Production Efficiency and Total Protein Yield in Quinoa Grown under Water Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Establishment and Experimental Design
2.2. Input and Output Indicators
2.3. The Model
2.4. Statistical Analysis
3. Results
3.1. Seed Yield
3.2. Total Protein Content
3.3. Globulin and Albumin Yield
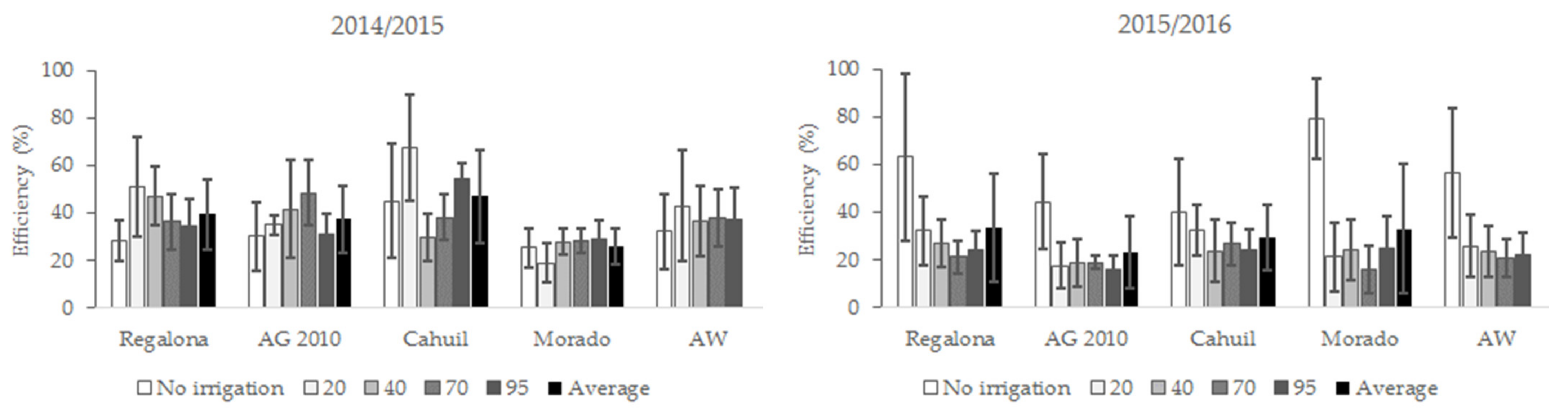
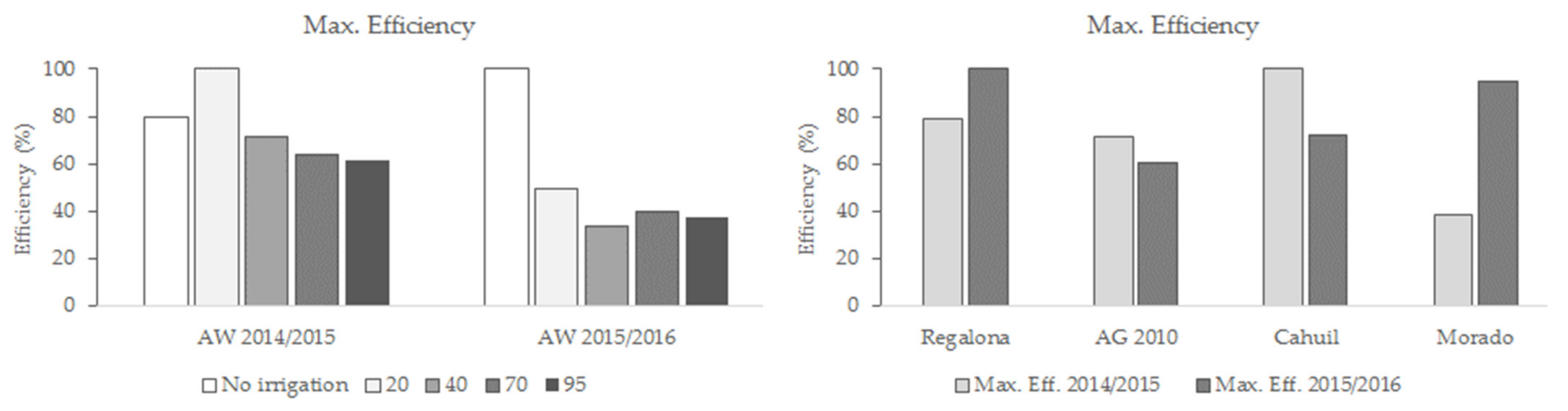
3.4. Seeds Yield Efficiency
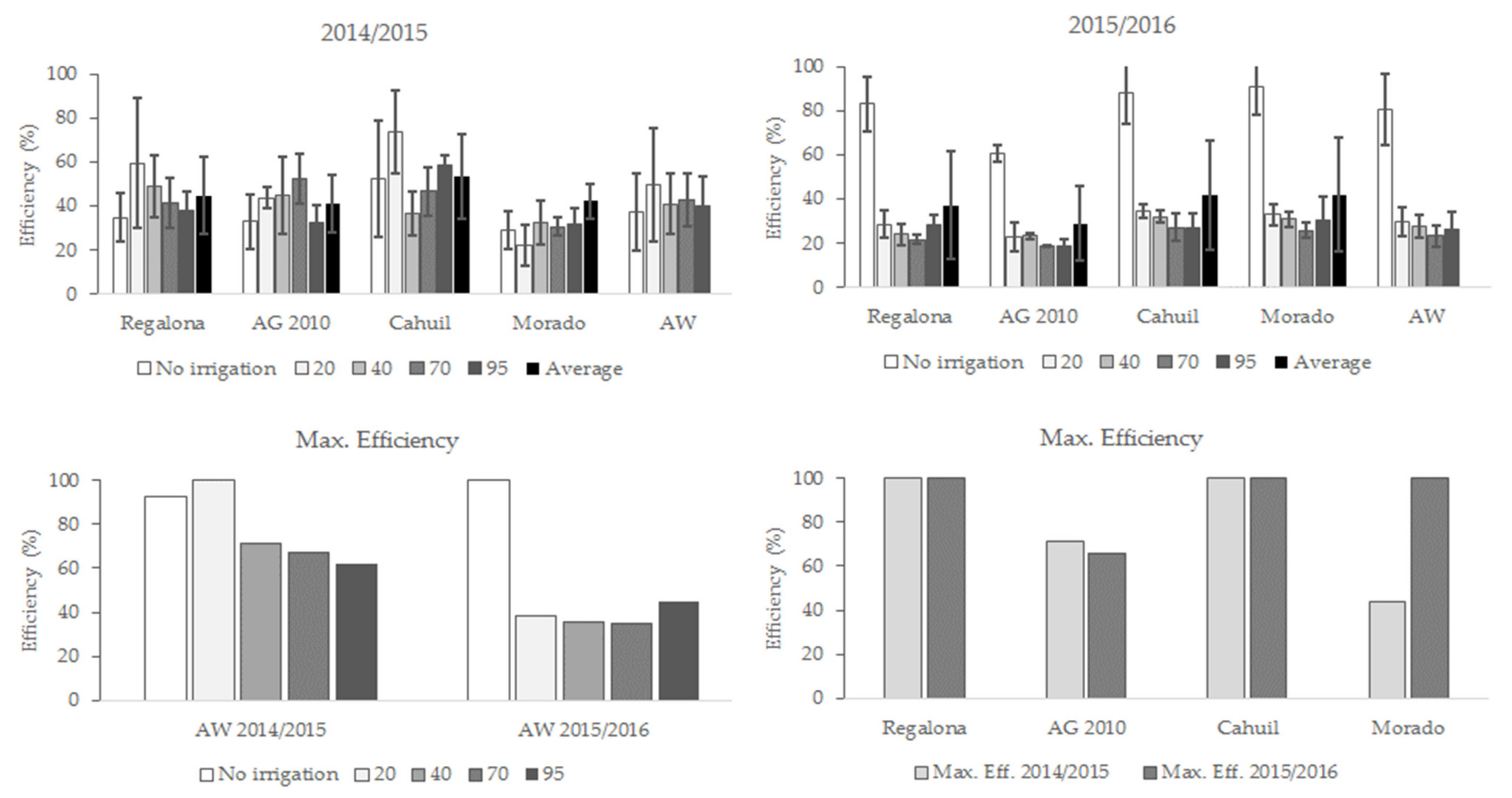
3.5. Technical Efficiency in Globulin and Albumin Yield
4. Discussion
4.1. Seed Yield and Seeds Yield Efficiency
4.2. Total Protein Content, Globulins, and Albumins Yield Efficiency
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schewe, J.; Heinke, J.; Gerten, D.; Haddeland, I.; Arnell, N.W.; Clark, D.B.; Dankers, R.; Eisner, S.; Fekete, B.M.; Colón-González, F.J. Multimodel assessment of water scarcity under climate change. Proc. Natl. Acad. Sci. USA 2014, 111, 3245–3250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoekstra, A.Y. Water scarcity challenges to business. Nat. Clim. Chang. 2014, 4, 318–320. [Google Scholar] [CrossRef]
- Gosling, S.N.; Arnell, N.W. A global assessment of the impact of climate change on water scarcity. Clim. Chang. 2016, 134, 371–385. [Google Scholar] [CrossRef] [Green Version]
- Fellmann, T.; Witzke, P.; Weiss, F.; Doorslaer, B.; Drabik, D.; Huck, I.; Salputra, G.; Jansson, T.; Leip, A. Major challenges of integrating agriculture into climate change mitigation policy frameworks. Mitig. Adapt. Strat. Glob. Chang. 2018, 23, 451–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil, J.D.B.; Reidsma, P.; Giller, K.; Todman, L.; Whitmore, A.; Van Ittersum, M. Sustainable development goal 2: Improved targets and indicators for agriculture and food security. Ambio 2019, 48, 685–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, X.C.; Shu, R.; Guo, X.P.; Wang, W.G. Scarce water resources and priority irrigation schemes from agronomic crops. Mitig. Adapt. Strat. Glob. Chang. 2017, 24, 399–417. [Google Scholar] [CrossRef]
- Sala, S.; McLaren, S.J.; Notarnicola, B.; Saouter, E.; Sonesson, U. In quest of reducing the environmental impacts of food production and consumption. J. Clean. Prod. 2017, 140, 387–398. [Google Scholar] [CrossRef]
- Pravst, I. Functional Foods in Europe: A Focus on Health Claims. Scientific, Health and Social Aspects of the Food Industry. Available online: https://www.intechopen.com/books/scientific-health-and-social-aspects-of-the-food-industry/functional-foods-in-europe-a-focus-on-health-claims (accessed on 1 June 2021).
- Daliu, P.; Santini, A.; Novellino, E. From pharmaceuticals to nutraceuticals: Bridging disease prevention and management. Expert Rev. Clin. Pharmacol. 2019, 12, 1–7. [Google Scholar] [CrossRef]
- Lionetti, V.; Tuana, B.S.; Casieri, V.; Parikh, M.; Pierce, G.N. Importance of functional food compounds in cardioprotection through action on the epigenome. Eur. Heart J. 2019, 40, 575–582. [Google Scholar] [CrossRef]
- Semba, R.D. The rise and fall of protein malnutrition in global health. Ann. Nutr. Metab. 2016, 69, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Aiking, H. Future protein supply. Trends Food Sci. Technol. 2011, 22, 112–120. [Google Scholar] [CrossRef]
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future protein supply and demand: Strategies and factors influencing a sustainable equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reedy, J.; Cudhea, F.; Shi, P.; Zhang, J.; Onopa, J.; Miller, V.; Mozaffarian, D. Global intakes of total protein and sub-types; findings from the 2015 global dietary database (P10-050-19). Curr. Dev. Nutr. 2018, 3 (Suppl. 1). [Google Scholar] [CrossRef] [Green Version]
- Gorinstein, S.; Pawelzik, E.; Delgado-Licon, E.; Haruenkit Weisz, M.; Trakhtenberg, S. Characterization of pseudocereal and cereal proteins by protein and amino acid analyses. J. Sci. Food Agric. 2002, 82, 886–891. [Google Scholar] [CrossRef]
- Zurita-Silva, A.; Fuentes, F.; Zamora, P.; Jacobsen, S.E.; Schwember, A.R. Breeding quinoa (Chenopodium quinoa Willd.): Potential and perspectives. Mol. Breed. 2014, 34, 13–30. [Google Scholar] [CrossRef]
- Fischer, S.; Wilckens, R.; Jara, J.; Aranda, M.; Valdivia, W.; Bustamante, L.; Graf, F.; Obal, I. Protein and antioxidant composition of quinoa (Chenopodium quinoa Willd.) sprout from seeds submitted to water stress, salinity and light conditions. Ind. Crop Prod. 2017, 107, 558–564. [Google Scholar] [CrossRef]
- Janssen, F.; Pauly, A.; Rombouts, I.; Jansens, K.J.; Deleu, L.J.; Delcour, J.A. Proteins of amaranth (Amaranthus spp.), buckwheat (Fagopyrum spp.), and quinoa (Chenopodium spp.): A food science and technology perspective. Compr. Rev. Food Sci. 2017, 16, 39–58. [Google Scholar] [CrossRef]
- Dakhili, S.; Abdolalizadeh, L.; Hosseini, S.M.; Shojaee-Aliabadi, S.; Mirmoghtadaie, L. Quinoa protein: Composition, structure and functional properties. Food Chem. 2019, 299, 125–161. [Google Scholar] [CrossRef]
- Abugoch, L.E.; Romero, N.; Tapia, C.A.; Silva, J.; Rivera, M. Study of some physicochemical and functional properties of quinoa (Chenopodium quinoa Willd) protein isolates. J. Agric. Food Chem. 2008, 56, 4745–4750. [Google Scholar] [CrossRef]
- Guo, H.; Hao, Y.; Yang, X.; Ren, G.; Richel, A. Exploration on bioactive properties of quinoa protein hydrolysate and peptides: A review. Crit. Rev. Food. 2021, 1–14. [Google Scholar] [CrossRef]
- López, D.N.; Galante, M.; Robson, M.; Boeris, V.; Spelzini, D. Amaranth, quinoa and chia protein isolates: Physicochemical and structural properties. Int. J. Biol. Macromol. 2018, 109, 152–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escudero, O.; Gonzalez, M.I.; Wells, G.; Fischer, S.; Hernández, J.M. Amino acid profile of the quinoa (Chenopodium quinoa Willd.) using near infrared spectroscopy and chemometric techniques. J. Cereal Sci. 2014, 60, 67–74. [Google Scholar] [CrossRef]
- Bertero, H.D.; de la Vega, A.J.; Correa, G.; Jacobsen, S.E.; Mujica, A. Genotype and genotype-by-environment interaction effects for grain yield and grain size of quinoa (Chenopodium quinoa Willd.) as revealed by pattern analysis of multi-environment trials. Field Crop Res. 2014, 89, 299–318. [Google Scholar] [CrossRef]
- Geerts, S.; Raes, D.; Garcia, M.; Mendoza, J.; Huanca, R. Crop water use indicators to quantify the flexible phenology of quinoa (Chenopodium quinoa Willd.) in response to drought stress. Field Crop Res. 2008, 108, 150–156. [Google Scholar] [CrossRef]
- Delatorre-Herrera, J.; Rojas-Urrutia, J.; Rojo, L.E.; Graf, B.L. Osmotic stress in Chenopodium quinoa Willd.: Variations in osmoprotectants at different phenological stages. Afr. J. Agric. Res. 2019, 14, 361–368. [Google Scholar] [CrossRef]
- Sellami, M.H.; Pulvento, C.; Lavini, A. Agronomic practices and performances of quinoa under field conditions: A systematic review. Plants 2021, 10, 72. [Google Scholar] [CrossRef]
- Khaitov, B.; Karimov, A.A.; Toderich, K.; Sultanova, Z.; Mamadrahimov, A.; Allanov, K.; Islamov, S. Adaptation, grain yield and nutritional characteristics of quinoa (Chenopodium quinoa) genotypes in marginal environments of the Aral Sea basin. J. Plant Nutr. 2021, 44, 1365–1379. [Google Scholar] [CrossRef]
- Da Silva, P.C.; Ribeiro, W.Q., Jr.; Ramos, M.L.G.; Celestino, S.M.C.; Silva, A.D.N.; Casari, R.; Vinson, C.C. Quinoa for the Brazilian Cerrado: Agronomic characteristics of elite genotypes under different water regimes. Plants 2021, 10, 1591. [Google Scholar] [CrossRef]
- Geerts, S.; Raes, D.; Garcia, M.; Vacher, J.; Mamani, R.; Mendoza, J.; Huanca, R.; Morales, B.; Miranda, R.; Cusicanqui, J.; et al. Introducing deficit irrigation to stabilize yields of quinoa (Chenopodium quinoa Willd.). Eur. J. Agron. 2008, 28, 427–436. [Google Scholar] [CrossRef]
- Dalla, M.A.; Eitzinger, J.; Kersebaum, K.C.; Todorovic, M.; Altobelli, F. Assessment and monitoring of crop water use and productivity in response to climate change. J. Agric. Sci. 2018, 156, 575–576. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Wang, F.X.; Shock, C.C.; Meng, C.B.; Huang, Z.J.; Gao, L.; Zhao, J.Y. Evaluating quinoa stem lodging susceptibility by a mathematical model and the finite element method under different agronomic practices. Field Crop Res. 2021, 271, 108241. [Google Scholar] [CrossRef]
- Geerts, S.; Raes, D. Deficit irrigation as an on-farm strategy to maximize crop water productivity in dry areas. Agric. Water Manag. 2009, 96, 1275–1284. [Google Scholar] [CrossRef] [Green Version]
- Zahoor, S.A.; Ahmad, S.; Ahmad, A.; Wajid, A.; Khaliq, T.; Mubeen, M.; Nasim, W. Improving water use efficiency in agronomic crop production. In Agronomic Crops; Hasanuzzaman, M., Ed.; Springer: Singapore, 2019; pp. 13–29. [Google Scholar] [CrossRef]
- Field, C.B.; Barros, V.; Stocker, T.F.; Dahe, Q. (Eds.) Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation: Special Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, MA, USA, 2012; Available online: https://www.ipcc.ch/report/managing-the-risks-of-extr (accessed on 10 June 2021). [CrossRef]
- Jaramillo Roman, V.; van de Zedde, R.; Peller, J.; Visser, R.G.; van der Linden, C.G.; van Loo, E.N. High-resolution analysis of growth and transpiration of quinoa under saline conditions. Front. Plant Sci. 2021, 1627. [Google Scholar] [CrossRef]
- Battese, G.E.; Coelli, T.J. Frontier production functions, technical efficiency and panel data: With application to paddy farmers in India. J. Prod. Anal. 1992, 3, 153–169. [Google Scholar] [CrossRef]
- Chavas, J.P.; Aliber, M. An analysis of economic efficiency in agriculture: A nonparametric approach. J. Agric. Res. Econ. 1993, 18, 1–16. [Google Scholar] [CrossRef]
- Latruffe, L.; Balcombe, K.; Davidova, S.; Zawalinska, K. Technical and scale efficiency of crop and livestock farms in Poland: Does specialization matter? Agric. Econ. 2005, 32, 281–296. [Google Scholar] [CrossRef]
- Hansson, H. Strategy factors as drivers and restraints on dairy farm performance: Evidence from Sweden. Agric. Syst. 2007, 94, 726–737. [Google Scholar] [CrossRef]
- Asmild, M.; Baležentis, T.; Hougaard, J.L. Multi-directional program efficiency: The case of Lithuanian family farms. J. Prod. Anal. 2016, 45, 23–33. [Google Scholar] [CrossRef]
- Laso, J.; Hoehn, D.; Margallo, M.; García-Herrero, I.; Batlle-Bayer, L.; Bala, A.; Aldaco, R. Assessing energy and environmental efficiency of the Spanish agri-food system using the LCA/DEA methodology. Energies 2018, 11, 3395. [Google Scholar] [CrossRef] [Green Version]
- Gamboa, C.; Bojacá, C.R.; Schrevens, E.; Maertens, M. Sustainability of small holder quinoa production in the Peruvian Andes. J. Clean. Prod. 2020, 264, 121657. [Google Scholar] [CrossRef]
- Llyas, H.M.A.; Safa, M.; Bailey, A.; Rauf, S.; Khan, A. Energy efficiency outlook of New Zealand dairy farming systems: An application of data envelopment analysis (DEA) approach. Energies 2020, 13, 251. [Google Scholar] [CrossRef] [Green Version]
- Amiryousefi, M.; Tadayon, M.R.; Ebrahimi, R. Energy and exergy efficiencies assessment for two quinoa cultivars production. Energy Rep. 2021, 7, 2324–2331. [Google Scholar] [CrossRef]
- Charnes, A.; Cooper, W.W.; Rhodes, E. Measuring the efficiency of decision making units. Eur. J. Oper. Res. 1978, 2, 429–444. [Google Scholar] [CrossRef]
- Coelli, T.J.; Rao, D.S.P.; O’Donnell, C.J.; Battese, G.E. An Introduction to Efficiency and Productivity Analysis, 2nd ed.; Springer: New York, NY, USA, 2005. [Google Scholar] [CrossRef]
- Seiford, L.M.; Thrall, R.M. Recent developments in DEA: The mathematical programming approach to frontier analysis. J. Econ. 1990, 46, 7–38. [Google Scholar] [CrossRef]
- Liu, J.S.; Lu, L.Y.; Lu, W.M.; Lin, B.J. A survey of DEA applications. Omega 2013, 41, 893–902. [Google Scholar] [CrossRef]
- Resti, A. Efficiency measurement for multi-product industries: A comparison of classic and recent techniques based on simulated data. Eur. J. Oper. Res. 2000, 121, 559–578. [Google Scholar] [CrossRef]
- Zhang, T. Decomposing allocative efficiency for multi-product production systems. EM Ekon. Manag. 2010, 13, 71–77. [Google Scholar]
- Hosseinzadeh-Bandbafha, H.; Safarzadeh, D.; Ahmadi, E.; Nabavi-Pelesaraei, A. Optimization of energy consumption of dairy farms using data envelopment analysis—A case study: Qazvin city of Iran. J. Saudi Soc. Agric. Sci. 2018, 17, 217–228. [Google Scholar] [CrossRef] [Green Version]
- Stolpe, N. Descripciones de los principales suelos de la VIII Región de Chile. In Publicaciones Departamento de Suelos y Recursos Naturales; Universidad de Concepción: Chillán, Chile, 2006. [Google Scholar]
- Del Pozo, A.; del Canto, P. Áreas agroclimáticas y sistemas productivos en la VII y VIII Regiones. In Serie Quilamapu N◦ 113; INIA Quilamapu: Chillán, Chile, 1999. [Google Scholar]
- Klute, A. Water Retention: Laboratory Methods. Methods of Soil Analysis Part. 5-Mineralogical Methods; American Society of Agronomy, Inc.: Madison, WI, USA; Soil Science Society of America, Inc.: Madison, WI, USA, 1986; pp. 635–662. [Google Scholar] [CrossRef]
- Fischer, S.; Wilckens, R.; Jara, J.; Aranda, M. Variation in antioxidant capacity of quinoa (Chenopodium quinoa Willd) subjected to drought stress. Ind. Crop Prod. 2013, 46, 341–349. [Google Scholar] [CrossRef]
- Valdivia-Cea, W.; Bustamante, L.; Jara, J.; Fischer, S.; Holzapfel, E.; Wilckens, R. Effect of soil water availability on physiological parameters, yield, and seed quality in four quinoa genotypes (Chenopodium quinoa Willd.). Agronomy 2021, 11, 1012. [Google Scholar] [CrossRef]
- Berti, M.T.; Serri, H.; Wilckens, R.; Urbina, A.; Figueroa, I. Determination of physiological maturity and optimal harvest date in quinoa (Chenopodium quinoa Willd.). Agro Cienc. 1997, 13, 135–141. [Google Scholar]
- AOAC International. Official Methods of Analysis, 20th ed.; AOAC International: Rockville, MD, USA, 2016; Available online: www.aoac.org (accessed on 1 June 2021).
- Fujihara, S.; Sasaki, H.; Aoyagi, Y.; Sugahara, T. Nitrogen-to-protein conversion factors for some cereal products in Japan. J. Food Sci. 2008, 73, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Stegemann, H.; Pietsch, G. Methods for characterization of the seed proteins in cereals and legumes. In Seed Proteins; Gottschalk, W., Müller, H.P., Eds.; Springer: Dordrecht, The Netherlands, 1983; pp. 45–75. [Google Scholar] [CrossRef]
- Wilckens, R.; Hevia, F.; Tapia, M.; Albarrán, R. Caracterización de dos genotipos de quinoa (Chenopodium quinoa Willd) chilena. I. Electroforesis de proteínas de semilla. Agro Cienc. 1996, 12, 51–56. [Google Scholar]
- Tone, K. A slacks-based measure of efficiency in data envelopment analysis. Eur. J. Oper. Res. 2011, 130, 498–509. [Google Scholar] [CrossRef] [Green Version]
- SAS Institute. SAS User´s Guide: Statististics; SAS Institute: Cary, NC, USA, 2014. [Google Scholar]
- Curti, R.N.; de la Vega, A.J.; Andrade, A.J.; Bramardi, S.J.; Bertero, H.D. Multi-environmental evaluation for grain yield and its physiological determinants of quinoa genotypes across Northwest Argentina. Field Crop Res. 2014, 166, 46–57. [Google Scholar] [CrossRef]
- Rezaei, E.E.; Webber, H.; Gaiser, T.; Naab, J.; Ewert, F. Heat stress in cereals: Mechanisms and modelling. Eur. J. Agron. 2015, 64, 98–113. [Google Scholar] [CrossRef]
- Deng, N.; Ling, X.; Sun, Y.; Zhang, C.; Fahad, S.; Peng, S.; Huang, J. Influence of temperature and solar radiation on grain yield and quality in irrigated rice system. Eur. J. Agron. 2015, 64, 37–46. [Google Scholar] [CrossRef]
- Farooq, M.; Bramley, H.; Palta, J.A.; Siddique, K.H. Heat stress in wheat during reproductive and grain-filling phases. Crit. Rev. Plant Sci. 2011, 30, 491–507. [Google Scholar] [CrossRef]
- Matías, J.; Cruz, V.; Reguera, M. Heat stress impact on yield and composition of quinoa straw under Mediterranean field conditions. Plants 2021, 10, 955. [Google Scholar] [CrossRef]
- Manaa, A.; Goussi, R.; Derbali, W.; Cantamessa, S.; Essemine, J.; Barbato, R. Photosynthetic performance of quinoa (Chenopodium quinoa Willd.) after exposure to a gradual drought stress followed by a recovery period. Bioch. Biophys. Acta Bioenerg. 2021, 1862, 148383. [Google Scholar] [CrossRef]
- Hirich, A.; Allah, R.C.; Jacobsen, S.E.; El Youssfi, L.; El Homaria, H. Using deficit irrigation with treated wastewater in the production of quinoa (Chenopodium quinoa Willd.) in Morocco. Rev. Científica UDO Agrícola 2012, 12, 570–583. [Google Scholar]
- García, M.; Raes, D.; Jacobsen, S.E. Evapotranspiration analysis and irrigation requirements of quinoa (Chenopodium quinoa) in the Bolivian highlands. Agric. Water Manag. 2003, 60, 119–134. [Google Scholar] [CrossRef]
- Killi, D.; Haworth, M. Diffusive and metabolic constraints to photosynthesis in quinoa during drought and salt stress. Plants 2017, 6, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winkel, T.; Méthy, M.; Thenot, F. Radiation use efficiency, chlorophyll fluorescence, and reflectance indices associated with ontogenic changes in water-limited Chenopodium quinoa leaves. Photosynthesis 2002, 40, 227–232. [Google Scholar] [CrossRef]
- Hinojosa, L.; González, J.; Barrios-Masias, F.; Fuentes, F.; Murphy, K. Quinoa abiotic stress responses: A review. Plants 2018, 7, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naz, H.; Akram, N.A.; Kong, H. Assessment of secondary metabolism involvement in water stress tolerance of quinoa (Chenopodium quinoa Willd.) Subjected to varying water regimes. Pak. J. Bot. 2020, 52, 1553–1559. [Google Scholar] [CrossRef]
- Miranda, M.; Vega-Gálvez, A.; Quispe-Fuentes, I.; Rodríguez, M.J.; Maureira, H.; Martínez, E.A. Nutritional aspects of six quinoa (Chenopodium quinoa Willd.) ecotypes from three geographical areas of Chile. Chil. J. Agric. Res. 2012, 72, 175. [Google Scholar] [CrossRef] [Green Version]
- Föste, M.; Elgeti, D.; Brunner, A.K.; Jekle, M.; Becker, T. Isolation of quinoa protein by milling fractionation and solvent extraction. Food Bioprod. Process. 2015, 96, 20–26. [Google Scholar] [CrossRef]
- Filho, A.M.M.; Pirozi, M.R.; Borges, J.T.D.S.; Pinheiro Sant’Ana, H.M.; Chaves, J.B.P.; Coimbra, J.S.D.R. Quinoa: Nutritional, functional, and antinutritional aspects. Crit. Rev. Food Sci. Nutr. 2017, 57, 1618–1630. [Google Scholar] [CrossRef]
- Bazile, D.; Jacobsen, S.-E.; Verniau, A. The global expansion of quinoa: Trends and limits. Front. Plant Sci. 2014, 7, 622. [Google Scholar] [CrossRef] [Green Version]
- Bascuñán-Godoy, L.; Reguera, M.; Abdel-Tawab, Y.M.; Blumwald, E. Water deficit stress-induced changes in carbon and nitrogen partitioning in Chenopodium quinoa Willd. Planta 2016, 243, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, H.; Zhu, H.; Tao, Y.; Zhang, G.; Zhang, L.; Ma, Z. Classification and expression diversification of wheat dehydrin genes. Plant Sci. 2014, 214, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Gambín, B.L.; Borrás, L. Resource distribution and the trade-off between seed number and seed weight: A comparison across crop species. Ann. Appl. Biol. 2010, 156, 91–102. [Google Scholar] [CrossRef]
- Li, P.; Chen, J.; Wu, P. Agronomic characteristics and grain yield of 30 spring wheat genotypes under drought stress and non stress conditions. Agron. J. 2011, 103, 1619–1628. [Google Scholar] [CrossRef] [Green Version]
- Lindeboom, N. Studies on the Characterization, Biosynthesis and Isolation of Starch and Protein from Quinoa (Chenopodium quinoa Willd.). Ph.D. Thesis, College of Graduate Studies and Research, University of Saskatchewan, Saskatoon, SK, Canada, 2005. [Google Scholar]
- Vilcacundo, R.; Martínez-Villaluenga, C.; Hernández-Ledesma, B. Release of dipeptidyl peptidase IV, α-amylase and α-glucosidase inhibitory peptides from quinoa (Chenopodium quinoa Willd.) during in vitro simulated gastrointestinal digestion. J. Funct. Foods 2017, 35, 531–539. [Google Scholar] [CrossRef] [Green Version]
- Burrieza, H.P.; Rizzo, A.J.; Vale, E.M.; Silveira, V.; Maldonado, S. Shotgun proteomic analysis of quinoa seeds reveals novel lysine-rich seed storage globulins. Food Chem. 2019, 293, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Sathe, S.; Hamaker, B.; Szetao, K.; Venkatachalam, M. Isolation, purification, and biochemical characterization of a novel water soluble protein from Inca peanut (Plukenetia volubilis L.). J. Agric. Food Chem. 2002, 50, 4906–4908. [Google Scholar] [CrossRef]
- Aranda-Olmedo, I.; Ruiz, R.; Peinado, M.; Rubio, L. A pea (Pisum sativum L.) seed albumin extract prevents colonic DSS induced dysbiosis in mice. J. Funct. Foods. 2017, 35, 279–294. [Google Scholar] [CrossRef]
- Campos, D.C.; Costa, A.S.; Luz, P.B.; Soares, P.M.; Alencar, N.M.; Oliveira, H.D. Morinda citrifolia lipid transfer protein 1 exhibits anti-inflammatory activity by modulation of pro- and anti-inflammatory cytokines. Int. J. Biol. Macromol. 2017, 103, 1121–1129. [Google Scholar] [CrossRef]
- Nadaud, I.; Tasleem-Tahir, A.; Chateigner-Boutin, A.L.; Chambon, C.; Viala, D.; Branlard, G. Proteome evolution of wheat (Triticum aestivum L.) aleurone layer at fifteen stages of grain development. J. Proteom. 2015, 123, 29–41. [Google Scholar] [CrossRef]
- Triboï, E.; Martre, P.; Triboï-Blondel, A.M. Environmentally-induced changes in protein composition in developing grains of wheat are related to changes in total protein content. J. Exp. Bot. 2003, 54, 1731–1742. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, C.V.; Guerrero, G.R.; Piedras, J.J.G. Respuesta morfoagronómica y calidad en proteína de tres accesiones de quinua (Chenopodium quinoa Willd.) en la sabana norte de Bogotá. Rev. UDCA Actual. Divulg. Científica 2016, 19, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Monteil, V.; Kwon, H.; Prado, P.; Hagelkrüys, A.; Wimmer, R.A.; Stahl, M.; Penninger, J.M. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 2020, 181, 905–913. [Google Scholar] [CrossRef]
- Rangaswamy, A.N.; Ashok, A.; Hanumanthappa, P.; Chandrashekaramurthy, A.S.; Kumbaiah, M.; Hiregouda, P.; Sosalegowda, A.H. Identification of potential peptide inhibitors of ACE-2 target of SARS-CoV-2 from buckwheat and quinoa. Int. J. Pept. Res. Ther. 2021, 27, 799–813. [Google Scholar] [CrossRef] [PubMed]
- Brinegar, C.; Goundan, S. Isolation and characterization of chenopodin, the 11S seed storage protein of quinoa (Chenopodium quinoa). J. Agric. Food Chem. 1993, 41, 182–185. [Google Scholar] [CrossRef]
- Brinegar, C.; Sine, B.; Nwokocha, L. High-cysteine 2S seed storage proteins from quinoa (Chenopodium quinoa). J. Agric. Food Chem. 1996, 44, 1621–1623. [Google Scholar] [CrossRef]
- Liu, J.; Wang, R.; Liu, W.; Zhang, H.; Guo, Y.; Wen, R. Genome-wide characterization of heat-shock protein 70S from Chenopodium quinoa and expression analyses of Cqhsp70s in response to drought stress. Genes 2018, 9, 35. [Google Scholar] [CrossRef] [Green Version]
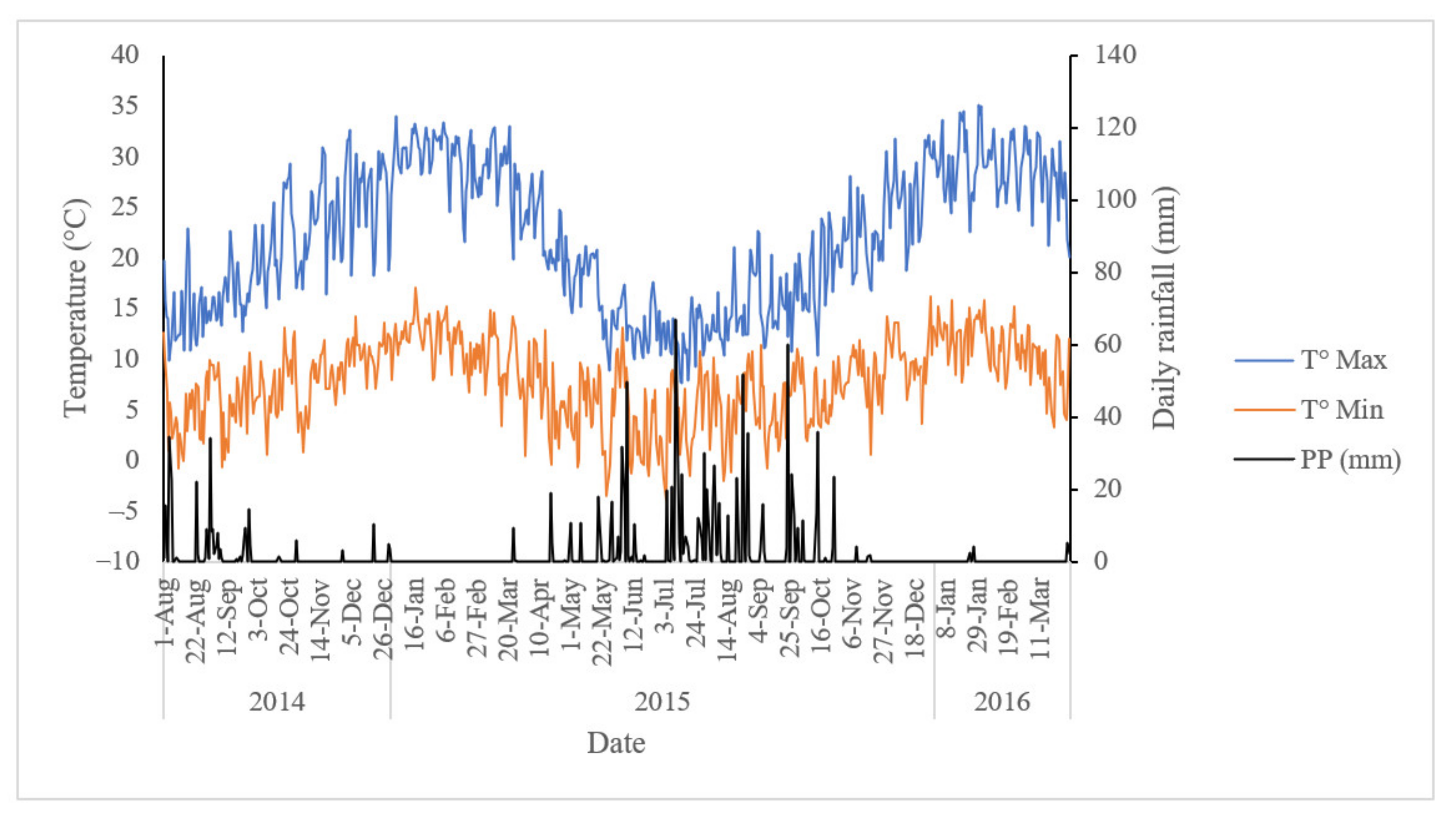
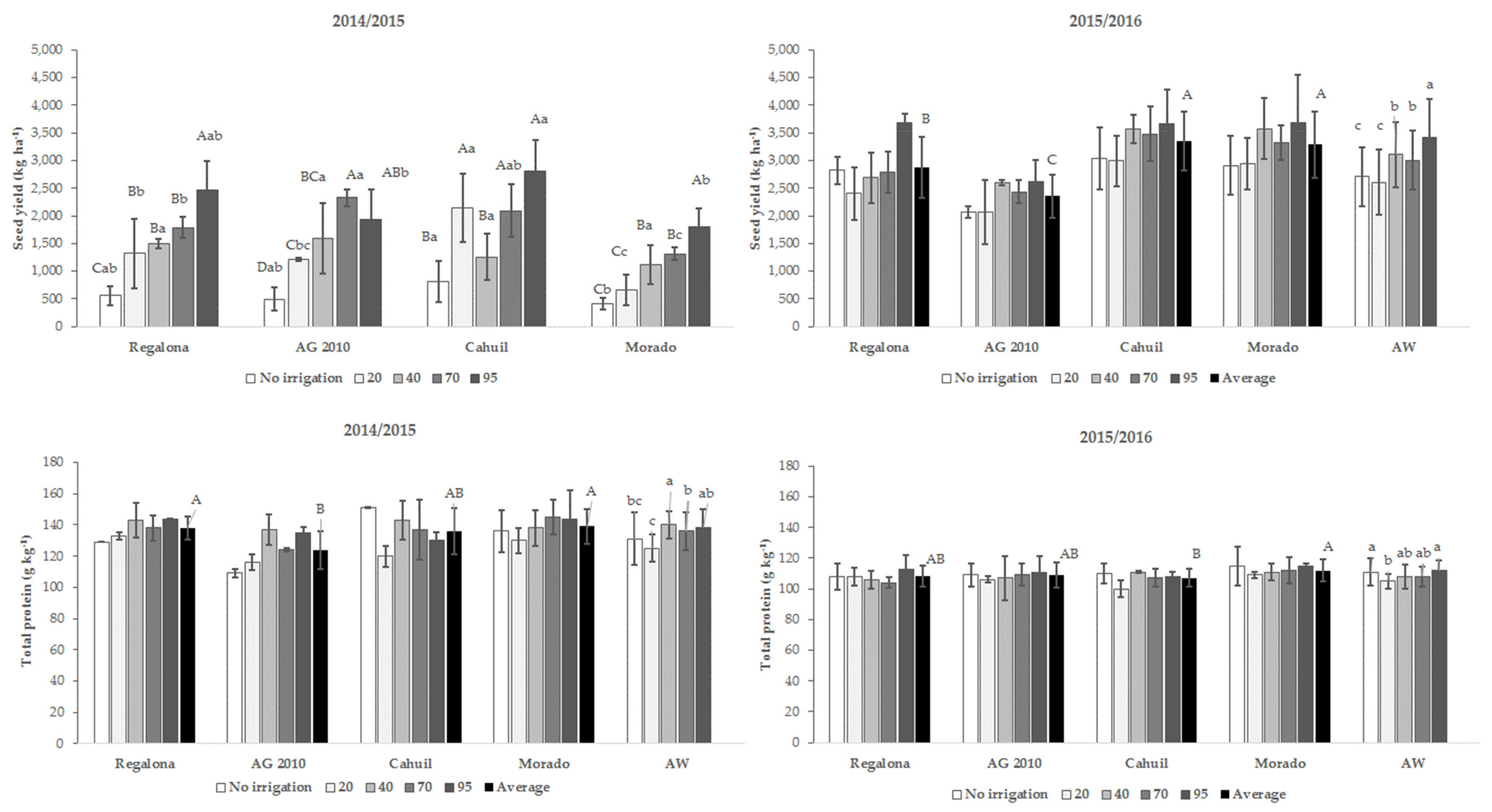

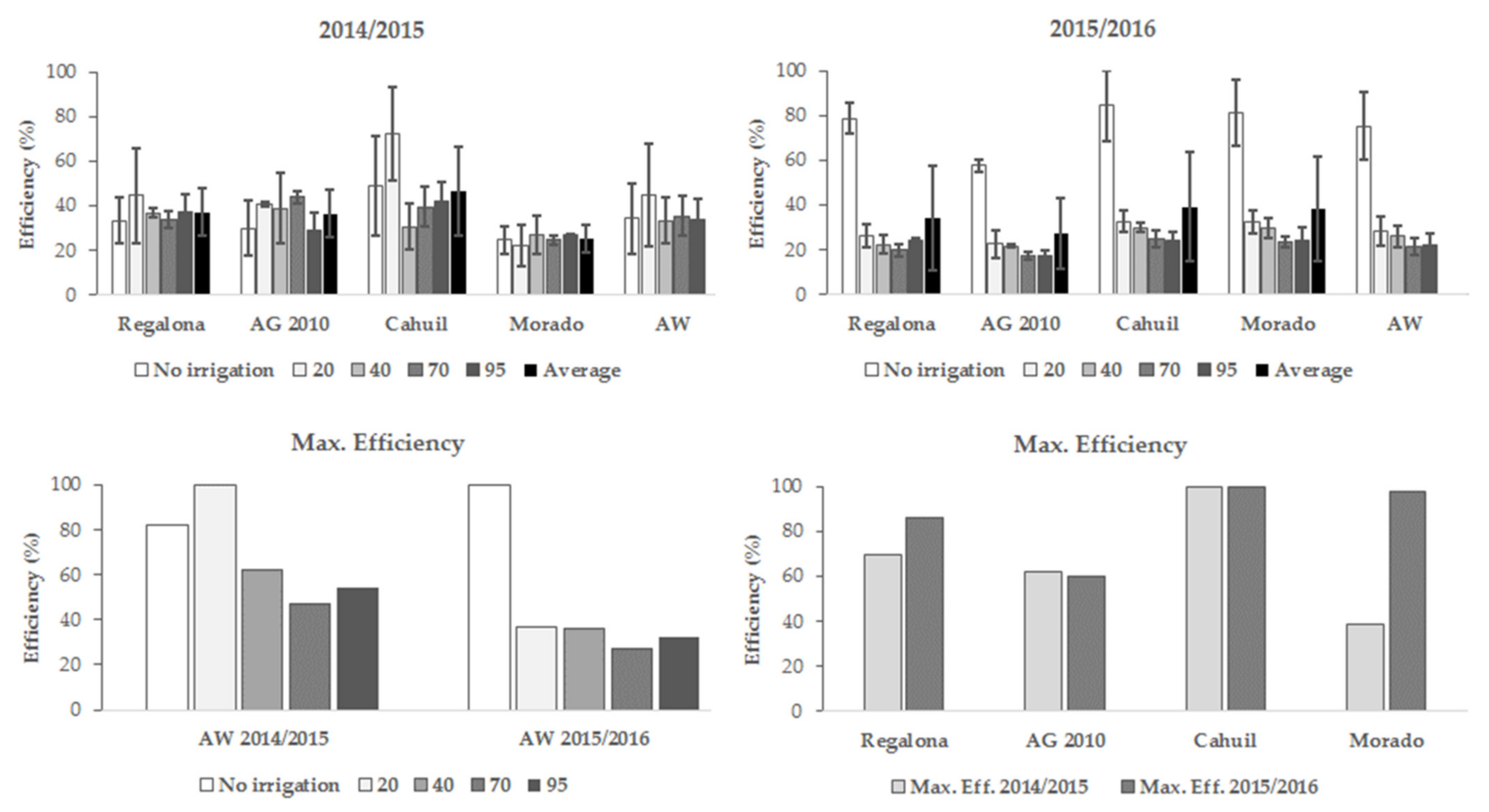
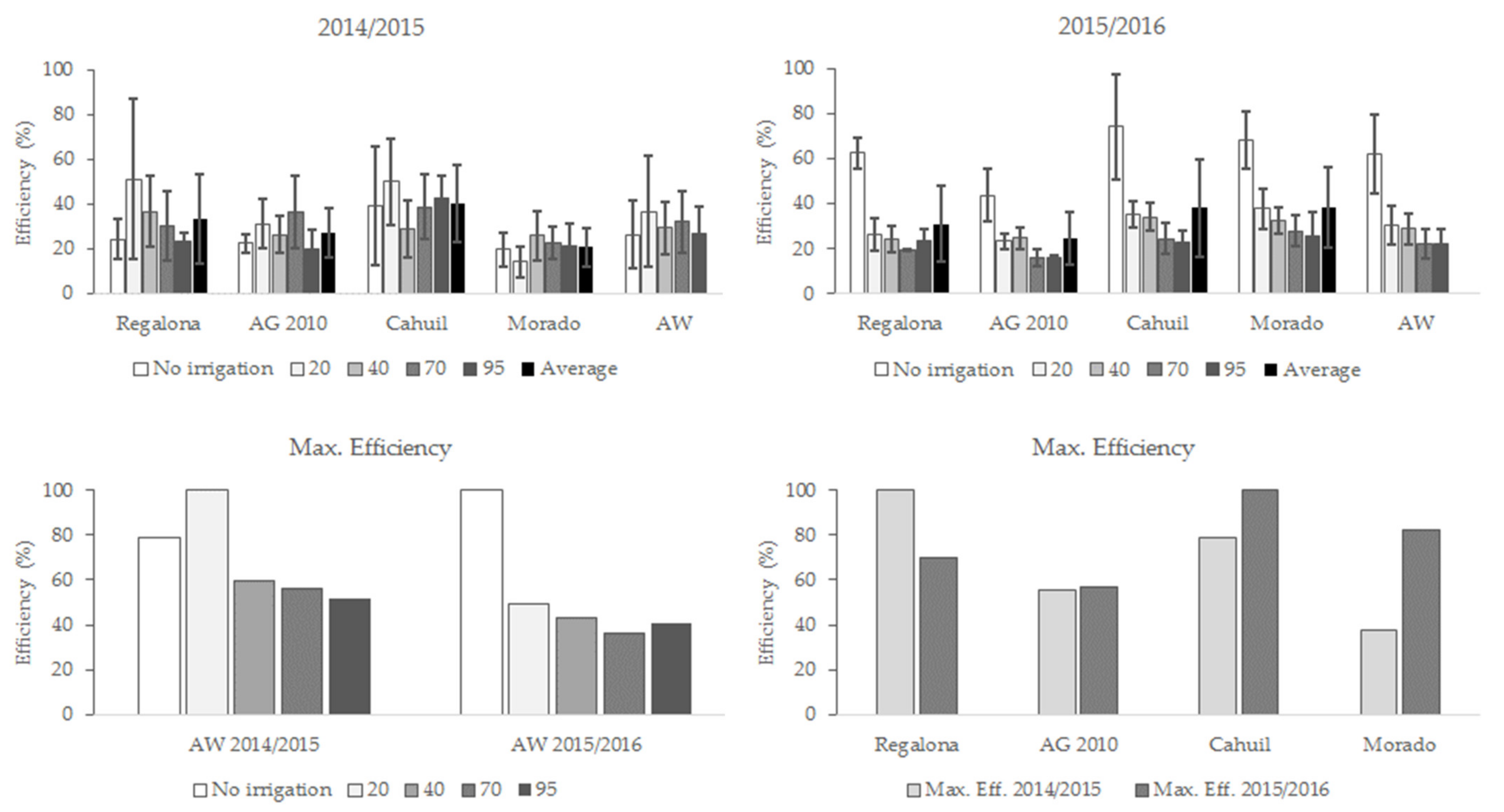
| Season | pH | OM g kg−1 | Pm g kg−1 | Km g kg−1 | NO3-N mg kg−1 |
|---|---|---|---|---|---|
| 2014–2015 | 6.6 | 6.2 | 39.5 | 398.6 | 21.8 |
| 2015–2016 | 6.6 | 6.2 | 36.2 | 378.4 | 19.5 |
| Source of Variation | df | Seed Yield | Total Protein | Globulin | Albumin |
|---|---|---|---|---|---|
| 2014/2015 | |||||
| Rep | 3 | 146,989 | 3.5 | 5.1 | 1.8 |
| AW | 4 | 6,506,713 | 309.8 | 71.1 * | 77.2 |
| AWx Rep | 12 | 214,418 | 89.5 | 4.9 | 1.6 |
| Genotype | 3 | 1,969,361 | 440.4 * | 30.2 * | 19.8 |
| Genotype × AW | 12 | 367,172 * | 141.9 | 7.0 | 6.9 * |
| Error | 45 | 130,449 | 91.9 | 4.4 | 2.4 |
| CV, % | 24 | 7.15 | 43.8 | 33.2 | |
| 2015/2016 | |||||
| Rep | 3 | 367,931 | 11.3 | 2.3 | 7.2 |
| AW | 4 | 1,675,102 * | 103.9 | 16.1 * | 9.8 * |
| AWx Rep | 12 | 308,874 | 75.5 | 3.8 | 2.4 |
| Genotype | 3 | 4,195,026 * | 83.8 | 22.9 * | 4.4 * |
| Genotype × AW | 12 | 144,262 | 30.4 | 1.1 | 1.6 |
| Error | 164,946 | 48.6 | 1.5 | 1.5 | |
| CV, % | 14 | 6.4 | 21.9 | 38.9 | |
| Available Water (AW) | Regalona | AG 2010 | Cahuil | Morado | Total Average |
|---|---|---|---|---|---|
| 2014/2015 | |||||
| Seed yield efficiency (%) | |||||
| No irrigation | 33.5 ± 10.5 | 29.9 ± 12.4 | 48.7 ± 22.4 | 24.6 ± 6.4 | 34.2 ± 15.7 |
| 20 | 44.6 ± 21.2 | 40.8 ± 1.1 | 72.2 ± 20.9 | 22.4 ± 9.3 | 45.0 ± 23.1 |
| Globulin production efficiency (%) | |||||
| No irrigation | 24.4 ± 8.9 | 22.5 ± 4.0 | 39.4 ± 26.4 | 19.7 ± 7.8 | 26.5 ± 15.3 |
| 20 | 51.2 ± 35.8 | 31.3 ± 11.0 | 50.0 ± 19.4 | 14.3 ± 6.9 | 36.7 ± 24.7 |
| Albumin production efficiency (%) | |||||
| No irrigation | 28.4 ± 8.8 | 30.1 ± 14.6 | 45.1 ± 24.0 | 25.3 ± 8.3 | 32.2 ± 15.8 |
| 20 | 51.1 ± 20.8 | 34.9 ± 4.1 | 67.5 ± 22.3 | 19.0 ± 8.1 | 43.1 ± 23.5 |
| Multiproducts efficiency (%) | |||||
| No irrigation | 34.7 ± 10.9 | 33.0 ± 12.5 | 52.5 ± 26.7 | 29.0 ± 8.5 | 37.3 ± 17.3 |
| 20 | 59.6 ± 29.8 | 43.7 ± 4.8 | 73.8 ± 19.1 | 22.4 ± 9.3 | 49.9 ± 25.7 |
| 2015/2016 | |||||
| Seed yield efficiency (%) | |||||
| No irrigation | 78.6 ± 6.8 | 57.6 ± 2.9 | 84.6 ± 15.8 | 81.0 ± 14.8 | 75.4 ± 14.9 |
| 20 | 26.2 ± 5.2 | 22.6 ± 6.4 | 32.6 ± 4.9 | 32.2 ± 5.1 | 28.4 ± 6.5 |
| Globulin production efficiency (%) | |||||
| No irrigation | 62.4 ± 7.1 | 43.6 ± 11.6 | 74.1 ± 23.2 | 67.9 ± 12.7 | 62.0 ± 17.8 |
| 20 | 26.2 ± 7.1 | 23.4 ± 3.3 | 35.2 ± 5.7 | 37.7 ± 8.9 | 30.6 ± 8.5 |
| Albumin production efficiency (%) | |||||
| No irrigation | 63.2 ± 34.9 | 44.5 ± 20.1 | 40.1 ± 22.2 | 79.4 ± 16.9 | 56.8 ± 27.2 |
| 20 | 32.2 ± 14.6 | 17.7 ± 9.6 | 32.4 ± 10.5 | 21.3 ± 14.6 | 25.9 ± 13.1 |
| Multiproducts efficiency (%) | |||||
| No irrigation | 83.0 ± 12.4 | 60.6 ± 4.0 | 88.2 ± 13.9 | 90.7 ± 12.3 | 80.6 ± 15.9 |
| 20 | 28.4 ± 6.2 | 22.8 ± 6.3 | 34.6 ± 3.1 | 32.9 ± 4.9 | 29.7 ± 6.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, A.A.; Fischer, S.; Wilckens, R.; Bustamante, L.; Berti, M.T. Production Efficiency and Total Protein Yield in Quinoa Grown under Water Stress. Agriculture 2021, 11, 1089. https://doi.org/10.3390/agriculture11111089
Pinto AA, Fischer S, Wilckens R, Bustamante L, Berti MT. Production Efficiency and Total Protein Yield in Quinoa Grown under Water Stress. Agriculture. 2021; 11(11):1089. https://doi.org/10.3390/agriculture11111089
Chicago/Turabian StylePinto, Antonio A., Susana Fischer, Rosemarie Wilckens, Luis Bustamante, and Marisol T. Berti. 2021. "Production Efficiency and Total Protein Yield in Quinoa Grown under Water Stress" Agriculture 11, no. 11: 1089. https://doi.org/10.3390/agriculture11111089
APA StylePinto, A. A., Fischer, S., Wilckens, R., Bustamante, L., & Berti, M. T. (2021). Production Efficiency and Total Protein Yield in Quinoa Grown under Water Stress. Agriculture, 11(11), 1089. https://doi.org/10.3390/agriculture11111089







