Methodology for Measurement of Ammonia Emissions from Intensive Pig Farming
Abstract
:1. Introduction
2. Materials and Methods
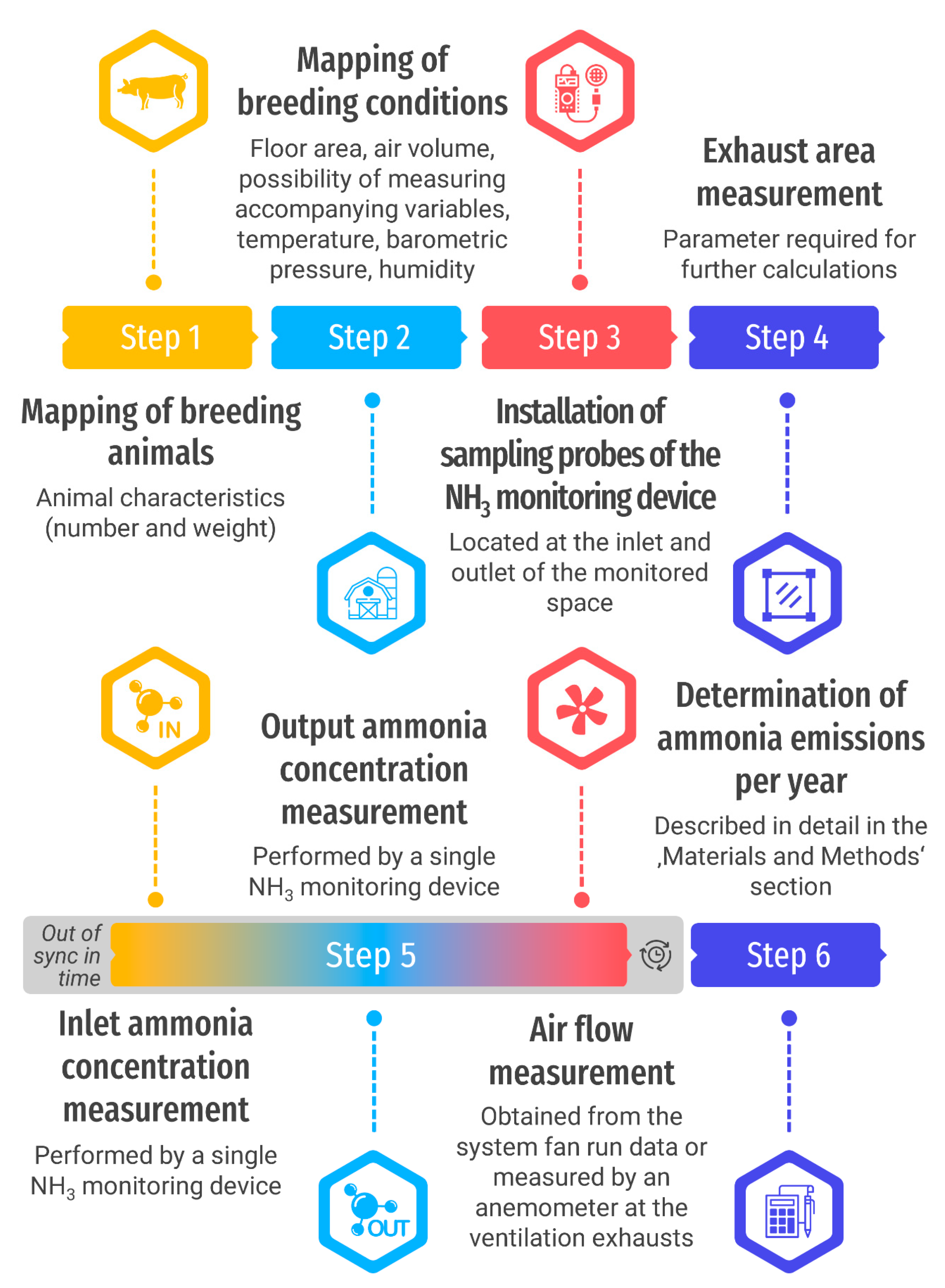
2.1. Scheme of Methodology
2.2. Experimental Pig House
2.3. Methodology for Measurements of the Ammonia Concentration in the Pig Farm
2.4. Methodology for Calculation of NH3 Emissions
2.5. Creation of Model Data for Method Testing
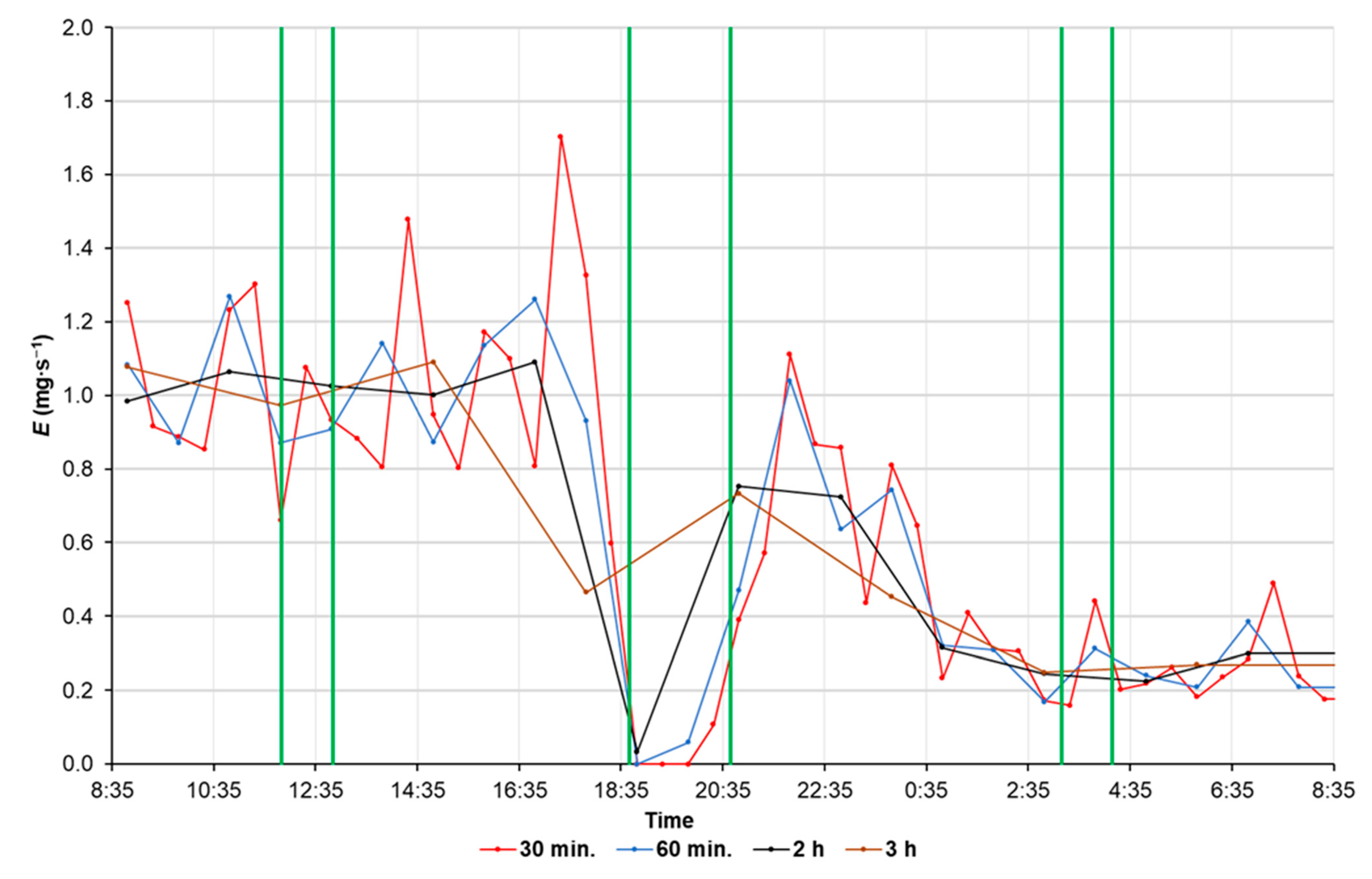
2.6. Testing the E Evaluation Method
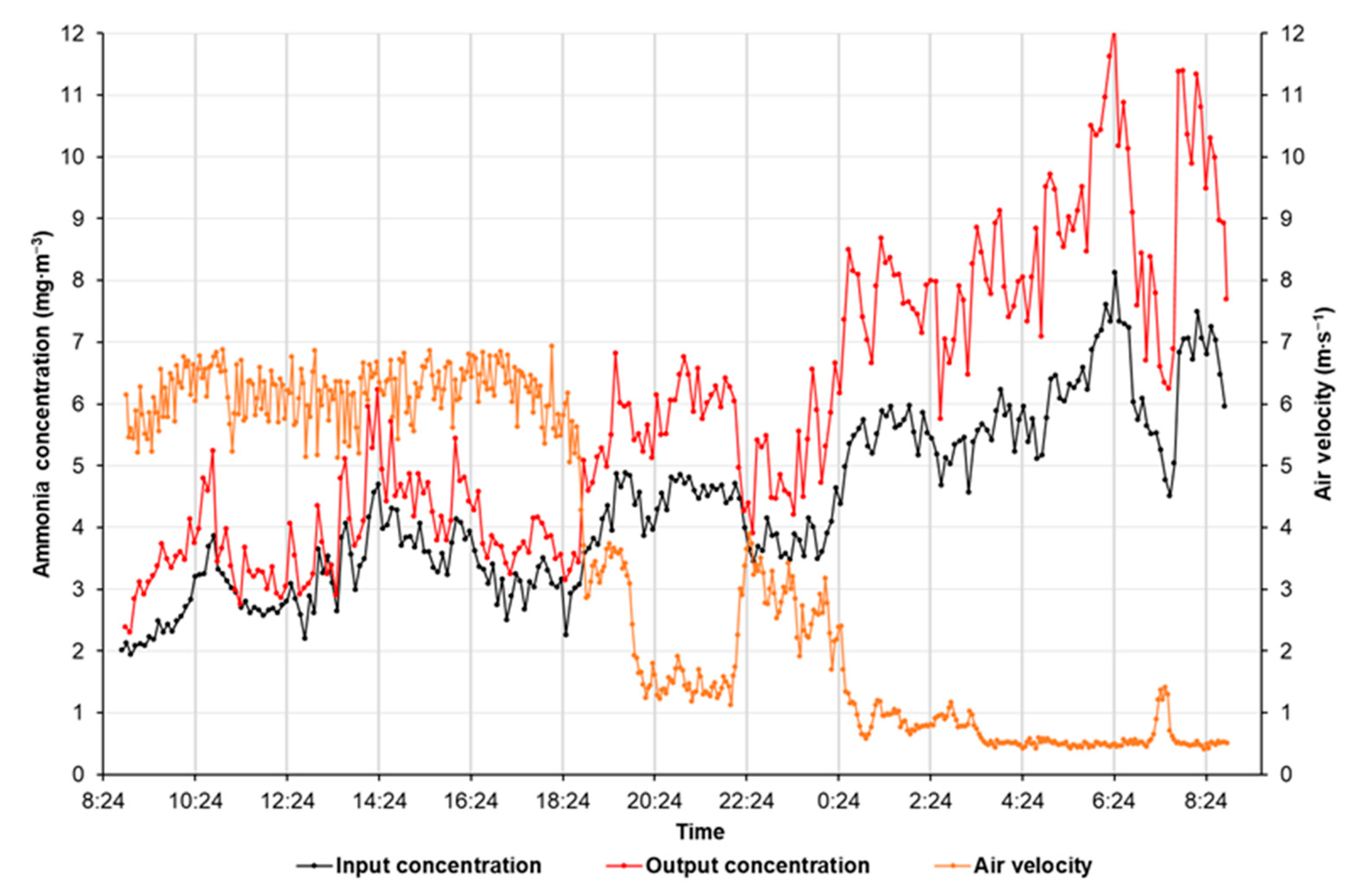
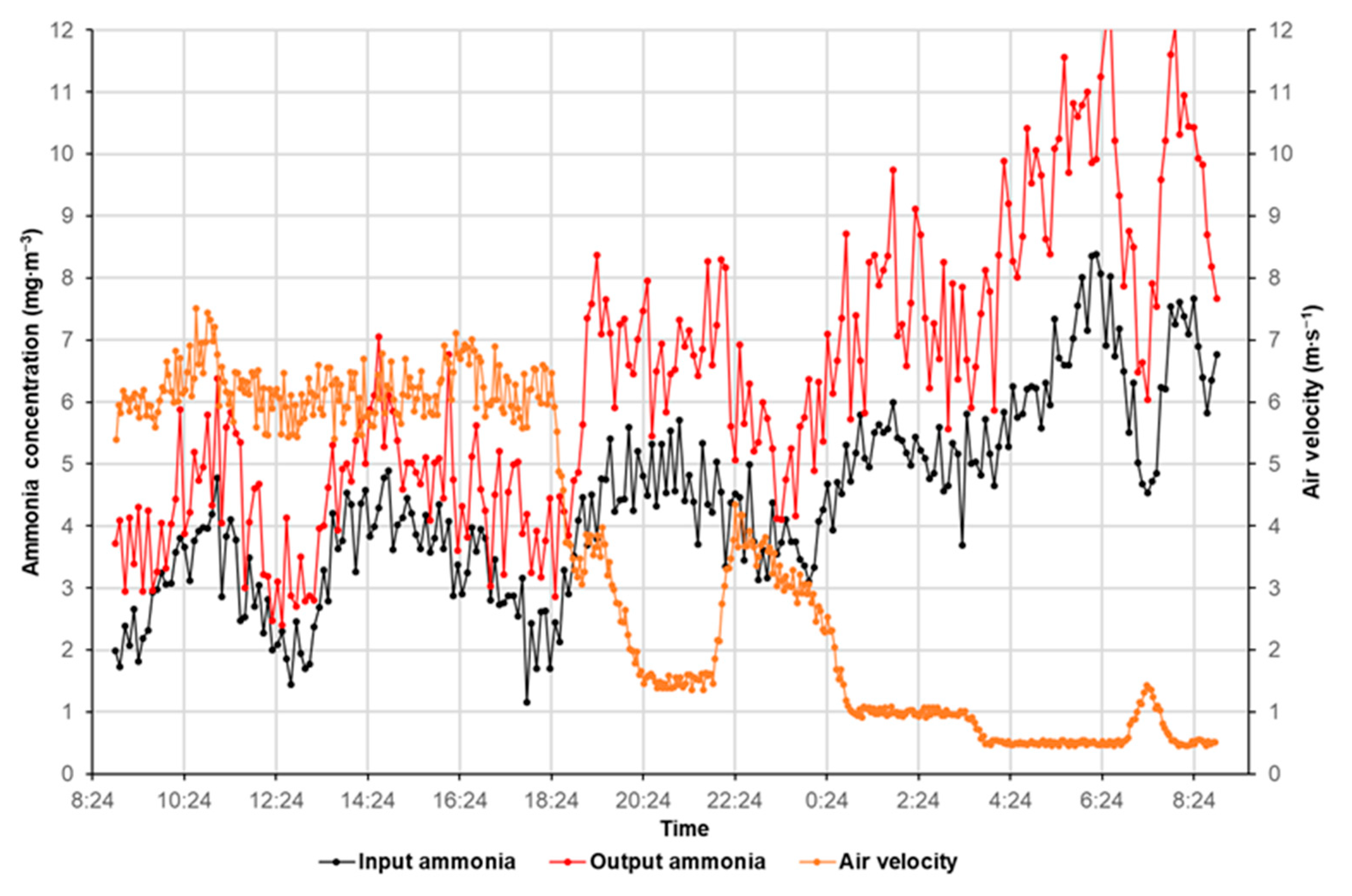
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guthrie, S.; Giles, S.; Dunkerley, F.; Tabaqchali, H.; Harshfield, A.; Ioppolo, B.; Manville, C. The Impact of Ammonia Emissions from Agriculture on Biodiversity; The Royal Society Reports; Rand Corporation: Cambridge, UK, 2018; Available online: https://www.rand.org/content/dam/rand/pubs/research_reports/RR2600/RR2695/RAND_RR2695.pdf (accessed on 26 June 2021).
- Krupa, S. Effects of atmospheric ammonia (NH3) on terrestrial vegetation: A review. Environ. Pollut. 2003, 124, 179–221. [Google Scholar] [CrossRef]
- Renard, J.J.; Calidonna, S.E.; Henley, M.V. Fate of ammonia in the atmosphere—A review for applicability to hazardous releases. J. Hazard. Mater. 2004, 108, 29–60. [Google Scholar] [CrossRef] [PubMed]
- Brunekreef, B.; Holgate, S.T. Air pollution and health. Lancet 2002, 360, 1233–1242. [Google Scholar] [CrossRef]
- Visek, W. Some Aspects of Ammonia Toxicity in Animal Cells. J. Dairy Sci. 1968, 51, 286–295. [Google Scholar] [CrossRef]
- Griffiths, R.; Megson, L. The effect of uncertainties in human toxic response on hazard range estimation for ammonia and chlorine. Atmos. Environ. 1984, 18, 1195–1206. [Google Scholar] [CrossRef]
- Naseem, S.; King, A.J. Ammonia production in poultry houses can affect health of humans, birds, and the environment—Techniques for its reduction during poultry production. Environ. Sci. Pollut. Res. 2018, 25, 15269–15293. [Google Scholar] [CrossRef]
- Donham, K.J. The Concentration of Swine Production: Effects on Swine Health, Productivity, Human Health, and the Environment. Vet.-Clin. N. Am. Food Anim. Pract. 2000, 16, 559–597. [Google Scholar] [CrossRef]
- Kim, K.Y.; Ko, H.J.; Kim, H.T.; Kim, C.N.; Byeon, S.H. Association between pig activity and environmental factors in pig confinement buildings. Aust. J. Exp. Agric. 2008, 48, 680–686. [Google Scholar] [CrossRef]
- ElAssouli, S.M.; Alqahtani, M.H.; Milaat, W. Genotoxicity of Air Borne Particulates Assessed by Comet and the Salmonella Mutagenicity Test in Jeddah, Saudi Arabia. Int. J. Environ. Res. Public Health 2007, 4, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Claxton, L.D.; Woodall, G.M., Jr. A review of the mutagenicity and rodent carcinogenicity of ambient air. Mutat. Res. Rev. Mutat. Res. 2007, 636, 36–94. [Google Scholar] [CrossRef]
- van Zelm, R.; Huijbregts, M.; Hollander, H.A.D.; van Jaarsveld, H.A.; Sauter, F.J.; Struijs, J.; van Wijnen, H.J.; van de Meent, D. European characterization factors for human health damage of PM10 and ozone in life cycle impact assessment. Atmos. Environ. 2008, 42, 441–453. [Google Scholar] [CrossRef]
- EEA. European Environment Agency. Ammonia (NH3) Emissions. European Environment Agency Retrieved 02/01/2019. 2019. Available online: http://www.eea.europa.eu/data-and-maps/indicators/eea-32-ammonia-nh3-emissions-1/assessment-4 (accessed on 10 July 2021).
- EUROSTAT. Agriculture, Forestry and Fishery Statistics; European Union: Brussels, Belgium, 2016. [Google Scholar]
- Clarisse, L.; Clerbaux, C.; Dentener, F.J.; Hurtmans, D.; Coheur, P.-F. Global ammonia distribution derived from infrared satellite observations. Nat. Geosci. 2009, 2, 479–483. [Google Scholar] [CrossRef]
- Waldrip, H.; Cole, N.A.; Todd, R.W. Review: Nitrogen sustainability and beef cattle feedyards: II Ammonia emissions. Prof. Anim. Sci. 2015, 31, 395–411. [Google Scholar] [CrossRef] [Green Version]
- Groenestein, C.; Van Faassen, H. Volatilization of Ammonia, Nitrous Oxide and Nitric Oxide in Deep-litter Systems for Fattening Pigs. J. Agric. Eng. Res. 1996, 65, 269–274. [Google Scholar] [CrossRef]
- Insausti, M.; Timmis, R.; Kinnersley, R.; Rufino, M. Advances in sensing ammonia from agricultural sources. Sci. Total Environ. 2020, 706, 135124. [Google Scholar] [CrossRef]
- Olivier, J.; Bouwman, L.; Van der Hoek, K.; Berdowski, J. Global air emission inventories for anthropogenic sources of NOx, NH3 and N2O in 1990. Environ. Pollut. 1998, 102, 135–148. [Google Scholar] [CrossRef]
- Misselbrook, T.; van der Weerden, T.; Pain, B.; Jarvis, S.; Chambers, B.; Smith, K.; Phillips, V.; Demmers, T. Ammonia emission factors for UK agriculture. Atmos. Environ. 2000, 34, 871–880. [Google Scholar] [CrossRef]
- Canh, T.T.; Aarnink, A.J.A.; Mroz, Z.; Jongbloed, A.W.; Schrama, J.W.; Verstegen, M.W.A. Influence of electrolyte balance and acidifying calcium salts in the diet of growing-finishing pigs on urinary pH, slurry pH and ammonia volatilisation from slurry. Livest. Prod. Sci. 1998, 56, 1–13. [Google Scholar] [CrossRef]
- Arogo, J.; Westerman, P.W.; Heber, A.J.; Robarge, W.P.; Classen, J.J. Ammonia Emissions from Animal Feeding Operations; Report: White Papers; National Center for Manure & Animal Waste Management: Raleigh, NC, USA, 2001. [Google Scholar]
- Faulkner, W.; Shaw, B. Review of ammonia emission factors for United States animal agriculture. Atmos. Environ. 2008, 42, 6567–6574. [Google Scholar] [CrossRef]
- Santonja, G.G.; Georgitzikis, K.; Scalet, B.M.; Montobbio, P.; Roudier, S.; Sancho, L.D. Best Available Techniques (BAT) Reference Document for the Intensive Rearing of Poultry or Pigs; EUR 28674 EN; Publications Office of the European Union: Luxembourg, 2017. [Google Scholar] [CrossRef]
- U.S. EPA. EPA’s Report on the Environment (ROE); U.S. Environmental Protection Agency: Washington, DC, USA, 2008.
- Council Directive 2010/75/EU of 15 February 2017 Establishing Best Available Techniques (BAT) Conclusions, under Directive 2010/75/EU of the European Parliament and of the Council, for the Intensive Rearing of Poultry or Pigs. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv%3AOJ.L_.2017.043.01.0231.01.ENG (accessed on 27 October 2021).
- Xie, Q.; Ni, J.-Q.; Su, Z. A prediction model of ammonia emission from a fattening pig room based on the indoor concentration using adaptive neuro fuzzy inference system. J. Hazard. Mater. 2017, 325, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Zong, C.; Feng, Y.; Zhang, G.; Hansen, M.J. Effects of different air inlets on indoor air quality and ammonia emission from two experimental fattening pig rooms with partial pit ventilation system—Summer condition. Biosyst. Eng. 2014, 122, 163–173. [Google Scholar] [CrossRef]
- Ye, Z.; Zhu, S.; Kai, P.; Li, B.; Blanes-Vidal, V.; Pan, J.; Wang, C.; Zhang, G. Key factors driving ammonia emissions from a pig house slurry pit. Biosyst. Eng. 2011, 108, 195–203. [Google Scholar] [CrossRef]
- Saha, C.; Zhang, G.; Kai, P.; Bjerg, B. Effects of a partial pit ventilation system on indoor air quality and ammonia emission from a fattening pig room. Biosyst. Eng. 2010, 105, 279–287. [Google Scholar] [CrossRef]
- Blanes-Vidal, V.; Hansen, M.; Pedersen, S.; Rom, H. Emissions of ammonia, methane and nitrous oxide from pig houses and slurry: Effects of rooting material, animal activity and ventilation flow. Agric. Ecosyst. Environ. 2008, 124, 237–244. [Google Scholar] [CrossRef]
- Liu, S.; Ni, J.-Q.; Radcliffe, J.S.; Vonderohe, C.E. Mitigation of ammonia emissions from pig production using reduced dietary crude protein with amino acid supplementation. Bioresour. Technol. 2017, 233, 200–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeppsson, K.-H.; Olsson, A.-C.; Nasirahmadi, A. Cooling growing/finishing pigs with showers in the slatted area: Effect on animal occupation area, pen fouling and ammonia emission. Livest. Sci. 2021, 243, 104377. [Google Scholar] [CrossRef]
- Ni, J.-Q.; Shi, C.; Liu, S.; Richert, B.T.; Vonderohe, C.E.; Radcliffe, J.S. Effects of antibiotic-free pig rearing on ammonia emissions from five pairs of swine rooms in a wean-to-finish experiment. Environ. Int. 2019, 131, 104931. [Google Scholar] [CrossRef]
- Phillips, V.; Holden, M.; Sneath, R.; Short, J.; White, R.; Hartung, J.; Seedorf, J.; Schröder, M.; Linkert, K.; Pedersen, S.; et al. The Development of Robust Methods for Measuring Concentrations and Emission Rates of Gaseous and Particulate Air Pollutants in Livestock Buildings. J. Agric. Eng. Res. 1998, 70, 11–24. [Google Scholar] [CrossRef]
- Kim, K.Y.; Ko, H.J.; Kim, H.T.; Kim, Y.S.; Roh, Y.M.; Lee, C.M.; Kim, C.N. Quantification of ammonia and hydrogen sulfide emitted from pig buildings in Korea. J. Environ. Manag. 2008, 88, 195–202. [Google Scholar] [CrossRef]
- Hayes, E.; Curran, T.; Dodd, V. Odour and ammonia emissions from intensive pig units in Ireland. Bioresour. Technol. 2006, 97, 940–948. [Google Scholar] [CrossRef] [Green Version]
- Vranken, E.; Claes, S.; Hendriks, J.; Darius, P.; Berckmans, D. Intermittent Measurements to determine Ammonia Emissions from Livestock Buildings. Biosyst. Eng. 2004, 88, 351–358. [Google Scholar] [CrossRef]





| NH3 Monitoring System | Experiment Duration | Sampling Frequency (Records per Hour) | Statistical Analysis | Reference |
|---|---|---|---|---|
| Innova 1312 | 37 days | 6 | Total mean, standard deviation | [31] |
| Air sampling pump Gilian Instrument 7lG9 | 8 h | 1 | Total mean, min. and max. values | [36] |
| Innova 1412 | N/A 1 | N/A 1 | Total mean, min. and max. value | [27] |
| Innova 1312 | 20 h | 1.18 | Mean of individual measurement, standard deviation | [29,30] |
| Innova 1412 | 24 h | N/A1 | Total mean, standard deviation | [28] |
| N/A 1 | 345 days | 5 | Determination of factors to make predictions | [38] |
| NOx analyzer with a thermal converter | 24 h | 8.6 | Total mean, hourly means, day and night means, min. and max. values | [35] |
| Innova 1412 | 155 days | 6 | Daily means | [32] |
| Innova 1412 | 24 h | 2 | Daily means | [33] |
| Innova 1412 | 146–154 days | 5 | Daily means | [34] |
| iTX Multi-gas monitor | 43–165 days | 12 | Daily means | [37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kriz, P.; Kunes, R.; Smutny, L.; Cerny, P.; Havelka, Z.; Olsan, P.; Xiao, M.; Stehlik, R.; Dolan, A.; Bartos, P. Methodology for Measurement of Ammonia Emissions from Intensive Pig Farming. Agriculture 2021, 11, 1073. https://doi.org/10.3390/agriculture11111073
Kriz P, Kunes R, Smutny L, Cerny P, Havelka Z, Olsan P, Xiao M, Stehlik R, Dolan A, Bartos P. Methodology for Measurement of Ammonia Emissions from Intensive Pig Farming. Agriculture. 2021; 11(11):1073. https://doi.org/10.3390/agriculture11111073
Chicago/Turabian StyleKriz, Pavel, Radim Kunes, Lubos Smutny, Pavel Cerny, Zbynek Havelka, Pavel Olsan, Maohua Xiao, Radim Stehlik, Antonin Dolan, and Petr Bartos. 2021. "Methodology for Measurement of Ammonia Emissions from Intensive Pig Farming" Agriculture 11, no. 11: 1073. https://doi.org/10.3390/agriculture11111073
APA StyleKriz, P., Kunes, R., Smutny, L., Cerny, P., Havelka, Z., Olsan, P., Xiao, M., Stehlik, R., Dolan, A., & Bartos, P. (2021). Methodology for Measurement of Ammonia Emissions from Intensive Pig Farming. Agriculture, 11(11), 1073. https://doi.org/10.3390/agriculture11111073











