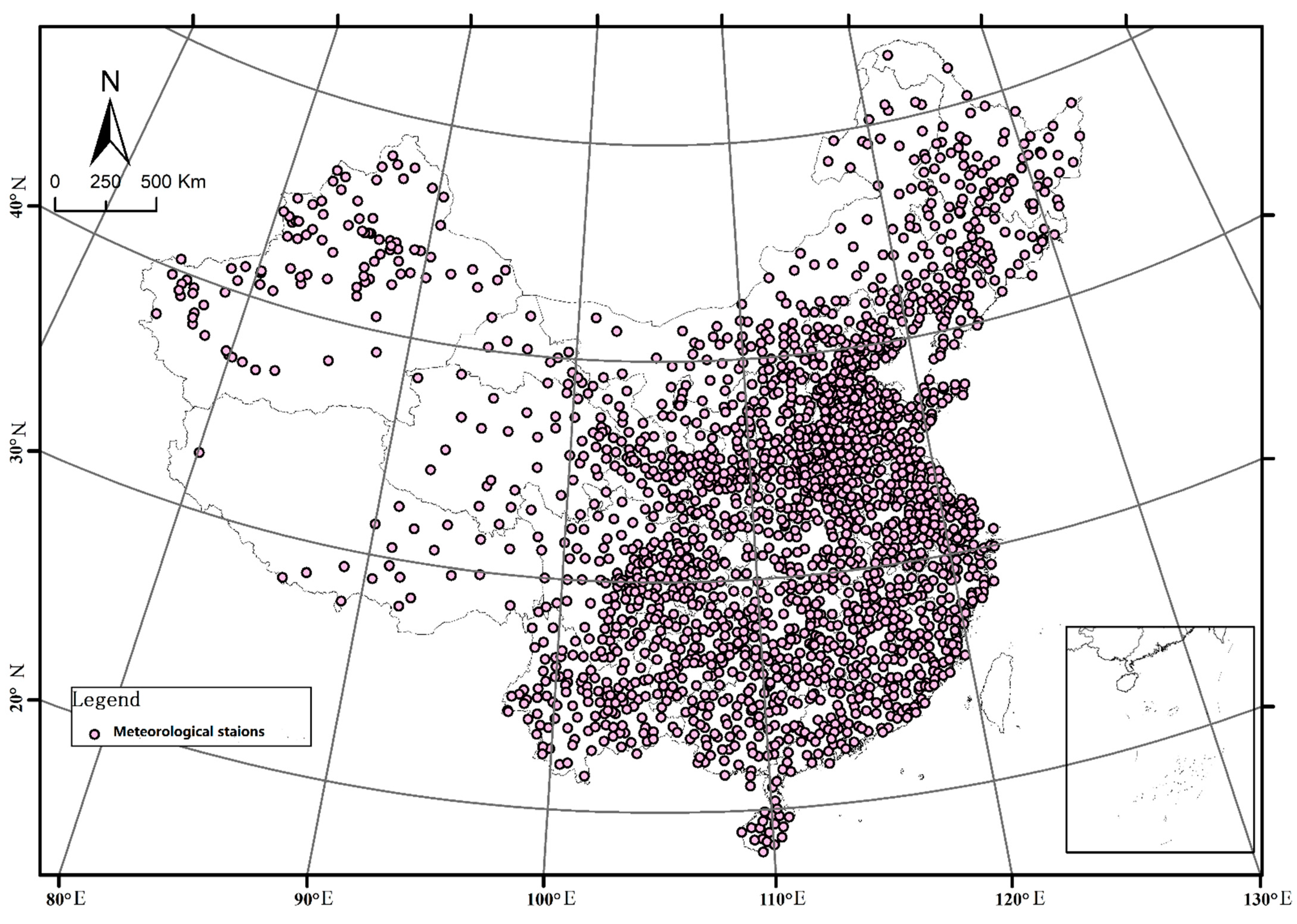
2.1. Sampling
2.2. Low Temperature Treatment and LT50 Determination
2.3. Clustering of Cold Resistance of Wine Grape Germplasms (V. vinifera)
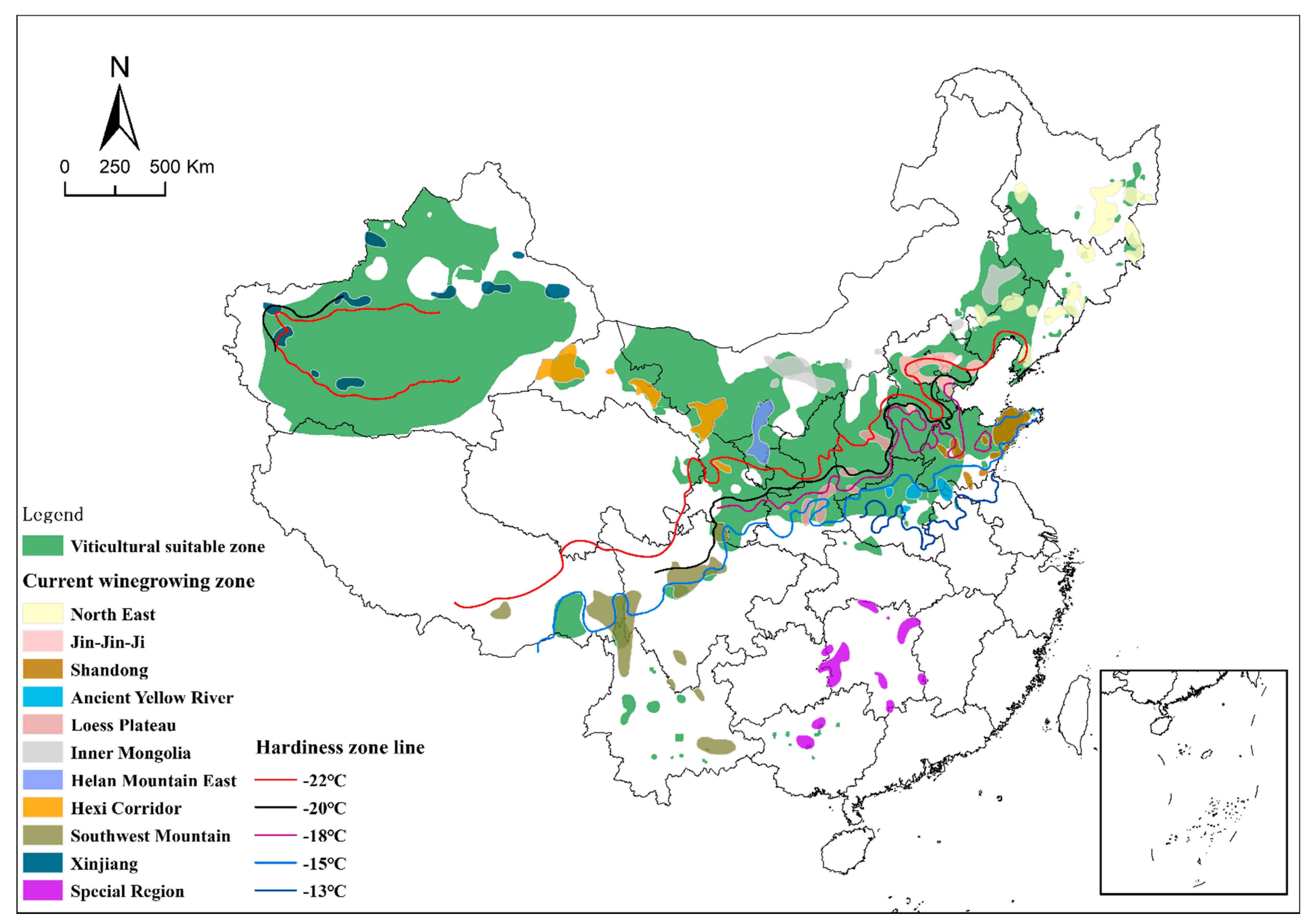
2.4. Zoning of Cold Resistance of Wine Grape Germplasms (V. vinifera)
2.5. Cold Regionalization of Wine Grape Germplasms (V. vinifera) in China
2.6. Data Analysis
3. Results
3.1. LT50 Values
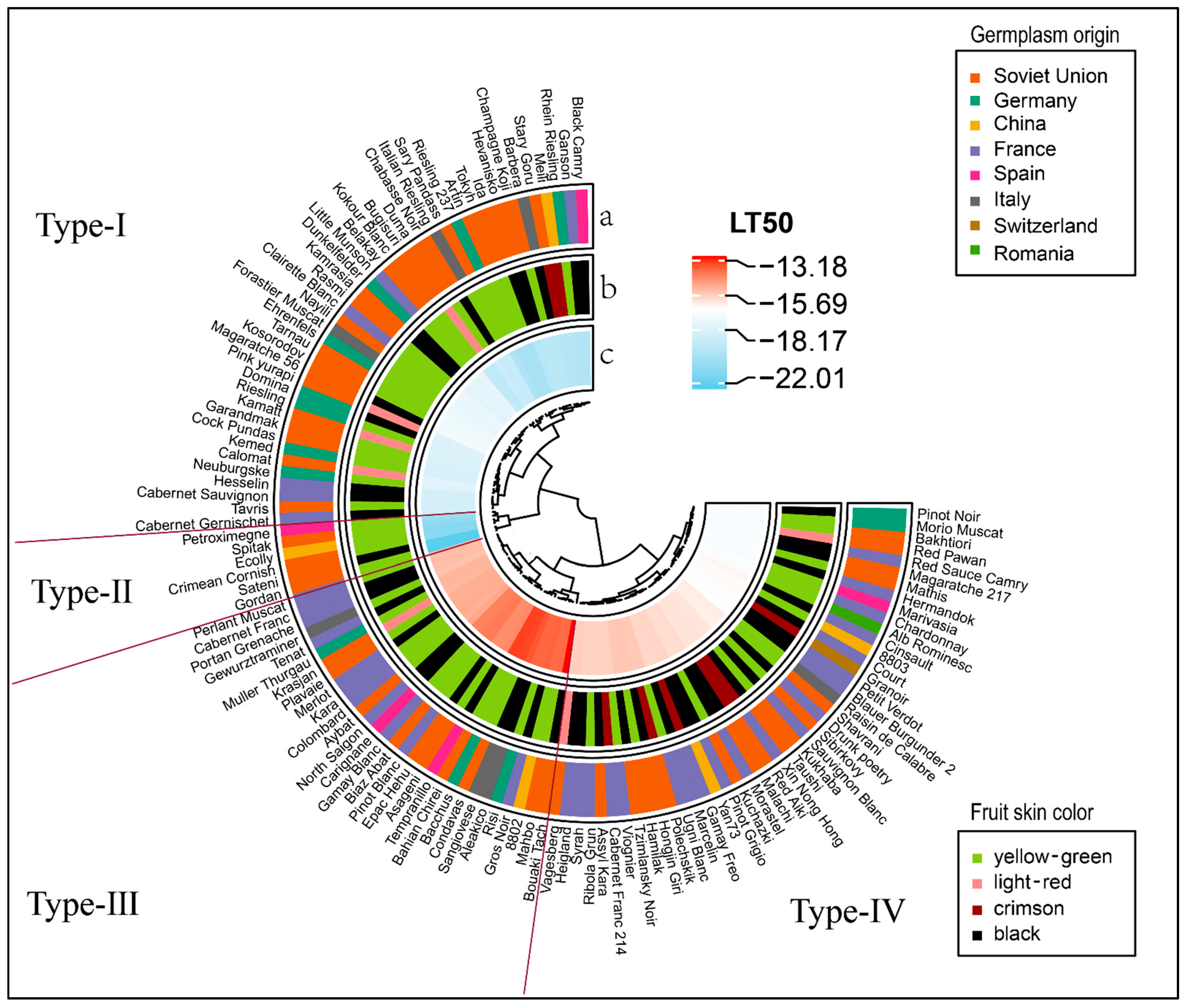
3.2. ClusterAanalysis of LT50 Values
3.3. Hardiness Zones of Wine Grape Germplasms for Different Origin Areas
3.4. Cold Regionalization of Wine Grape Germplasms (V. vinifera) in China
4. Discussion
4.1. Evaluation of Cold Resistance of Wine Grape Germplasms (V. vinifera)
4.2. Cold Regionalization of Wine Grape Germplasms (V. vinifera) in China
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leolini, L.; Moriondo, M.; Fila, G.; Costafreda-Aumedes, S.; Ferrise, R.; Bindi, M. Late spring frost impacts on future grapevine distribution in Europe. Field Crops Res. 2018, 222, 197–208. [Google Scholar] [CrossRef]
- Kretschmer, M.; Coumou, D.; Agel, L.; Barlow, M.; Tziperman, E.; Cohen, J. More-persistent weak stratospheric polar vortex states linked to cold extremes. Bull. Am. Meteorol. Soc. 2018, 99, 49–60. [Google Scholar] [CrossRef]
- Toffolatti, S.L.; De Lorenzis, G.; Costa, A.; Maddalena, G.; Passera, A.; Bonza, M.C.; Pindo, M.; Stefani, E.; Cestaro, A.; Casati, P.; et al. Unique resistance traits against downy mildew from the center of origin of grapevine (Vitis vinifera). Sci. Rep. 2018, 8, 12523. [Google Scholar] [CrossRef]
- Huang, X.; Shao, Y.; Gao, A.; Feng, J.; Xu, L. The analysis of domestic documents about Vitis vinifera L. in China. J. Libr. Inf. Sci. Agric. 2005, 17, 136–139. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z. Minor resistant genes’ accumulation by replacement in the grape-powdery mildew pathosystem. Acta Bot. Boreal. Occident. Sin. 1995, 15, 120–124. [Google Scholar]
- Hou, L.; Zhang, G.; Zhao, F.; Zhu, D.; Fan, X.; Zhang, Z.; Liu, X. VvBAP1 is involved in cold tolerance in Vitis vinifera L. Front. Plant. Sci 2018, 9, 726. [Google Scholar] [CrossRef]
- Sun, Q.; Gates, M.J.; Lavin, E.H.; Acree, T.E.; Sacks, G.L. Comparison of odor-active compounds in grapes and wines from vitis vinifera and non-foxy American grape species. J. Agric. Food Chem. 2011, 59, 10657–10664. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, L.; Zhao, K.; Niu, R.; Zhai, H.; Zhang, J. VaERD15, a transcription factor gene associated with cold-tolerance in Chinese wild Vitis amurensis. Front. Plant Sci. 2017, 8, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chai, F.; Liu, W.; Xiang, Y.; Meng, X.; Sun, X.; Cheng, C.; Liu, G.; Duan, L.; Xin, H.; Li, S. Comparative metabolic profiling of Vitis amurensis and Vitis vinifera during cold acclimation. Hortic. Res. 2019, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Kaifan, L.U.; Fang, Z.; Jiang, L.U.; Zhu, L.; Zhang, Y. Investigation of 136 grape germplasms overwintering without burying in Beijing. Sino-Overseas Grapevine Wine 2019, 6, 1–11. [Google Scholar] [CrossRef]
- Xue, T.; Han, X.; Zhang, H.; Wang, Y.; Wang, H.; Li, H. Effects of a biodegradable liquid film on winter chill protection of winegrape cultivars. Sci. Hortic. 2019, 246, 398–406. [Google Scholar] [CrossRef]
- Sgubin, G.; Swingedouw, D.; Dayon, G.; García de Cortázar-Atauri, I.; Ollat, N.; Pagé, C.; van Leeuwen, C. The risk of tardive frost damage in French vineyards in a changing climate. Agric. For. Meteorol 2018, 250–251, 226–242. [Google Scholar] [CrossRef]
- Mosedale, J.R.; Wilson, R.J.; Maclean, I.M. Climate change and crop exposure to adverse weather: Changes to frost risk and grapevine flowering conditions. PLoS ONE 2015, 10, e0141218. [Google Scholar] [CrossRef] [Green Version]
- Bucur, G.M.; Babes, A.C. Research on trends in extreme weather events and their effects on grapevine in Romanian viticulture. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Hort. J. 2016, 73, 126. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Zhang, X.; Cui, P.; Feng, Y.; Su, L.; Li, R.; Lou, S.; Xu, Z. Investigation on late frost of wine grapes in east foot area of Helan Mountain in April 2020. Ningxia J. Agric. For. Sci. Technol. 2020, 61, 51–53. [Google Scholar] [CrossRef]
- Zhai, Y.; Chen, Y.; Zhang, L.; Yang, Y.; He, B. Analysis on formation conditions of different types of frost in grape planting area of east Helan Mountain. J. Nat. Disaster 2019, 28, 134–142. [Google Scholar]
- Chai, F. Evaluation of Cold Hardiness in Grape Germplasm Resources and Screening of Candidate Genes Related to Cold. Ph.D. Thesis, University of Chinese Academy of Sciences, Wuhan, China, 2018. [Google Scholar]
- Wang, S.; Li, H.; Wang, H. Wind erosion prevention effect of suspending shoots on wires after winter pruning in soil-burying zones over-wintering. Trans. Chin. Soc. Agric. Eng. 2015, 31, 206–212. [Google Scholar] [CrossRef]
- Xue, T.; Han, X.; Zhang, H.; Li, H. Study on wind erosion control of grapes by different methods in wind tunnel experiments. J. Sedi Res. 2018, 43, 58–64. [Google Scholar] [CrossRef]
- Fennell, A. Freezing Tolerance and Injury in Grapevines. J. Crop. Improv. 2004, 10, 201–235. [Google Scholar] [CrossRef]
- Karimi, R.; Ershadi, A. Role of exogenous abscisic acid in adapting of ‘Sultana’ grapevine to low-temperature stress. Acta Physiol. Plant. 2015, 37, 151. [Google Scholar] [CrossRef]
- Zabadal, T.; Dami, I.; Goffinet, M.; Martison, T.; Chien, M. Winter Injury to Grapevines and Methods of Protect; Michigan State University: Ann Arbor, MI, USA, 2007; Volume 1, pp. 7–8. [Google Scholar]
- Ferrandino, A.; Lovisolo, C. Abiotic stress effects on grapevine (Vitis vinifera L.): Focus on abscisic acid-mediated consequences on secondary metabolism and berry quality. Environ. Exp. Bot. 2014, 103, 138–147. [Google Scholar] [CrossRef]
- Hatterman-Valenti, H.M.; Auwarter, C.P.; Stenger, J.E. Evaluation of cold-hardy grape cultivars for North Dakota and the North Dakota State University germplasm enhancement project. Acta Hortic. 2016, 1115, 13–22. [Google Scholar] [CrossRef]
- Köycü, N.D.; Stenger, J.E.; Hatterman-Valenti, H.M. Cold climate winegrape cultivar sensitivity to sulfur in the northern great plains region of the United States. HortTechnology 2017, 27, 235–239. [Google Scholar] [CrossRef]
- Lisek, J.; Lisek, A. Cold hardiness of primary buds of wine and table grape cultivars in Poland. South. Afr. J. Enol. Vitic. 2020, 41, 189–196. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Wei, Q.; Lu, R.; Gao, Z. Achievements and prospect of world cold-resistant grape breeding. J. Fruit Sci. 2004, 21, 461–466. [Google Scholar] [CrossRef]
- Nenko, N.I.; Ilyina, I.A.; Kiseleva, G.K.; Yablonskaya, E.K. Low-temperature stress tolerance of grapevine varieties of different ecological and geographical origin. Proc. Latv. Acad. Sci. B Nat. Exact. Appl. Sci. 2019, 73, 56–65. [Google Scholar] [CrossRef] [Green Version]
- Schrader, J.A.; Cochran, D.R.; Domoto, P.A.; Nonnecke, G.R. Phenology and winter hardiness of cold-climate grape cultivars and advanced selections in Iowa climate. Horttechnology 2019, 29, 906–922. [Google Scholar] [CrossRef] [Green Version]
- Vool, E.; Ratsep, R.; Karp, K. Effect of genotype on grape quality parameters in cool climate conditions. Acta Hortic. 2015, 1082, 353–358. [Google Scholar] [CrossRef]
- Qin, Y. Summary of research on cold resistance breeding of grape in Minnesota of America. Sino-Overseas Grapevine Wine 2004, 2, 64–65. [Google Scholar] [CrossRef]
- Wang, J.; Song, R.; Yi, L.; Sun, K. The traits and heredity of hybrid offspring of grape Vitis amurensis Rupr and Vitis vinifera L. Spec. Wild Econ. Anim. Plant Res. 1997, 4, 5–9. [Google Scholar]
- Atucha, A.; Hedtcke, J.; Workmaster, B.A. Evaluation of cold-climate interspecific hybrid wine grape cultivars for the Upper Midwest. Agric. Week J. Am. Pomol. Soc. 2018, 72, 80–93. [Google Scholar]
- Manso-Martínez, C.; Sáenz-Navajas, M.P.; Hernández, M.M.; Menéndez, C.M. Sensory profiling and quality assessment of wines derived from Graciano × Tempranillo selections. LWT 2020, 127, 109394. [Google Scholar] [CrossRef]
- Roca, P.; Weinmann, E.; Boos, M.; Ehret, P.; Flubacher, L.; Schneider, C.; Veith, L. Breeding of new disease-tolerant grape varieties—Viticulture in times of climatic change. BIO Web Conf. 2019, 01035. [Google Scholar] [CrossRef]
- Celik, H.; Rustem, C.; Kse, B. Determination of fox grape genotypes (Vitis labrusca L.) grown in Northeastern Anatolia. Hortic. Sci. 2008, 4, 162–170. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhang, Z.; Wang, H.; Liu, Y. A new grape variety—’Ecolly’. Acta Hortic. Sin. 2000, 27, 75. [Google Scholar]
- Zhang, Z.-W.; Wang, H.; Fang, Y.-L.; Xi, Z.-M.; Li, H. A new high quality wine-grape cultivar ‘Meili’ with disease resistance. Acta Hortic. Sin. 2013, 40, 1611–1612. [Google Scholar]
- Li, H. Grape Breeding for High Quality Disease Resistance—Study on Theory and Method; China Agriculture Press: Beijing, China, 1999; pp. 72–76. [Google Scholar]
- Li, E. Study on Characteristics of New Wine Grape Strain 8804. Bachelor’s Thesis, Northwest A&F University, Yangling, China, 2007. [Google Scholar]
- Li, N. Study on Brandy Brewine of Main White Wine Grape Varieties in Yangling. Bachelor’s Thesis, Northwest A&F University, Yangling, China, 2017. [Google Scholar]
- He, Y. Investigation of the Comprehensive Characters of the Main Wine Grapes in Yangling. Bachelor’s Thesis, Northwest A&F University, Yangling, China, 2015. [Google Scholar]
- Shu, T. Adaptability of Meili and Other Red Wine Grape Varieties and Its Cultivation in Yangling. Bachelor’s Thesis, Northwest A&F University, Yangling, China, 2018. [Google Scholar]
- Li, H.; Wang, H.; Liu, L.-P.; Wang, Z.-Q.; Shu, Y. Analysis of aroma components of Ecolly dry white wine by GC/MS. Sci. Agric. Sin. 2005, 38, 1250–1254. [Google Scholar] [CrossRef]
- Wang, X.; Liang, Y.; Li, N.; Du, H.; Yang, C.; LI, H.; Wang, H. Analysis of the aroma components of distilled spirits from main grape varieties in Yangling area. Chin. Brew. 2018, 37, 161–167. [Google Scholar] [CrossRef]
- Gómez-Plaza, E.; Gil-Muñoz, R.; Hernández-Jiménez, A.; López-Roca, J.M.; Ortega-Regules, A.; Martínez-Cutillas, A. Studies on the anthocyanin profile of Vitis Vinifera intraspecific hybrids (Monastrell×Cabernet Sauvignon). Eur. Food Res. Technol. 2008, 227, 479–484. [Google Scholar] [CrossRef]
- Wang, Z.L.; Xue, T.T.; Gao, F.F.; Zhang, L.; Han, X.; Wang, Y.; Hui, M.; Wu, D.; Li, H.; Wang, H. Intraspecific recurrent selection in V. vinifera: An effective method for breeding of high quality, disease-, cold-, and drought -resistant grapes. Euphytica 2021, 217, 111. [Google Scholar] [CrossRef]
- Wu, X. Study on Cold Resistance of Grape Resources. Bachelor’s Thesis, Northwest A&F University, Yangling, China, 2011. [Google Scholar]
- Zhao, Y.; Wang, Z.-X.; Yang, Y.-M.; Liu, H.-S.; Shi, G.-L.; Ai, J. Analysis of the cold tolerance and physiological response differences of amur grape (Vitis amurensis) germplasms during overwintering. Sci Hortic. 2020, 259. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, X.; Niu, R.; Liu, Y.; Wang, Y. Cold-resistance evaluation in 25 wild grape species. Vitis 2012, 51, 153–160. [Google Scholar]
- Zhan, J.; Li, D. Wine Grape Varieties; China Agricultural University Press: Beijing, China, 2015; pp. 45–106. ISBN 978-7-81117-954-5. [Google Scholar]
- Liu, X.; Chang, W.; Lu, J.; Zhang, Y. The investigation for botanical characters, berry quality and plant resistance of introduced America wine grapes grown in Beijing. J. Plant Genet. Resour. 2015, 16, 307–314. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, J.; Fan, X.; Sun, H.; Li, M.; Liu, C. Overview for introduction of grape varieties from overseas since 2000. Sino-Overseas Grapevine Wine 2018, 2, 54–59. [Google Scholar]
- Zhong, Y.; Zhao, S.; Liu, J.; Zhang, Q. Introduction and breeding of wine grape varieties in China. Bull. Agric. Sci. Technol. 2020, 214–220. [Google Scholar]
- Wang, L.; Li, H.; Wang, H. Climatic regionalization of grape in China I: Indexes and methods. Chin. Sci. Bull. 2017, 62, 1527–1538. [Google Scholar] [CrossRef]
- Wang, L.; Li, H.; Wang, H. Climatic regionalization of grape in China II: Wine grape varieties regionalization. Chin. Sci. Bull. 2017, 62, 1539–1554. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Li, H.; Chen, W.; Zhang, L.; Su, L.; Wang, J. Ecological regionalization of wine grape varieties in Ningxia. Chin. J. Ecol. 2014, 33, 3112–3119. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, G. The climatic suitability and climatic impact factors affecting the wine grapes (Vitis vinifera L.) planting distribution in China. Acta Ecol. Sin. 2021, 41, 2418–2427. [Google Scholar] [CrossRef]
- Yue, H.; He, B.; Jiang, J.; Pan, X.; Zhang, Y.; Liu, C.; Yang, Y. Risk Analysis of spring frost in main grape producing areas. Chin. J. Agrometeorol. 2021, 42, 221–229. [Google Scholar] [CrossRef]
- Qin, W.; Han, Y.; Wang, L.; Cheng, W.; Li, Q. Risk assessment and zoning of freezing damage of wine grapes in Ningxia. Jiangsu Agric. Sci. 2019, 47, 129–133. [Google Scholar] [CrossRef]
- Jin, M.; Xu, J.; Zhang, G. Relation between electrical impedance spectroscopy parameters and frost hardiness in shoots of apple rootstocks. Acta Hortic. Sin. 2011, 38, 1045–1051. [Google Scholar]
- Liu, Y.; Jiang, J.; Fan, X.; Zhang, Y.; Wu, J.; Wang, L.; Liu, C. Identification of heat tolerance in Chinese wildgrape germplasm resources. Horticulturae 2020, 6, 68. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Chen, R.; Liu, Z.; Li, F.; Feng, R.; Wang, J.; Li, H. Comparing the cold resistance of roots of different wine grape varieties. Chin. J. Eco-Agric. 2020, 28, 558–565. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, D.; Jiang, J.; Pan, X.; Zhang, Y. Relationship between LT50 and cold adaptability of five grape varieties. J. Northwest AF Univ. 2013, 41, 149–154. [Google Scholar] [CrossRef]
- Zeng, L.; Zheng, X.; Zhang, T.; Luo, J. Comparison of cold resistance of 6 apple rootstocks by conductance method and logistic equation. Jiangsu Agric. Sci. 2017, 45, 119–121. [Google Scholar] [CrossRef]
- Han, X.; Xue, T.; Liu, X.; Wang, Z.; Zhang, L.; Wang, Y.; Yao, F.; Wang, H.; Li, H. A sustainable viticulture method adapted to the cold climate zone in China. Horticulturae 2021, 7, 150. [Google Scholar] [CrossRef]
- Li, G.; Lian, Y.; Shanshan, C.; Xin, T.; Niu, S.; Huiling, H. Analysis of cold resistance of different seedless grape varieties under low temperature stress. Southwest China J. Agric. Sci. 2018, 31, 2399–2406. [Google Scholar] [CrossRef]
- Mills, L.; Ferguson, J.; Keller, M. Cold-hardiness evaluation of grapevine buds and cane tissues. Am. J. Enol. Vitic. 2006, 57. [Google Scholar] [CrossRef]
- He, W.; Ai, J.; Yang, Y.; Fan, S.; Wang, Z. Study on the semi-lethal temperature of germplasm resources of Vitis amurensis branches. North. Hortic. 2014, 19–22. [Google Scholar]
- Zhao, Y. Evaluation for Cold Tolerance in Germplasms of Vitis amurensis and Study on Mechanisms of Cold Tolerance. Ph.D. Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2018. [Google Scholar]
- Byard, S.; Wisniewski, M.; Li, J.H.; Karlson, D. Interspecific analysis of xylem freezing responses in acer and betula. HortScience 2010, 45, 165–168. [Google Scholar] [CrossRef] [Green Version]
- He, P.; Chao, W. Studies on the cold hardiness of eight species of V.vinifera L growing wild in China. Acta Hortic. Sin. 1982, 9, 17–21. [Google Scholar]
- Wang, L. Climatic Zoning and Variety Regionalization of Wine Grape in China Based on dem. Ph.D. Thesis, Northwest A&F University, Yangling, China, 2017. [Google Scholar]
- Xue, T. Research of Free-Buried Mode Based on Biodegradable Liquid Film in Grapevine Soil-Burial Areas. Ph.D. Thesis, Northwestern A&F University, Yangling, China, 2018. [Google Scholar]
| Number | Sink Color | Cultivars or Species | Number | Sink Color | Cultivars or Species |
|---|---|---|---|---|---|
| USSR1 | yellow-green | Artin | GER 8 | yellow-green | Riesling 237 |
| USSR2 | yellow-green | Asageni | GER 9 | yellow-green | Risi |
| USSR3 | yellow-green | Aybat ♀ | GER 10 | yellow-green | Muller Thurgau |
| USSR4 | black | Epac Hehu | GER 11 | yellow-green | Morio Muscat |
| USSR5 | yellow-green | Bakhtiori | GER 12 | yellow-green | Neuburgske |
| USSR6 | yellow-green | Kokour Blanc | GER 13 | black | Dunkelfelder |
| USSR7 | yellow-green | Bahian Chirei | CHN1 | yellow-green | 8802 |
| USSR8 | yellow-green | Biaz Abat | CHN2 | yellow-green | 8803 |
| USSR9 | yellow-green | Belakay | CHN3 | yellow-green | Ecolly |
| USSR10 | yellow-green | Plavaie | CHN4 | crimson | Meili |
| USSR11 | crimson | Polechskik ♀ | CHN5 | black | Yan73 |
| USSR12 | Black | Bouaki Tach | FRA1 | yellow-green | Pinot Blanc |
| USSR13 | light-red | Bugisuri | FRA2 | yellow-green | Gamay Blanc |
| USSR14 | yellow-green | Duma ♀ | FRA3 | yellow-green | Clairette Blanc |
| USSR15 | light-red | Pink yurapi | FRA4 | yellow-green | Ugni Blanc |
| USSR16 | yellow-green | Garandmak | FRA5 | black | North Saigon |
| USSR17 | yellow-green | Gordan | FRA6 | black | Portan Grenache |
| USSR18 | yellow-green | Hamilak | FRA7 | black | Cabernet Sauvignon |
| USSR19 | black | Assyl Kara | FRA8 | black | Gros Noir |
| USSR20 | crimson | Stary Goru | FRA9 | black | Blauer Burgunder 2 |
| USSR21 | black | Tzimlansky Noir | FRA10 | crimson | Gamay Freo |
| USSR22 | black | Chabasse Noir ♀ | FRA11 | black | Morastel |
| USSR23 | black | Hevanisko | FRA12 | black | Ganson |
| USSR24 | crimson | Red Aiki ♀ | FRA13 | yellow-green | Colombard |
| USSR25 | black | Hongjin Giri | FRA14 | yellow-green | Ribola Grun |
| USSR26 | light-red | Red Pawan | FRA15 | black | Heigland |
| USSR27 | light-red | Calomat | FRA16 | black | Hermandok |
| USSR28 | light-red | Kamatt | FRA17 | black | Hesselin |
| USSR29 | black | Kamrasia | FRA18 | black | Red Sauce Camry |
| USSR30 | yellow-green | Condavas | FRA19 | black | Pinot Grigio |
| USSR31 | yellow-green | Kukhaba | FRA20 | yellow-green | Kara |
| USSR32 | yellow-green | Cock Pundas | FRA21 | black | Court |
| USSR33 | yellow-green | Kosorodov | FRA22 | black | Marcelin |
| USSR34 | light-red | Krasjan | FRA23 | black | Merlot |
| USSR35 | yellow-green | Crimean Cornish ♀ | FRA24 | black | Cabernet Franc |
| USSR36 | yellow-green | Kuchazki | FRA25 | crimson | Cabernet Franc 214 |
| USSR37 | yellow-green | Rasmi | FRA26 | black | Cabernet Gernischet |
| USSR38 | black | Magaratche 217 | FRA27 | yellow-green | Perlant Muscat |
| USSR39 | black | Magaratche 56 | FRA28 | black | Sauvignon Blanc |
| USSR40 | yellow-green | Mahbo | FRA29 | black | Tenat |
| USSR41 | black | Malachi | FRA30 | yellow-green | Viognier |
| USSR42 | yellow-green | Mathis | FRA31 | black | Petit Verdot |
| USSR43 | yellow-green | Nayili | FRA32 | black | Syrah |
| USSR44 | black | Sateni | FRA33 | yellow-green | Chardonnay |
| USSR45 | yellow-green | Sary Pandass | FRA34 | yellow-green | Little Munson |
| USSR46 | yellow-green | Spitak | FRA35 | crimson | Xin Nong Hong |
| USSR47 | yellow-green | Tarnau | FRA36 | black | Cinsault |
| USSR48 | yellow-green | Tavris | FRA37 | black | Drunk poetry |
| USSR49 | black | Taushi | ESP1 | yellow-green | Petroximegne |
| USSR50 | yellow-green | Tokyh | ESP2 | black | Black Camry |
| USSR51 | light-red | Vagesberg | ESP3 | black | Carignane |
| USSR52 | yellow-green | Sibirkovy | ESP4 | yellow-green | Marivasia |
| USSR53 | yellow-green | Shavrani | ESP5 | black | Tempranillo |
| USSR54 | yellow-green | Champagne Koji | ITA1 | yellow-green | Gewurztraminer |
| USSR55 | black | Ida | ITA2 | black | Aleakico |
| GER 1 | yellow-green | Ehrenfels | ITA3 | black | Barbera |
| GER 2 | yellow-green | Bacchus | ITA4 | yellow-green | Forastier Muscat |
| GER 3 | black | Domina | ITA5 | yellow-green | Italian Riesling |
| GER 4 | black | Pinot Noir | ITA6 | yellow-green | Raisin de Calabre |
| GER 5 | yellow-green | Kemed | ITA7 | black | Sangiovese |
| GER 6 | yellow-green | Rhein Riesling | SUI1 | crimson | Granoir |
| GER 7 | yellow-green | Riesling | ROU1 | yellow-green | Alb Rominesc |
| Number | Regression Equation | R2 | LT50 | Number | Regression Equation | R2 | LT50 |
|---|---|---|---|---|---|---|---|
| USSR1 | Y = 0.08x + 1.62 | 0.69 | −19.91 | GER 8 | Y = 0.08x + 1.62 | 0.69 | −19.95 |
| USSR2 | Y = 0.19x + 2.75 | 0.90 | −14.65 | GER 9 | Y = 0.22x + 3.06 | 0.74 | −14.05 |
| USSR3 | Y = 0.18x + 2.75 | 0.79 | −15.28 | GER 10 | Y = 0.20x + 3.11 | 0.66 | −15.35 |
| USSR4 | Y = 0.19x + 2.82 | 0.64 | −14.67 | GER 11 | Y = 0.15x + 2.64 | 0.92 | −17.03 |
| USSR5 | Y = 0.15x + 2.64 | 0.95 | −17.07 | GER 12 | Y = 0.19x + 3.59 | 0.94 | −18.01 |
| USSR6 | Y = 0.05x + 0.90 | 0.95 | −19.09 | GER 13 | Y = 0.26x + 4.61 | 0.97 | −17.78 |
| USSR7 | Y = 0.19x + 2.52 | 0.94 | −13.58 | CHN1 | Y = 0.20x + 2.79 | 0.72 | −14.15 |
| USSR8 | Y = 0.20x + 2.90 | 0.89 | −14.37 | CHN2 | Y = 0.23x + 3.64 | 0.98 | −16.68 |
| USSR9 | Y = 0.16x + 2.84 | 0.93 | −17.76 | CHN3 | Y = 0.15x + 3.14 | 0.93 | −20.92 |
| USSR10 | Y = 0.19x + 2.98 | 0.88 | −15.23 | CHN4 | Y = 0.17x + 3.31 | 0.89 | −19.48 |
| USSR11 | Y = 0.13x + 2.04 | 0.99 | −15.73 | CHN5 | Y = 0.25x + 3.93 | 0.99 | −15.95 |
| USSR12 | Y = 0.19x + 2.53 | 0.90 | −13.18 | FRA1 | Y = 0.13x + 1.88 | 0.90 | −14.35 |
| USSR13 | Y = 0.18x + 3.54 | 0.82 | −19.20 | FRA2 | Y = 0.17x + 2.53 | 0.90 | −14.45 |
| USSR14 | Y = 0.17x + 3.22 | 0.82 | −18.74 | FRA3 | Y = 0.10x + 1.75 | 0.98 | −17.61 |
| USSR15 | Y = 0.13x + 2.40 | 0.74 | −18.09 | FRA4 | Y = 0.14x + 2.20 | 0.99 | −15.72 |
| USSR16 | Y = 0.20x + 3.59 | 0.94 | −18.18 | FRA5 | Y = 0.15x + 2.25 | 0.84 | −15.09 |
| USSR17 | Y = 0.16x + 3.66 | 0.87 | −22.01 | FRA6 | Y = 0.14x + 2.20 | 0.99 | −15.53 |
| USSR18 | Y = 0.13x + 2.04 | 0.99 | −15.69 | FRA7 | Y = 0.07x + 1.39 | 0.65 | −18.48 |
| USSR19 | Y = 0.12x + 1.84 | 0.92 | −15.89 | FRA8 | Y = 0.31x + 4.38 | 0.95 | −14.01 |
| USSR20 | Y = 0.10x + 2.04 | 0.85 | −19.51 | FRA9 | Y = 0.23x + 3.25 | 0.98 | −16.72 |
| USSR21 | y = 0.30x + 4.70 | 0.99 | −15.71 | FRA10 | Y = 0.12x + 1.89 | 0.61 | −15.99 |
| USSR22 | Y = 0.20x + 3.38 | 0.96 | −18.95 | FRA11 | Y = 0.14x + 2.20 | 0.99 | −16.09 |
| USSR23 | Y = 0.19x + 3.76 | 0.83 | −19.36 | FRA12 | Y = 0.07x + 1.51 | 0.62 | −19.64 |
| USSR24 | Y = 0.15x + 2.50 | 0.99 | −16.22 | FRA13 | Y = 0.30x + 4.60 | 0.98 | −15.19 |
| USSR25 | Y = 0.18x + 2.79 | 0.63 | −15.68 | FRA14 | Y = 0.24x + 3.84 | 0.99 | −15.90 |
| USSR26 | Y = 0.17x + 2.96 | 0.66 | −17.10 | FRA15 | Y = 0.10x + 1.55 | 0.59 | −15.79 |
| USSR27 | Y = 0.27x + 4.85 | 0.99 | −17.94 | FRA16 | Y = 0.19x + 3.22 | 0.89 | −16.97 |
| USSR28 | Y = 0.25x + 4.60 | 0.85 | −18.17 | FRA17 | Y = 0.07x + 1.39 | 0.65 | −18.49 |
| USSR29 | Y = 0.16x + 2.78 | 0.98 | −17.86 | FRA18 | Y = 0.21x + 3.09 | 0.99 | −16.99 |
| USSR30 | Y = 0.27x + 3.70 | 0.95 | −13.67 | FRA19 | Y = 0.21x + 3.36 | 0.99 | −15.95 |
| USSR31 | Y = 0.11x + 1.85 | 0.51 | −16.24 | FRA20 | Y = 0.26x + 3.98 | 0.92 | −15.21 |
| USSR32 | Y = 0.14x + 2.54 | 0.86 | −18.29 | FRA21 | Y = 0.23x + 3.81 | 0.84 | −16.66 |
| USSR33 | Y = 0.12x + 2.14 | 0.57 | −17.29 | FRA22 | Y = 0.26x + 4.10 | 0.99 | −15.98 |
| USSR34 | Y = 0.23x + 3.48 | 0.66 | −15.41 | FRA23 | Y = 0.12x + 1.89 | 0.93 | −15.22 |
| USSR35 | Y = 0.05x + 1.06 | 0.93 | −20.88 | FRA24 | Y = 0.11x + 1.74 | 0.85 | −15.51 |
| USSR36 | Y = 0.30x + 4.80 | 0.95 | −16.09 | FRA25 | Y = 0.25 + 3.93 | 0.99 | −15.89 |
| USSR37 | Y = 0.16x + 2.84 | 0.94 | −17.62 | FRA26 | Y = 0.18x + 3.32 | 0.98 | −18.46 |
| USSR38 | Y = 0.21x + 3.09 | 0.99 | −16.99 | FRA27 | Y = 0.14x + 2.17 | 0.65 | −15.51 |
| USSR39 | Y = 0.17x + 2.96 | 0.66 | −17.28 | FRA28 | Y = 0.18x + 2.86 | 0.97 | −16.26 |
| USSR40 | Y = 0.22x + 3.05 | 0.95 | −14.11 | FRA29 | Y = 0.25x + 3.89 | 0.96 | −15.36 |
| USSR41 | Y = 0.13x + 2.03 | 0.93 | −16.06 | FRA30 | Y = 0.22x + 3.06 | 0.65 | −15.87 |
| USSR42 | Y = 0.15x + 2.62 | 0.99 | −16.95 | FRA31 | Y = 0.27x + 4.47 | 0.80 | −16.83 |
| USSR43 | Y = 0.29x + 5.21 | 0.97 | −17.63 | FRA32 | Y = 0.12x + 1.84 | 0.92 | −15.90 |
| USSR44 | Y = 0.14x + 3.05 | 0.99 | −21.95 | FRA33 | Y = 0.15x + 2.62 | 0.99 | −16.90 |
| USSR45 | Y = 0.08x + 1.62 | 0.69 | −19.75 | FRA34 | Y = 0.26x + 4.62 | 0.99 | −17.77 |
| USSR46 | Y = 0.03x + 0.67 | 0.96 | −20.68 | FRA35 | Y = 0.16x + 2.57 | 0.99 | −16.21 |
| USSR47 | Y = 0.15x + 2.60 | 0.91 | −17.29 | FRA36 | Y = 0.07x + 1.13 | 0.81 | −16.58 |
| USSR48 | Y = 0.75x + 1.39 | 0.65 | −18.49 | FRA37 | Y = 0.14x + 2.29 | 0.78 | −16.41 |
| USSR49 | Y = 0.13x + 2.03 | 0.93 | −16.19 | ESP1 | Y = o.11x + 2.34 | 0.65 | −20.46 |
| USSR50 | Y = 0.17x + 3.37 | 0.98 | −19.91 | ESP2 | Y = 0.16x + 3.14 | 0.86 | −19.64 |
| USSR51 | Y = 0.08x + 1.23 | 0.82 | −15.82 | ESP3 | Y = 0.13x + 1.92 | 0.79 | −14.48 |
| USSR52 | Y = 0.20x + 3.22 | 0.95 | −16.42 | ESP4 | Y = 0.14x + 2.38 | 0.76 | −16.97 |
| USSR53 | Y = 0.18x + 3.01 | 0.77 | −16.38 | ESP5 | Y = 0.18x + 2.67 | 0.75 | −14.58 |
| USSR54 | Y = 0.18x + 3.55 | 0.82 | −19.35 | ITA1 | Y = 0.13x + 2.04 | 0.99 | −15.60 |
| USSR55 | Y = 0.14x + 2.72 | 0.87 | −19.43 | ITA2 | Y = 0.25x + 3.54 | 0.99 | −14.07 |
| GER 1 | y = 0.35x + 6.00 | 0.87 | −17.40 | ITA3 | Y = 0.14x + 2.61 | 0.98 | −19.35 |
| GER 2 | y = 0.16x + 2.21 | 0.93 | −13.61 | ITA4 | Y = 0.17x + 3.00 | 0.97 | −17.49 |
| GER 3 | y = 0.25x + 4.69 | 0.85 | −18.09 | ITA5 | Y = 0.18x + 3.48 | 0.95 | −18.92 |
| GER 4 | Y = 0.17x + 2.90 | 0.90 | −17.03 | ITA6 | Y = 0.31x + 5.20 | 0.99 | −16.77 |
| GER 5 | Y = 0.25x + 4.60 | 0.85 | −18.27 | ITA7 | Y = 0.25x + 3.54 | 0.99 | −13.85 |
| GER 6 | Y = 0.10x + 2.04 | 0.85 | −19.59 | SUI1 | Y = 0.14x + 2.33 | 0.79 | −16.66 |
| GER 7 | Y = 0.17x + 3.01 | 0.59 | −18.08 | ROU1 | Y = 0.16x + 2.71 | 0.88 | −16.94 |
| Item | LT50 | |||
|---|---|---|---|---|
| Type II | Type I | Type IV | Type Ⅲ | |
| Temperature of germplasms origin | 0.580 | 0.224 | 0.280 * | 0.343 |
| Fruit skin color of germplasms | −0.591 | 0.249 | 0.336 ** | −0.099 |
| Origin Area | Average LT50 (°C) | Total Number | Hardiness Zone | Number of Germplasms | Frequency Distribution Histogram |
|---|---|---|---|---|---|
| Germany | 17.25 ± 1.20 b | 13 | Zone 2 | 3 | 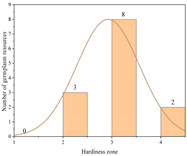 |
| Zone 3 | 8 | ||||
| Zone 4 | 2 | ||||
| France | 16.26 ± 1.21 a | 37 | Zone 2 | 4 | 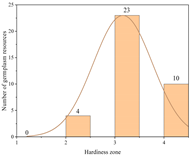 |
| Zone 3 | 23 | ||||
| Zone 4 | 10 | ||||
| Soviet Union | 17.29 ± 0.95 b | 55 | Zone 1 | 4 | 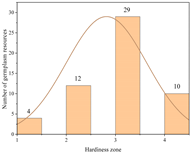 |
| Zone 2 | 12 | ||||
| Zone 3 | 29 | ||||
| Zone 4 | 10 | ||||
| Spain | 17.22 ± 1.61 b | 5 | Zone 1 | 1 | / |
| Zone 2 | 1 | ||||
| Zone 3 | 1 | ||||
| Zone 4 | 2 | ||||
| Italy | 16.58 ± 1.49 a | 7 | Zone 2 | 2 | 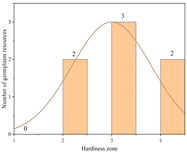 |
| Zone 3 | 3 | ||||
| Zone 4 | 2 | ||||
| China | 17.44 ± 1.79 b | 5 | Zone 1 | 1 | / |
| Zone 2 | 1 | ||||
| Zone 3 | 2 | ||||
| Zone 4 | 1 | ||||
| Romania | / | 1 | Zone 3 | 1 | / |
| Switzerland | / | 1 | Zone 3 | 1 | / |
| Division | LT50 (°C) | Germplasms |
|---|---|---|
| A | −22 ≤ LT50 ≤ −20 | USSR17, USSR44, CHN3, USSR35, USSR46, ESP1 |
| B | −20 ≤ LT50 ≤ −18 | GER8, USSR1, USSR50, USSR45, ESP2, FRA12, GER6, USSR20, CHN4, USSR55, USSR23, ITA3, USSR54, USSR13, USSR6, USSR22, ITA5, USSR14, USSR48, FRA17, FRA7, FRA26, USSR32, GER5, USSR16, USSR28, GER3, USSR15, GER7, GER12 |
| C | −18 ≤ LT50 ≤ −15 | USSR27, USSR29, GER13, FRA34, USSR9, USSR43, USSR37, FRA3, ITA4, GER1, USSR47, USSR33, USSR39, USSR26, USSR5, GER11, GER4, USSR38, FRA18, FRA16, ESP4, USSR42, ROU1, FRA33, FRA31, ITA6, FRA9, CHN2, SUI1, FRA21, FRA36, USSR52, FRA37, USSR53, FRA28, USSR31, USSR24, FRA35, USSR49, FRA11, USSR36, USSR41, FRA10, FRA22, CHN5, FRA19, FRA32, FRA14, USSR19, FRA25, FRA30, USSR51, FRA15, USSR11, FRA4, USSR21, USSR18, USSR25, ITA1, FRA6, FRA27, FRA24, USSR34, FRA29, GER10, USSR3, USSR10, FRA23, FRA20, FRA13, FRA5 |
| D | −15 ≤ LT50 ≤ −13 | USSR4, USSR2, ESP5, ESP3, FRA2, USSR8, FRA1, CHN1, USSR40, ITA2, GER9, FRA8, ITA7, USSR30, GER2, USSR7, USSR12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wang, Y.; Wu, D.; Hui, M.; Han, X.; Xue, T.; Yao, F.; Gao, F.; Cao, X.; Li, H.; et al. Identification and Regionalization of Cold Resistance of Wine Grape Germplasms (V. vinifera). Agriculture 2021, 11, 1117. https://doi.org/10.3390/agriculture11111117
Wang Z, Wang Y, Wu D, Hui M, Han X, Xue T, Yao F, Gao F, Cao X, Li H, et al. Identification and Regionalization of Cold Resistance of Wine Grape Germplasms (V. vinifera). Agriculture. 2021; 11(11):1117. https://doi.org/10.3390/agriculture11111117
Chicago/Turabian StyleWang, Zhilei, Ying Wang, Dong Wu, Miao Hui, Xing Han, Tingting Xue, Fei Yao, Feifei Gao, Xiao Cao, Hua Li, and et al. 2021. "Identification and Regionalization of Cold Resistance of Wine Grape Germplasms (V. vinifera)" Agriculture 11, no. 11: 1117. https://doi.org/10.3390/agriculture11111117
APA StyleWang, Z., Wang, Y., Wu, D., Hui, M., Han, X., Xue, T., Yao, F., Gao, F., Cao, X., Li, H., & Wang, H. (2021). Identification and Regionalization of Cold Resistance of Wine Grape Germplasms (V. vinifera). Agriculture, 11(11), 1117. https://doi.org/10.3390/agriculture11111117







