A Comparison of the Ability of Some Commercially Produced Biological Control Agents to Protect Strawberry Plants against the Plant Pathogen Phytophthora cactorum
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Growth Test of BCAs
2.2. Discriminant Analysis—Statistical Analysis of Multicriterial Data
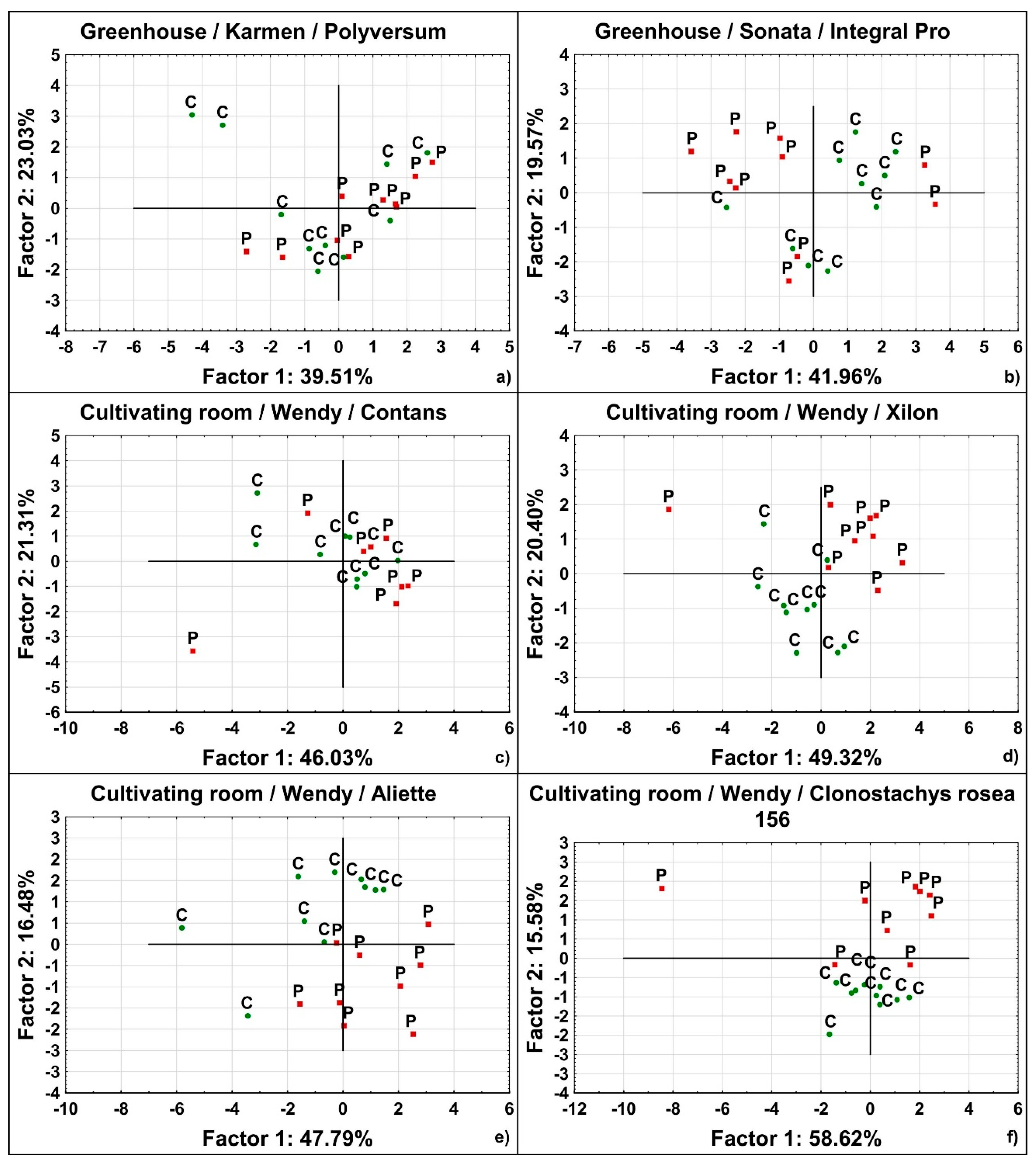
2.3. Principal Component Analysis—Visualization of Differences between Variants
2.4. A Comparison of the Most Important Variables for Differentiating between the Influence of Each BCA and of the Pathogen on Plant Growth
3. Results
3.1. Discriminant Analysis
3.2. Principal Component Analysis
3.3. A Comparison of the Weight of Roots, the Weight of Leaves, and Their Ratio
3.3.1. Influence of the Tested BCAs Alone on the Growth of Plants
3.3.2. Joint Influence of BCAs and the Pathogen on the Plants
3.3.3. Influence of BCAs on Plants with the Pathogen
3.3.4. Influence of the Pathogen on Plants with BCAs
3.3.5. Comparison of the Influence of Each BCA with That of the Pathogen
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Delmas, C.E.L.; Mazet, I.D.; Jolivet, J.; Delière, L.; Delmotte, F. Simultaneous quantification of sporangia and zoospores in a biotrophic oomycete with an automatic particle analyzer: Disentangling dispersal and infection potentials. J. Microbiol. Methods 2014, 107, 169–175. [Google Scholar] [CrossRef]
- Raftoyannis, Y.; Dick, M.W. Zoospore encystment and pathogenicity of Phytophthora and Pythium species on plant roots. Microbiol. Res. 2006, 161, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Darmono, T.W.; Parke, J.L.L. Chlamydospores of Phytophthora cactorum: Their production, structure, and infectivity. Can. J. Bot. 1990, 68, 640–645. [Google Scholar] [CrossRef]
- Harris, D.C. Survival of Phytophthora syringae oospores in and on apple orchard soil. Trans. Br. Mycol. Soc. 1985, 85, 153–155. [Google Scholar] [CrossRef]
- Sneh, B.; McIntosh, D.L. Studies on the behavior and survival of Phytophthora cactorum in soil. Can. J. Bot. 1974, 52, 795–802. [Google Scholar] [CrossRef]
- Stein, J.M.; Kirk, W.W. The generation and quantification of resistance to dimethomorph in Phytophthora infestans. Plant Dis. 2004, 88, 930–934. [Google Scholar] [CrossRef]
- Thomidis, T.; Tsipouridis, K. Effectiveness of metalaxyl, fosetyl-Al, dimethomorph, and cymoxanil against Phytophthora cactorum and P. citrophthora of peach tree. Phytopathol. Mediterr. 2001, 40, 253–259. [Google Scholar]
- Hill, S.N.; Hausbeck, M.K. Virulence and fungicide sensitivity of Phytophthora cactorum isolated from American ginseng gardens in Wisconsin and Michigan. Plant Dis. 2008, 92, 1183–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeffers, S.N.; Schnabel, G.; Smith, J.P. First report of resistance to mefenoxam in Phytophthora cactorum in The United States and elsewhere. Plant Dis. 2004, 88, 576. [Google Scholar] [CrossRef] [PubMed]
- Utkhede, R.S.; Smith, E.M. Long-term effects of chemical and biological treatments on crown and root rot of apple trees caused by Phytophthora cactorum. Soil Biol. Biochem. 1993, 25, 383–386. [Google Scholar] [CrossRef]
- Chabane, K.; Leroux, P.; Bompeix, G. Resistance to fungicides and streptomycin in Phytophthora parasitica : Genetic determinism and use in hybrid determination. Phytopath. Medit. 1996, 35, 82–90. [Google Scholar]
- Chang, T.T.; Ko, W.H. Resistance to fungicides and antibiotics in Phytophthora parasitica: Genetic nature and use in hybrid determination. Phytopathology 1990, 80, 1414–1421. [Google Scholar] [CrossRef]
- Davidse, L.C. Phenylamide fungicides–Biochemical action and resistance. In Modern Selective Fungicides, 2nd ed.; Lyr, H., Braun, P., Eds.; Gustav Fischer Verlag: New York, NY, USA, 1995; pp. 347–354. [Google Scholar]
- Davidse, L.C. Resistance to acylalanines in Phytophthora infestans in The Netherlands. EPPO Bull. 1985, 15, 403–409. [Google Scholar] [CrossRef]
- Matheron, M.E.; Porchas, M. Impact of azoxystrobin, dimethomorph, fluazinam, fosetyl-al, and metalaxyl on growth, sporulation, and zoospore cyst germination of three Phytophthora spp. Plant Dis. 2000, 84, 454–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rekanović, E.; Potočnik, I.; Milijašević-Marčić, S.; Stepanović, M.; Todorović, B.; Mihajlović, M. Toxicity of metalaxyl, azoxystrobin, dimethomorph, cymoxanil, zoxamide and mancozeb to Phytophthora infestans isolates from Serbia. J. Environ. Sci. Health Part B 2012, 47, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Utkhede, R.S.; Gupta, V.K. In vitro selection of strains of Phytophthora cactorum resistant to metalaxyl. J. Phytopathol. 1988, 122, 35–44. [Google Scholar] [CrossRef]
- Anjum, M.Z.; Adnan, M.; Ali, S.M.; Bilal, H.M.; Javaid, H. Antifungal potential of biocontrol agents against Phytophthora capsici causing chili fruit rot. Agric. Res. Technol. 2019, 22, 156–159. [Google Scholar]
- Aryantha, I.N.; Guest, D. Mycoparasitic and antagonistic inhibition on Phytophthora cinnamomi rands by microbial agents isolated from manure composts. Plant Pathol. J. 2006, 5, 291–298. [Google Scholar]
- da Silva Costa, J.L.; Menge, J.A.; Casale, W.L. Biological control of Phytophthora root rot of avocato with microorganisms grown in organic mulches. Braz. J. Microbiol. 2000, 31, 239–246. [Google Scholar]
- Iqbal, M.; Jamshaid, M.; Zahid, M.A.; Andreasson, E.; Vetukuri, R.; Stenberg, J. Biological control of strawberry crown rot, root rot and grey mould by the beneficial fungus Aureobasidium pullulans. Res. Sq. 2021, 66, 535–545. [Google Scholar]
- Akgül, D.S.; Mirik, M. Biocontrol of Phytophthora capsici on pepper plants by Bacillus megaterium strains. J. Plant Pathol. 2008, 90, 29–34. [Google Scholar]
- Lim, J.; Kim, S. Biocontrol of Phytophthora blight of red pepper caused by Phytophthora capsici using Bacillus subtilis AH18 and B. licheniformis K11 Formulations. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 766–773. [Google Scholar] [CrossRef]
- Moradi, M.; Nejad, F.J.; Hosein, G.; Bonjar, S.; Fani, S.R. Efficacy of Bacillus subtilis native strains for biocontrol of Phytophthora crown and root rot of pistachio in Iran. Trop. Plant Pathol. 2018, 43, 306–313. [Google Scholar] [CrossRef]
- Oh, B.T.; Hur, H.; Lee, K.J.; Shanthi, K.; Soh, B.Y.; Lee, W.J.; Myung, H.; Kamala-Kannan, S. Biocontrol science and technology suppression of Phytophthora blight on pepper (Capsicum annuum L.) by bacilli isolated from brackish environment. Biocontrol Sci. Technol. 2011, 21, 1297–1311. [Google Scholar] [CrossRef]
- Saberi-Riseh, R.; Sharifi-Tehrani, A.; Khezri, M.; Ahmadzadeh, M.; Nikkhah, M.J. Study on biocontrol of Phytophthora citrophthora, the causal agent of pistachio gummosis. Acta Hortic. 2006, 726, 627–630. [Google Scholar] [CrossRef]
- Syed-Ab-Rahman, S.F.; Carvalhais, L.C.; Chua, E.; Xiao, Y.; Wass, T.J.; Schenk, P.M. Identification of soil bacterial isolates suppressing different Phytophthora spp. and promoting plant growth. Front. Plant Sci. 2018, 9, 1–18. [Google Scholar] [CrossRef]
- Utkhede, R.S. Antagonism of isolates of Bacillus subtilis to Phytophthora cactorum. Can. J. Bot. 1984, 62, 1032–1035. [Google Scholar] [CrossRef]
- Anandhakumar, J.; Zeller, W. Biological control of red stele (Phytophthora fragariae var fragariae) and crown rot (P. cactorum ) disease of strawberry with rhizobacteria. J. Plant Dis. Prot. 2008, 115, 49–56. [Google Scholar]
- Okamoto, H.; Sat, M.; Miyata, Y.; Yoshikawa, M.; Isaka, M. Biocontrol of Phytophthora root rot of angelica trees by Enterobacter cloacae and Serratia ficaria strains. J. Gen. Plant Pathol. 2000, 66, 86–94. [Google Scholar] [CrossRef]
- Hautsalo, J.; Vestberg, M.; Parikka, P.; Finni, S.; Karhu, S.; Tahvonen, R. Biological control of strawberry crown rot is substrate dependent phenomenon. J. Berry Res. 2016, 6, 65–79. [Google Scholar] [CrossRef] [Green Version]
- Krauss, U.; ten Hoopen, M.; Rees, R.; Stirrup, T.; Argyle, T.; George, A.; Arroyo, C.; Corrales, E.; Casanoves, F. Mycoparasitism by Clonostachys byssicola and Clonostachys rosea on Trichoderma spp. from cocoa (Theobroma cacao) and implication for the design of mixed biocontrol agents. Biol. Control. 2013, 67, 317–327. [Google Scholar] [CrossRef]
- Rogerson, C.T.; Stephenson, S.L. Myxomyceticolous Fungi. Mycologia 1993, 85, 456–469. [Google Scholar] [CrossRef]
- Møller, K.; Jensen, B.; Andersen, H.P.; Stryhn, H.; Hockenhull, J. Biocontrol of Pythium tracheiphilum in Chinese cabbage by Clonostachys rosea under field conditions. Biocontrol Sci. Technol. 2003, 13, 171–182. [Google Scholar] [CrossRef]
- Sutton, J.C.; Liu, W.; Ma, J.; Brown, W.G.; Stewart, J.F.; Walker, G.D. Evaluation of the fungal endophyte Clonostachys rosea as an inoculant to enhance growth, fitness and productivity of crop plants. Acta Hortic. 2008, 782, 279–286. [Google Scholar] [CrossRef]
- Afshari, N.; Hemmati, R. First report of the occurrence and pathogenicity of Clonostachys rosea on faba bean. Australas. Plant Pathol. 2017, 46, 231–234. [Google Scholar] [CrossRef]
- Ivanová, H.; Hamarová, L.; Pristaš, P. Clonostachys rosea associated with ponderosa and Coulter pine needles in Slovakia. Biologia 2017, 72, 1258–1263. [Google Scholar] [CrossRef]
- Moraga-Suazo, P.; Sanfuentes, E. Induced systemic resistance triggered by Clonostachys rosea against Fusarium circinatum in Pinus radiata. For. Res. Open Access 2016, 5, 174. [Google Scholar]
- Sun, Z.B.; Li, S.D.; Ren, Q.; Xu, J.L.; Lu, X.; Sun, M.H. Biology and applications of Clonostachys rosea. J. Appl. Microbiol. 2020, 129, 486–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toledo, A.V.; Virla, E.; Humber, R.A.; Paradell, S.L.; Lastra, C.C.L. First record of Clonostachys rosea (Ascomycota: Hypocreales) as an entomopathogenic fungus of Oncometopia tucumana and Sonesimia grossa (Hemiptera: Cicadellidae) in Argentina. J. Invertebr. Pathol. 2006, 92, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Berger, G.; Czarnocka, K.; Cochard, B.; Oszako, T.; Lefort, F. Biocontrol endotherapy with Trichoderma spp. and Bacillus amyloliquefaciens against Phytophthora spp.: A comparative study with phosphite treatment on Quercus robur and Fagus sylvatica. J. Agric. Sci. Technol. A 2015, 5, 428–439. [Google Scholar]
- Höfte, M.; Altier, N. Fluorescent pseudomonads as biocontrol agents for sustainable agricultural systems. Res. Microbiol. 2010, 161, 464–471. [Google Scholar] [CrossRef]
- Agustí, L.; Bonaterra, A.; Moragrega, C.; Camps, J.; Montesinos, E. Biocontrol of root rot of strawberry caused by Phytophthora cactorum with a combination of two Pseudomonas fluorescens strains. J. Plant Pathol. 2011, 93, 363–372. [Google Scholar]
- Farzaneh, M.; Sharifi-Tehrani, A.; Ahmadzadeh, M.; Zad, J. Biocontrol of Phytophthora cactorum the causal agent of root and crown rot on apple (Malus domestica) by formulated Pseudomonas fluorescens. Commun. Agric. Appl. Biol. Sci. 2007, 72, 891–900. [Google Scholar] [PubMed]
- Rath, M.; Mitchell, T.R.; Gold, S.E. Volatiles produced by Bacillus mojavensis RRC101 act as plant growth modulators and are strongly culture-dependent. Microbiol. Res. 2018, 208, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Whipps, J.M.; Gerlagh, M. Biology of Coniothyrium minitans and its potential for use in disease biocontrol. Mycol. Res. 1992, 96, 897–907. [Google Scholar] [CrossRef]
- Zeng, W.; Wang, D.; Kirk, W.; Hao, J. Use of Coniothyrium minitans and other microorganisms for reducing Sclerotinia Sclerotiorum. Biol. Control. 2012, 60, 225–232. [Google Scholar] [CrossRef]
- Tomprefa, N.; McQuilken, M.P.; Hill, R.A.; Whipps, J.M. Antimicrobial activity of Coniothyrium minitans and its macrolide antibiotic macrosphelide A. J. Appl. Microbiol. 2009, 106, 2048–2056. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gong, X.; Zheng, J.; Jiang, D.; Fu, Y.; Hou, M. Transformation of Coniothyrium minitans, a parasite of Sclerotinia sclerotiorum, with Agrobacterium tumefaciens. FEMS Microbiol. Lett. 2005, 243, 323–329. [Google Scholar] [CrossRef] [Green Version]
- McQuilken, M.P.; Gemmell, J.; Whipps, J.M. Some nutritional factors affecting production of biomass and antifungal metabolites of Coniothyrium minitans. Biocontrol Sci. Technol. 2002, 12, 443–454. [Google Scholar] [CrossRef]
- Ampuero, J.; Latorre, B.A.; Torres, R.; Chávez, E.R. Identification of Phytophthora cryptogea as the cause of rapid decline of Petunia ( Petunia × hybrida ) in Chile. Plant Dis. 2008, 92, 1529–1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-González, E.I.; Gutiérrez-Soto, J.G.; Olivares-Sáenz, E.; Gutiérrez-Díez, A.; Barrientos-Priego, A.F.; Ochoa-Ascencio, S. Screening progenies of mexican race avocado genotypes for resistance to Phytophthora cinnamomi rands. HortScience 2019, 54, 809–813. [Google Scholar] [CrossRef] [Green Version]
- Schafleitner, S.; Bonnet, A.; Pedeprat, N.; Rocca, D.; Chartier, P.; Denoyes, B. Genetic variation of resistance of the cultivated strawberry to crown rot caused by Phytophthora cactorum. J. Berry Res. 2013, 3, 79–91. [Google Scholar] [CrossRef] [Green Version]
- Luo, W.; Jiang, Y.; Lü, X.; Wang, X.; Li, M.-H.; Bai, E.; Han, X.; Xu, Z. Patterns of plant biomass allocation in temperate grasslands across a 2500-km transect in Northern China. PLoS ONE 2013, 8, e71749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agathokleous, E.; Belz, R.G.; Kitao, M.; Koike, T.; Calabrese, E.J. Does the root to shoot ratio show a hormetic response to stress? An ecological and environmental perspective. J. For. Res. 2019, 30, 1569–1580. [Google Scholar] [CrossRef] [Green Version]
- Jamieson, A.R.; Kempler, C. Strawberry genotypes differ in their ratio of shoots to roots, based on dry weight. Acta Hortic. 2009, 842, 589–592. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Aguilar-Trigueros, C.A.; Flaig, I.C.; Rillig, M.C. Root trait responses to drought are more heterogeneous than leaf trait responses. Funct. Ecol. 2020, 34, 2224–2235. [Google Scholar] [CrossRef]
- Zhou, G.; Zhou, X.; Nie, Y.; Bai, S.H.; Zhou, L.; Shao, J.; Cheng, W.; Wang, J.; Hu, F.; Fu, Y. Drought-induced changes in root biomass largely result from altered root morphological traits: Evidence from a synthesis of global field trials. Plant Cell Environ. 2018, 41, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Barraud, J.; Tojo, M. Suppressive effects of Pythium oligandrum on soybean damping off caused by P. aphanidermatum and P. myriotylum. Annu. Rep. Kansai Plant Prot. Soc. 2019, 61, 9–13. [Google Scholar] [CrossRef] [Green Version]
- El-Katatny, D.M.H.; Abdelzaher, H.M.A.; Shoulkamy, M.A. Antagonistic actions of Pythium oligandrum and Trichoderma harzianum against phytopathogenic fungi (Fusarium oxysporum and Pythium ultimum var ultimum). Arch. Phytopathol. Plant Prot. 2006, 39, 289–301. [Google Scholar] [CrossRef]
- Takenaka, S.; Sekiguchi, H.; Nakaho, K.; Tojo, M.; Masunaka, A.; Takahashi, H. Colonization of Pythium oligandrum in the tomato rhizosphere for biological control of bacterial wilt disease analyzed by real-time PCR and confocal laser-scanning microscopy. Phytopathology 2008, 98, 187–195. [Google Scholar] [CrossRef] [Green Version]
- Benhamou, N.; Le Floch, G.; Vallance, J.; Gerbore, J.; Grizard, D.; Rey, P. Pythium oligandrum: An example of opportunistic success. Microbiology 2012, 158, 2679–2694. [Google Scholar] [CrossRef] [PubMed]
- Le Floch, G.; Rey, P.; Benizri, E.; Benhamou, N.; Tirilly, Y. Impact of auxin-compounds produced by the antagonistic fungus Pythium oligandrum or the minor pathogen Pythium group F on plant growth. Plant Soil 2003, 257, 459–470. [Google Scholar] [CrossRef]
- Bae, S.J.; Mohanta, T.K.; Young Chung, J.; Ryu, M.; Park, G.; Shim, S.; Hong, S.B.; Seo, H.; Bae, D.W.; Bae, I.; et al. Trichoderma metabolites as biological control agents against Phytophthora pathogens. Biol. Control. 2015, 92, 128–138. [Google Scholar]
- Jiang, H.; Zhang, L.; Zhang, J.; Ojaghian, M.R.; Hyde, K.D. Antagonistic interaction between Trichoderma asperellum and Phytophthora capsici in vitro. J. Zhejiang Univ. Sci. B 2016, 17, 271–281. [Google Scholar] [CrossRef] [Green Version]
- La Spada, F.; Stracquadanio, C.; Riolo, M.; Pane, A.; Cacciola, S.O. Trichoderma counteracts the challenge of Phytophthora nicotianae infections on tomato by modulating plant defense mechanisms and the expression of crinkler, necrosis-inducing Phytophthora protein 1, and cellulose-binding elicitor lectin pathogenic effectors. Front. Plant Sci. 2020, 11, 1653. [Google Scholar]
- Sid Ahmed, A.; Pérez-Sánchez, C.; Egea, C.; Candela, M.E. Evaluation of Trichoderma harzianum for controlling root rot caused by Phytophthora capsici in pepper plants. Plant Pathol. 1999, 48, 58–65. [Google Scholar] [CrossRef]
- Blom, D.; Fabbri, C.; Connor, E.C.; Schiestl, F.P.; Klauser, D.R.; Boller, T.; Eberl, L.; Weisskopf, L. Production of plant growth modulating volatiles is widespread among rhizosphere bacteria and strongly depends on culture conditions. Environ. Microbiol. 2011, 13, 3047–3058. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.C.; Elliott, L.F.; Young, F.L.; Douglas, C.L. Rhizobacteria suppressive to the weed downy brome. Soil Sci. Soc. Am. J. 1991, 55, 722–727. [Google Scholar] [CrossRef]
- Kim, B.; Moon, S.; Hwang, B. Isolation, identification, and antifungal activity of a macrolide antibiotic, oligomycin A, produced by Streptomyces libani. Can. J. Bot. 2011, 77, 850–858. [Google Scholar]
- Blom, D.; Fabbri, C.; Eberl, L.; Weisskopf, L. Volatile-mediated killing of Arabidopsis thaliana by bacteria is mainly due to hydrogen cyanide. Appl. Environ. Microbiol. 2011, 77, 1000–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudrappa, T.; Splaine, R.E.; Biedrzycki, M.L.; Bais, H.P. Cyanogenic pseudomonads influence multitrophic interactions in the rhizosphere. PLoS ONE 2008, 3, e2073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerbore, J.; Benhamou, N.; Vallance, J.; Le Floch, G.; Grizard, D.; Regnault-Roger, C.; Rey, P. Biological control of plant pathogens: Advantages and limitations seen through the case study of Pythium oligandrum. Environ. Sci. Pollut. Res. 2014, 21, 4847–4860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| BCA | Active Compound | Dilution (g/mL) | No of Spores (cells)/Pot |
|---|---|---|---|
| Gliorex | Trichoderma sp., Clonostachys sp. | 2 | 1.7 mil |
| Clonoplus | Clonostachys rosea | 2 | 0.3 mil |
| \ | Clonostachys rosea isolate 156 | \ | 0.3 mil |
| Contans | Coniothyrium minitans | 0.2 | 3.3 mil |
| Hirundo | Bacillus sp. | 0.05 | 0.8 mil |
| Prometheus | Pseudomonas | 0.05 | 0.8 mil |
| \ | Pseudomonas sp., isolate | \ | 0.8 mil |
| Polyversum | Pythium oligandrum | 0.15 | 2500 |
| Integral Pro | Bacillus amyloliquefaciens (MBI 600) | 0.05 | 18.3 mil |
| Xilon | Trichoderma asperellum kmen T34 | 0.5 | 83,000 |
| IDs of Phytophthora cactorum Isolates Used | NCBI GenBank Accession Numbers | Locality of Origin | |
| 17_04_12 | MW193106 | Kunratice/Central Bohemia | |
| 18_10_14a | MW193116 | Břežany II/Central Bohemia | |
| 17_12_20 | MW193108 | Plzeň/West Bohemia | |
| 17_15_10b | OK448179 | Holešov/South Moravia | |
| 17_23_19 | MW193104 | Lomec/West Bohemia | |
| 19_28_2 | OK257676 | Přelovice/East Bohemia | |
| 19_28_10 | OK257674 | Přelovice/East Bohemia | |
| 17_30_18 | OK257655 | Svárov/North Bohemia | |
| 17_34_7 | OK257657 | Lysá nad Labem/Central Bohemia | |
| 17_45_1b | OK257664 | Veselá u Semil/East Bohemia | |
| Comparison Mode | Assessed Variant | Control Variant | ||
|---|---|---|---|---|
| Presence of BCA | Presence of Pathogen | Presence of BCA | Presence of Pathogen | |
| a | P | A | A | A |
| b | P | P | A | A |
| c | P | P | A | P |
| d | P | P | P | A |
| e | P | A | A | P |
| Partial Dataset Used (Cultivating Space/Strawberry Cultivars) | Combination of Independent Variables Used for Grouping | λW | p-Values of Partial λW of Measured Variables | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Fruits | Number of Blossoms | Weight of Fruits | Weight of Blossoms | Weight of Roots | Weight of Rhizomes | Diameter of Rhizomes | Length of Roots | Number of Leaves | Weight of Leaves | |||
| G/S + K + W | BCA | 0.63764 | 0.01 | 0.83 | 0.02 | 0.46 | 0.00 | 0.00 | 0.00 | 0.00 | 0.16 | 0.00 |
| G/K | BCA | 0.36297 | 0.26 | 0.71 | 0.96 | 0.84 | 0.01 | 0.00 | 0.02 | 0.00 | 0.29 | 0.02 |
| G/S | BCA | 0.30766 | 0.25 | 0.42 | 0.03 | 0.07 | 0.00 | 0.00 | 0.00 | 0.00 | 0.17 | 0.07 |
| G/W | BCA | 0.34125 | 0.02 | 0.79 | 0.00 | 0.72 | 0.02 | 0.42 | 0.00 | 0.24 | 0.20 | 0.00 |
| C/S + W | BCA | 0.63135 | 0.35 | 0.06 | 0.00 | 0.02 | 0.00 | 0.13 | 0.16 | 0.32 | 0.21 | 0.31 |
| C/S | BCA | 0.40998 | 0.30 | 0.18 | 0.00 | 0.66 | 0.55 | 0.32 | 0.08 | 0.01 | 0.03 | 0.17 |
| C/W | BCA | 0.45809 | 0.76 | 0.20 | 0.55 | 0.20 | 0.00 | 0.07 | 0.16 | 0.85 | 0.03 | 0.17 |
| G/S + K + W | Cultivar | 0.27766 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| C/S + W | Cultivar | 0.60780 | 0.00 | 0.13 | 0.01 | 0.26 | 0.08 | 0.01 | 0.00 | 0.00 | 0.40 | 0.00 |
| G/S + K + W | Cultivar + BCA | 0.09009 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| C/S + W | Cultivar + BCA | 0.60780 | 0.00 | 0.13 | 0.01 | 0.26 | 0.08 | 0.01 | 0.00 | 0.00 | 0.40 | 0.00 |
| G/S + K + W | Cultivar + Pathogen | 0.20683 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| C/2 | Cultivar + Pathogen | 0.35366 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.02 | 0.48 | 0.00 |
| C/2 | Cultivar + Pathogen + BCA | 0.06205 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 |
| G/S + K + W | Pathogen | 0.88373 | 0.00 | 0.02 | 0.00 | 0.00 | 0.02 | 0.00 | 0.61 | 0.40 | 0.06 | 0.00 |
| G/K | Pathogen | 0.84736 | 0.65 | 0.22 | 0.10 | 0.96 | 0.21 | 0.01 | 0.60 | 0.07 | 0.01 | 0.00 |
| G/S | Pathogen | 0.62026 | 0.00 | 0.00 | 0.00 | 0.25 | 0.28 | 0.62 | 0.92 | 0.32 | 0.83 | 0.00 |
| G/W | Pathogen | 0.77976 | 1.00 | 0.80 | 0.56 | 0.40 | 0.24 | 0.00 | 0.13 | 0.42 | 0.20 | 0.73 |
| C/S + W | Pathogen | 0.66781 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.09 | 0.30 | 0.12 | 1.00 | 0.00 |
| C/S | Pathogen | 0.64577 | 0.10 | 0.00 | 0.00 | 0.00 | 0.60 | 0.72 | 0.14 | 0.42 | 0.31 | 0.10 |
| C/W | Pathogen | 0.60210 | 0.25 | 0.38 | 0.01 | 0.02 | 0.00 | 0.26 | 0.44 | 0.26 | 0.98 | 0.00 |
| G/S + K + W | Pathogen + BCA | 0.39665 | 0.00 | 0.09 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.05 | 0.00 |
| G/K | Pathogen + BCA | 0.15278 | 0.41 | 0.45 | 0.38 | 0.86 | 0.01 | 0.00 | 0.05 | 0.00 | 0.00 | 0.00 |
| G/S | Pathogen + BCA | 0.04398 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.28 | 0.00 |
| G/W | Pathogen + BCA | 0.09164 | 0.04 | 0.92 | 0.00 | 0.78 | 0.02 | 0.00 | 0.00 | 0.00 | 0.26 | 0.02 |
| G + C/S | Pathogen + BCA | 0.23271 | 0.74 | 0.00 | 0.00 | 0.12 | 0.00 | 0.01 | 0.00 | 0.00 | 0.01 | 0.00 |
| C/S + W | Pathogen + BCA | 0.27867 | 0.28 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 0.54 | 0.05 | 0.08 | 0.00 |
| C/S | Pathogen + BCA | 0.10628 | 0.40 | 0.00 | 0.00 | 0.05 | 0.04 | 0.09 | 0.07 | 0.02 | 0.06 | 0.05 |
| C/W | Pathogen + BCA | 0.12617 | 0.92 | 0.29 | 0.57 | 0.34 | 0.00 | 0.21 | 0.35 | 0.39 | 0.01 | 0.00 |
| G + C/W | Pathogen + BCA | 0.30120 | 0.25 | 0.23 | 0.15 | 0.23 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 |
| G + C/S | Cultivating space | 0.42850 | 0.02 | 0.93 | 0.50 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.00 | 0.10 |
| G + C/W | Cultivating space | 0.31110 | 0.51 | 0.00 | 0.02 | 0.91 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 |
| Cultivating Space Used | Strawberry Cultivar | BCA Tested | Covered Variability (%) | Estimated Differentiation Read from Plots | ||
|---|---|---|---|---|---|---|
| Factor 1 | Factor 2 | Sum of the First Two Factors | ||||
| C | S | Aliette | 62.58 | 15.94 | 78.52 | 2 |
| C | W | Aliette | 47.79 | 16.48 | 64.27 | 1 |
| C | S | Integral Pro | 47.03 | 21.6 | 68.63 | 5 |
| C | W | Integral Pro | 44.58 | 17.96 | 62.54 | 5 |
| G | S | Integral Pro | 41.96 | 19.57 | 61.53 | 5 |
| G | W | Integral Pro | 42.14 | 24.24 | 66.38 | 3 |
| C | S | Clonoplus | 55.81 | 14.78 | 70.59 | 3 |
| C | W | Clonoplus | 47.5 | 17.13 | 64.63 | 5 |
| G | S | Clonoplus | 51.29 | 22.2 | 73.49 | 4 |
| G | K | Clonoplus | 34.92 | 23.74 | 58.66 | 5 |
| G | W | Clonoplus | 38.59 | 18.87 | 57.46 | 5 |
| C | S | Clonostachys rosea isolate 156 | 54.01 | 17.54 | 71.55 | 2 |
| C | W | Clonostachys rosea isolate 156 | 58.62 | 15.58 | 74.2 | 1 |
| C | S | Contans | 55.34 | 21.27 | 76.61 | 5 |
| C | W | Contans | 46.03 | 21.31 | 67.34 | 5 |
| G | S | Contans | 54.85 | 21.65 | 76.5 | 5 |
| G | K | Contans | 35.86 | 26.23 | 62.09 | 5 |
| G | W | Contans | 42.38 | 20.88 | 63.26 | 2 |
| C | S | Control | 62.72 | 17.08 | 79.8 | 5 |
| C | W | Control | 53.3 | 15.85 | 69.15 | 3 |
| G | S | Control | 43.07 | 15.88 | 58.95 | 4 |
| G | K | Control | 35.16 | 21.76 | 56.92 | 5 |
| G | W | Control | 49.5 | 15.18 | 64.68 | 5 |
| C | S | Gliorex | 61.48 | 14.98 | 76.46 | 2 |
| C | W | Gliorex | 33.93 | 22.22 | 56.15 | 4 |
| G | S | Gliorex | 41.4 | 28.05 | 69.45 | 5 |
| G | W | Gliorex | 46.92 | 22.08 | 69 | 2 |
| C | S | Hirundo | 59.06 | 24.25 | 83.31 | 4 |
| C | W | Hirundo | 50.95 | 15.03 | 65.98 | 4 |
| G | S | Hirundo | 42.7 | 20.15 | 62.85 | 5 |
| G | K | Hirundo | 42.04 | 22.91 | 64.95 | 5 |
| G | W | Hirundo | 57.03 | 14.6 | 71.63 | 1 |
| C | S | Polyversum | 47.85 | 21.29 | 69.14 | 4 |
| C | W | Polyversum | 31.04 | 19.06 | 50.1 | 4 |
| G | S | Polyversum | 51.33 | 27.2 | 78.53 | 5 |
| G | K | Polyversum | 39.51 | 23.03 | 62.54 | 5 |
| G | W | Polyversum | 31.17 | 23.82 | 54.99 | 5 |
| C | S | Prometheus | 48.64 | 17.98 | 66.62 | 2 |
| C | W | Prometheus | 43.01 | 16.08 | 59.09 | 4 |
| G | S | Prometheus | 53.74 | 16.83 | 70.57 | 4 |
| G | K | Prometheus | 51.74 | 19.94 | 71.68 | 5 |
| G | W | Prometheus | 40.02 | 21.31 | 61.33 | 5 |
| C | S | Pseudomonas sp. | 73.77 | 11.91 | 85.68 | 5 |
| C | W | Pseudomonas sp. | 50.36 | 20.62 | 70.98 | 4 |
| G | S | Pseudomonas sp. | 37.38 | 22.99 | 60.37 | 3 |
| G | K | Pseudomonas sp. | 33.13 | 27.82 | 60.95 | 5 |
| G | W | Pseudomonas sp. | 50.93 | 20.83 | 71.76 | 5 |
| C | S | Xilon | 52.66 | 20.19 | 72.85 | 4 |
| C | W | Xilon | 49.32 | 20.4 | 69.72 | 2 |
| G | S | Xilon | 41.29 | 23.68 | 64.97 | 3 |
| G | K | Xilon | 29.13 | 20.97 | 50.1 | 5 |
| G | W | Xilon | 38.42 | 24.19 | 62.61 | 5 |
| Comparison Mode | (a) | (b) | (c) | (d) | (e) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control Variant | BCA-Free | BCA-Free | BCA-Free | BCA | BCA Free | |||||||||||
| Pathogen-Free | Pathogen-Free | Pathogen | Pathogen-Free | Pathogen | ||||||||||||
| Assessed Variant | BCA | BCA | BCA | BCA | BCA | |||||||||||
| Pathogen-Free | Pathogen | Pathogen | Pathogen | Pathogen Free | ||||||||||||
| Tested BCA | Active Component | Roots | Leaves | R:S | Roots | Leaves | R:S | Roots | Leaves | R:S | Roots | Leaves | R:S | Roots | Leaves | R:S |
| Aliette | Fosetyl-Al | 99.4 | 197.4 | 51.1 | 95.1 | 43.3 | 291.0 | 99.2 | 99.2 | 258.0 | 104.0 | 29.1 | 582.9 | 98.4 | 295.5 | 36.6 |
| Integral Pro | Bacillus amyloliquefaciens | 94.9 | 72.4 | 141.4 | 106.2 | 40.7 | 197.1 | 98.1 | 77.1 | 166.5 | 112.6 | 56.4 | 143.1 | 111.1 | 138.9 | 103.5 |
| Clonoplus | 4 strains of Clonostachys rosea | 117.3 | 184.5 | 98.9 | 88.9 | 82.0 | 160.1 | 116.0 | 112.1 | 132.6 | 89.6 | 43.2 | 181.5 | 93.6 | 261.3 | 89.6 |
| Clonostachys rosea | Clonostachys rosea isolate 156 | 93.0 | 207.2 | 47.9 | 116.3 | 77.7 | 162.1 | 93.0 | 118.3 | 106.5 | 133.3 | 39.5 | 333.6 | 119.9 | 294.7 | 32.8 |
| Contans | Coniothyrium minitans | 88.3 | 97.1 | 97.9 | 85.1 | 50.5 | 162.8 | 87.9 | 88.2 | 141.1 | 104.0 | 52.2 | 169.4 | 87.4 | 167.6 | 89.3 |
| Control | \ | 100.0 | 100.0 | 100.0 | 97.7 | 75.5 | 127.8 | 104.4 | 100.0 | 100.0 | 97.7 | 75.5 | 127.8 | 100.0 | 183.2 | 88.0 |
| Gliorex | Clonostachys rosea, Trichoderma asperellum | 98.9 | 207.3 | 77.9 | 92.3 | 53.9 | 160.3 | 99.3 | 118.6 | 147.8 | 97.6 | 34.8 | 262.1 | 94.8 | 304.1 | 69.8 |
| Hirundo | Bacillus amyloliquefaciens | 106.8 | 186.2 | 98.4 | 96.5 | 72.1 | 210.1 | 107.1 | 94.6 | 177.0 | 93.0 | 35.9 | 221.8 | 98.1 | 274.9 | 89.3 |
| Polyversum | Pythium oligandrum | 138.0 | 201.2 | 88.5 | 107.4 | 116.0 | 98.3 | 144.5 | 201.7 | 85.4 | 77.8 | 60.6 | 115.3 | 112.3 | 316.3 | 76.4 |
| Prometheus | Pseudomonas veronii | 125.3 | 178.2 | 85.8 | 109.0 | 54.2 | 145.5 | 131.1 | 108.0 | 129.3 | 87.4 | 33.8 | 198.4 | 111.1 | 283.8 | 73.7 |
| Pseoudomonas sp. | Pseudomonas sp. isolate | 114.1 | 143.3 | 115.7 | 108.4 | 100.8 | 306.9 | 112.6 | 147.0 | 268.7 | 112.3 | 67.6 | 231.0 | 113.3 | 234.6 | 106.4 |
| Xilon | Trichoderma asperellum | 139.8 | 205.1 | 102.1 | 103.8 | 102.3 | 195.4 | 143.3 | 132.2 | 173.7 | 73.8 | 45.6 | 197.6 | 105.7 | 311.1 | 90.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pánek, M.; Hanáček, A.; Wenzlová, J.; Maňasová, M.; Zouhar, M. A Comparison of the Ability of Some Commercially Produced Biological Control Agents to Protect Strawberry Plants against the Plant Pathogen Phytophthora cactorum. Agriculture 2021, 11, 1086. https://doi.org/10.3390/agriculture11111086
Pánek M, Hanáček A, Wenzlová J, Maňasová M, Zouhar M. A Comparison of the Ability of Some Commercially Produced Biological Control Agents to Protect Strawberry Plants against the Plant Pathogen Phytophthora cactorum. Agriculture. 2021; 11(11):1086. https://doi.org/10.3390/agriculture11111086
Chicago/Turabian StylePánek, Matěj, Aleš Hanáček, Jana Wenzlová, Marie Maňasová, and Miloslav Zouhar. 2021. "A Comparison of the Ability of Some Commercially Produced Biological Control Agents to Protect Strawberry Plants against the Plant Pathogen Phytophthora cactorum" Agriculture 11, no. 11: 1086. https://doi.org/10.3390/agriculture11111086
APA StylePánek, M., Hanáček, A., Wenzlová, J., Maňasová, M., & Zouhar, M. (2021). A Comparison of the Ability of Some Commercially Produced Biological Control Agents to Protect Strawberry Plants against the Plant Pathogen Phytophthora cactorum. Agriculture, 11(11), 1086. https://doi.org/10.3390/agriculture11111086






