Effects of Leaf Cutting on Fusarium Head Blight Disease Development, Photosynthesis Parameters and Yield of Wheat under F. graminearum Inoculation Condition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Cultivation
2.2. Leaf Cutting Treatment and Host FHB Resistance Screening
2.3. Measurement of Photosynthesis Parameters
2.4. Measurement of Yield Components and the Percent of Fusarium-Damaged Kernels
2.5. Statistical Analysis
3. Results
3.1. FHB Resistance of Wheat under Various Leaf Cutting Treatments
3.2. Effects of FHB on Photosynthesis Parameters under Various Leaf Cutting Treatments
3.3. Effects of FHB on Yield Components
| Year | Genotype | Treatments | N | NKS | TGW | GWS | PFDK |
|---|---|---|---|---|---|---|---|
| 2019 greenhouse | L661 | No leaf cutting | 29 | 26.3 ± 1.3 a C | 4.72 ± 0.43 b B | 0.13 ± 0.02 c C | 0.77 ± 0.05 a A |
| Flag leaf remains | 27 | 25.4 ± 1.2 a b B | 5.06 ± 0.39 b B | 0.13 ± 0.01 c B | 0.75 ± 0.06 a A | ||
| Cutting all leaves | 27 | 25.5 ± 1.5 a b C | 5.90 ± 0.67 b B | 0.15 ± 0.02 b c C | 0.67 ± 0.05 a A | ||
| L693 | No leaf cutting | 22 | 21.8 ± 2.2 b c B | 14.13 ± 3.17 a C | 0.31 ± 0.06 a B | 0.44 ± 0.30 b A | |
| Flag leaf remains | 24 | 21.0 ± 1.3 c B | 11.29 ± 1.64 a B | 0.24 ± 0.04 a b B | 0.46 ± 0.04 b A | ||
| Cutting all leaves | 26 | 20.0 ± 1.6 c B | 11.67 ± 1.30 a B | 0.25 ± 0.04 a B | 0.32 ± 0.07 b A | ||
| 2020 greenhouse | L661 | No leaf cutting | 29 | 16.0 ± 1.0 a D | 5.06 ± 0.42 c B | 0.08 ± 0.01 b C | 0.68 ± 0.04 a A |
| Flag leaf remains | 28 | 15.6 ± 1.1 a C | 4.98 ± 0.50 c B | 0.08 ± 0.01 b B | 0.66 ± 0.06 a A | ||
| Cutting all leaves | 31 | 10.8 ± 0.9 b D | 7.56 ± 1.48 b B | 0.06 ± 0.01 b C | 0.64 ± 0.06 a A | ||
| L693 | No leaf cutting | 27 | 16.9 ± 0.8 a B | 9.26 ± 1.35 a b B | 0.16 ± 0.03 a B | 0.52 ± 0.04 b A | |
| Flag leaf remains | 29 | 16.7 ± 1.0 a B | 10.59 ± 1.21 a b B | 0.19 ± 0.03 a B | 0.45 ± 0.04 b A | ||
| Cutting all leaves | 25 | 15.9 ± 0.8 a B | 11.99 ± 1.43 a B | 0.18 ± 0.02 a B | 0.40 ± 0.05 b A | ||
| 2021 field group 1 | L661 | No leaf cutting | 24 | 31.5 ± 2.7 c B | 25.61 ± 2.76 b A | 0.83 ± 0.13 c B | 0.30 ± 0.04 a B |
| Flag leaf remains | 19 | 32.5 ± 2.8 c A | 29.18 ± 2.80 b A | 0.98 ± 0.14 c A | 0.22 ± 0.04 b B | ||
| Cutting all leaves | 31 | 33.3 ± 2.0 c B | 26.62 ± 1.19 b A | 0.90 ± 0.07 c B | 0.11 ± 0.01 c B | ||
| L693 | No leaf cutting | 24 | 47.8 ± 2.2 a A | 49.14 ± 1.60 a A | 2.35 ± 0.14 a A | 0.05 ± 0.01 c B | |
| Flag leaf remains | 14 | 44.2 ± 3.4 a b A | 43.75 ± 2.99 a A | 1.94 ± 0.20 b A | 0.08 ± 0.03 c B | ||
| Cutting all leaves | 23 | 40.8 ± 1.6 b A | 44.53 ± 1.79 a A | 1.83 ± 0.10 b A | 0.05 ± 0.01 c B | ||
| 2021 field group 2 | L661 | No leaf cutting | 20 | 38.05 ± 3.02 c A | 29.29 ± 2.25 b A | 1.13 ± 0.13 c A | 0.24 ± 0.02 a b B |
| Flag leaf remains | 22 | 35.41 ± 2.99 d A | 31.21 ± 2.28 b A | 1.14 ± 0.14 c A | 0.26 ± 0.03 a B | ||
| Cutting all leaves | 23 | 39.96 ± 1.78 b c A | 29.61 ± 1.69 b A | 1.20 ± 0.10 c A | 0.2 ± 0.03 b B | ||
| L693 | No leaf cutting | 26 | 47.19 ± 1.72 a A | 51.17 ± 1.43 a A | 2.42 ± 0.11 a A | 0.08 ± 0.01 c B | |
| Flag leaf remains | 26 | 44.54 ± 2.24 a b A | 48.66 ± 1.49 a A | 2.16 ± 0.12 a b A | 0.06 ± 0.01 c B | ||
| Cutting all leaves | 16 | 43.44 ± 1.87 a b c A | 43.77 ± 2.05 a A | 1.90 ± 0.12 b A | 0.03 ± 0.00 c B |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bai, G.; Shaner, G. Management and resistance in wheat and barley to Fusarium head blight. Annu. Rev. Phytopathol. 2004, 42, 135–161. [Google Scholar] [CrossRef]
- Li, X.; Xiang, Z.; Chen, W.; Huang, Q.; Liu, T.; Li, Q.; Zhong, S.; Zhang, M.; Guo, J.; Lei, L. Reevaluation of Two Quantitative Trait Loci for Type II Resistance to Fusarium Head Blight in Wheat Germplasm PI 672538. Phytopathology 2017, 107, 92–99. [Google Scholar] [CrossRef] [Green Version]
- Sobrova, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and its toxicity. Interdiscip. Toxicol. 2010, 3, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Zwart, R.S.; Muylle, H.; Van Bockstaele, E.; Roldán-Ruiz, I. Evaluation of genetic diversity of Fusarium head blight resistance in European winter wheat. Theor. Appl. Genet. 2008, 117, 813–828. [Google Scholar] [CrossRef] [Green Version]
- Hill-Ambroz, K.; Webb, C.A.; Matthews, A.R.; Li, W.; Gill, B.S.; Fellers, J.P. Expression Analysis and Physical Mapping of a cDNA Library of Fusarium Head Blight Infected Wheat Spikes. Crop Sci. 2006, 46, S15. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.B.; Zhang, H.J.; Duan, G.Q.; Jing, X.W.; Chen, Z. Comparison of photosynthetic physiological and anatomical features of gene and virus controlled silver leaf mottling of summer squash. Chin. J. Eco-Agric. 2007, 15, 123–125. [Google Scholar]
- Stone, J.K.; Hood, I.A.; Watt, M.S.; Kerrigan, J.L. Distribution of Swiss needle cast in New Zealand in relation to winter temperature. Australas. Plant Pathol. 2007, 36, 445–454. [Google Scholar] [CrossRef]
- Moriondo, M.; Orlandini, S.; Giuntoli, A.; Bindi, M. The Effect of Downy and Powdery Mildew on Grapevine (Vitis vinifera L.) Leaf Gas Exchange. J. Phytopathol. 2005, 153, 350–357. [Google Scholar] [CrossRef]
- Dallagnol, L.J.; Rodrigues, F.A.; Martins, S.C.V.; Cavatte, P.C.; DaMatta, F.M. Alterations on rice leaf physiology during infection byBipolaris oryzae. Australas. Plant Pathol. 2011, 40, 360–365. [Google Scholar] [CrossRef]
- Rooney, J.M.; Hoad, G.V. Compensation in Growth and Photosynthesis of Wheat (Triticum aestivum L.) Following Early Inoculations with Septoria nodorum (Berk.) Berk. New Phytol. 1989, 113, 513–521. [Google Scholar] [CrossRef]
- Sturm, A.; Tang, G.-Q. The sucrose-cleaving enzymes of plants are crucial for development, growth and carbon partitioning. Trends Plant Sci. 1999, 4, 401–407. [Google Scholar] [CrossRef]
- Luo, P.G.; Luo, H.; Chang, Z.; Zhang, H.; Zhang, M.; Ren, Z. Characterization and chromosomal location of Pm40 in common wheat: A new gene for resistance to powdery mildew derived from Elytrigia intermedium. Theor. Appl. Genet. 2009, 118, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.G.; Ren, Z.L.; Zhang, H.Q.; Zhang, H.Y. Identification, Chromosome Location, and Diagnostic Markers for a New Gene (YrCN19) for Resistance to Wheat Stripe Rust. Phytopathology 2005, 95, 1266–1270. [Google Scholar] [CrossRef]
- Li, X.; Zhong, S.; Chen, W.; Fatima, S.A.; Huang, Q.; Li, Q.; Tan, F.; Luo, P. Transcriptome Analysis Identifies a 140 kb Region of Chromosome 3B Containing Genes Specific to Fusarium Head Blight Resistance in Wheat. Int. J. Mol. Sci. 2018, 19, 852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, X.; Li, H.; Jia, Q.; Feng, H.; Li, M.; Liang, H. Influence of wheat (Triticum aestivum L.) stripe rust infection on photosynthetic function and expression protein D1 of wheat leaves. Acta Ecol. Sin. 2008, 28, 669–676. [Google Scholar]
- Li, X.; Liu, T.; Chen, W.; Zhong, S.; Zhang, H.; Tang, Z.; Chang, Z.; Wang, L.; Zhang, M.; Li, L. Wheat WCBP1 encodes a putative copper-binding protein involved in stripe rust resistance and inhibition of leaf senescence. BMC Plant Biol. 2015, 15, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Zhong, S.; Liu, N.; Chen, W.; Liu, T.; Li, X.; Zhang, M.; Ren, Z.; Yang, J.; Luo, P. Gene expression profile and physiological and biochemical characterization of hexaploid wheat inoculated with Blumeria graminis f. sp. tritici. Physiol. Mol. Plant Pathol. 2015, 90, 39–48. [Google Scholar] [CrossRef]
- Yang, S.; Li, X.; Chen, W.; Liu, T.; Zhong, S.; Ma, L.; Zhang, M.; Zhang, H.; Yu, D.; Luo, P. Wheat resistance to fusarium head blight is associated with changes in photosynthetic parameters. Plant Dis. 2016, 100, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Li, X.; Chen, W.; Xiang, Z.; Zhong, S.; Chang, Z.; Zhang, M.; Zhang, H.; Tan, F.; Ren, Z. Genetic mapping of a putative Thinopyrum intermedium-derived stripe rust resistance gene on wheat chromosome 1B. Theor. Appl. Genet. 2014, 127, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xu, M.; Xiang, Z.; Li, X.; Chen, W.; Luo, P. Registration of the Novel Wheat Lines L658, L693, L696, and L699, with Resistance to Fusarium Head Blight, Stripe Rust, and Powdery Mildew. J. Plant Regist. 2015, 9, 121–124. [Google Scholar] [CrossRef]
- Gamm, M.; Héloir, M.-C.; Bligny, R.; Vaillant-Gaveau, N.; Trouvelot, S.; Alcaraz, G.; Frettinger, P.; Clément, C.; Pugin, A.; Wendehenne, D. Changes in carbohydrate metabolism in Plasmopara viticola-infected grapevine leaves. Mol. Plant-Microbe Interact. 2011, 24, 1061–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, C.; Wang, Y.; Zhu, Y.; Tang, J.; Hu, B.; Liu, L.; Ou, S.; Wu, H.; Sun, X.; Chu, J. OsNAP connects abscisic acid and leaf senescence by fine-tuning abscisic acid biosynthesis and directly targeting senescence-associated genes in rice. Proc. Natl. Acad. Sci. USA 2014, 111, 10013–10018. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.-M.; Lo, S.-F.; Ho, T.-H.D. Source–sink communication: Regulated by hormone, nutrient, and stress cross-signaling. Trends Plant Sci. 2015, 20, 844–857. [Google Scholar] [CrossRef]
- Chang, T.-G.; Zhu, X.-G. Source–sink interaction: A century old concept under the light of modern molecular systems biology. J. Exp. Bot. 2017, 68, 4417–4431. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.-H.; Xiu, W.; Chen, B.-C.; Jie, G. Effects of low light on agronomic and physiological characteristics of rice including grain yield and quality. Rice Sci. 2014, 21, 243–251. [Google Scholar] [CrossRef]
- Abdelrahman, M.; Burritt, D.J.; Gupta, A.; Tsujimoto, H.; Tran, L.-S.P. Heat stress effects on source–sink relationships and metabolome dynamics in wheat. J. Exp. Bot. 2019, 71, 543–554. [Google Scholar] [CrossRef]
- Luo, P.; Ren, Z.; Wu, X.; Zhang, H.; Zhang, H.; Feng, J. Structural and biochemical mechanism responsible for the stay-green phenotype in common wheat. Chin. Sci. Bull. 2006, 51, 2595–2603. [Google Scholar] [CrossRef]
- Luo, P.; Deng, K.; Hu, X.; Li, L.; Li, X.; Chen, J.; Zhang, H.; Tang, Z.; Zhang, Y.; Sun, Q. Chloroplast ultrastructure regeneration with protection of photosystem II is responsible for the functional ‘stay-green’ trait in wheat. Plant Cell Environ. 2013, 36, 683–696. [Google Scholar] [CrossRef]
- Thomas, H.; Howarth, C.J. Five ways to stay green. J. Exp. Bot. 2000, 51, 329–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, W.; Prithiviraj, B.; Smith, D.L. Photosynthetic responses of corn and soybean to foliar application of salicylates. J. Plant Physiol. 2003, 160, 485–492. [Google Scholar] [CrossRef]
- Lorenzini, G.; Guidi, L.; Nali, C.; Ciompi, S.; Soldatini, G.F. Photosynthetic response of tomato plants to vascular wilt diseases. Plant Sci. 1997, 124, 143–152. [Google Scholar] [CrossRef]
- Lamb, C.J.; Ryals, J.A.; Ward, E.R.; Dixon, R.A. Emerging Strategies for Enhancing Crop Resistance to Microbial Pathogens. Biotechnology 1992, 10, 1436–1445. [Google Scholar] [PubMed]
- Tao, Y.; Xie, Z.; Chen, W.; Glazebrook, J.; Katagiri, F. Quantitative Nature of Arabidopsis Responses during Compatible and Incompatible Interactions with the Bacterial Pathogen. Plant Cell 2003, 15, 317–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modrá, H.; Svobodová, Z. Incidence of animal poisoning cases in the Czech Republic: Current situation. Interdiscip. Toxicol. 2009, 2, 48–51. [Google Scholar] [CrossRef]
- Liu, W.-D.; Yin, J.; Zhu, G.-J. Effects of Leaf Removal on Dry Matter Accumulation and Grain Yield in Different Spike-type Wheat Varieties. Sci. Agric. Sin. 2007, 40, 1353–1360. [Google Scholar]
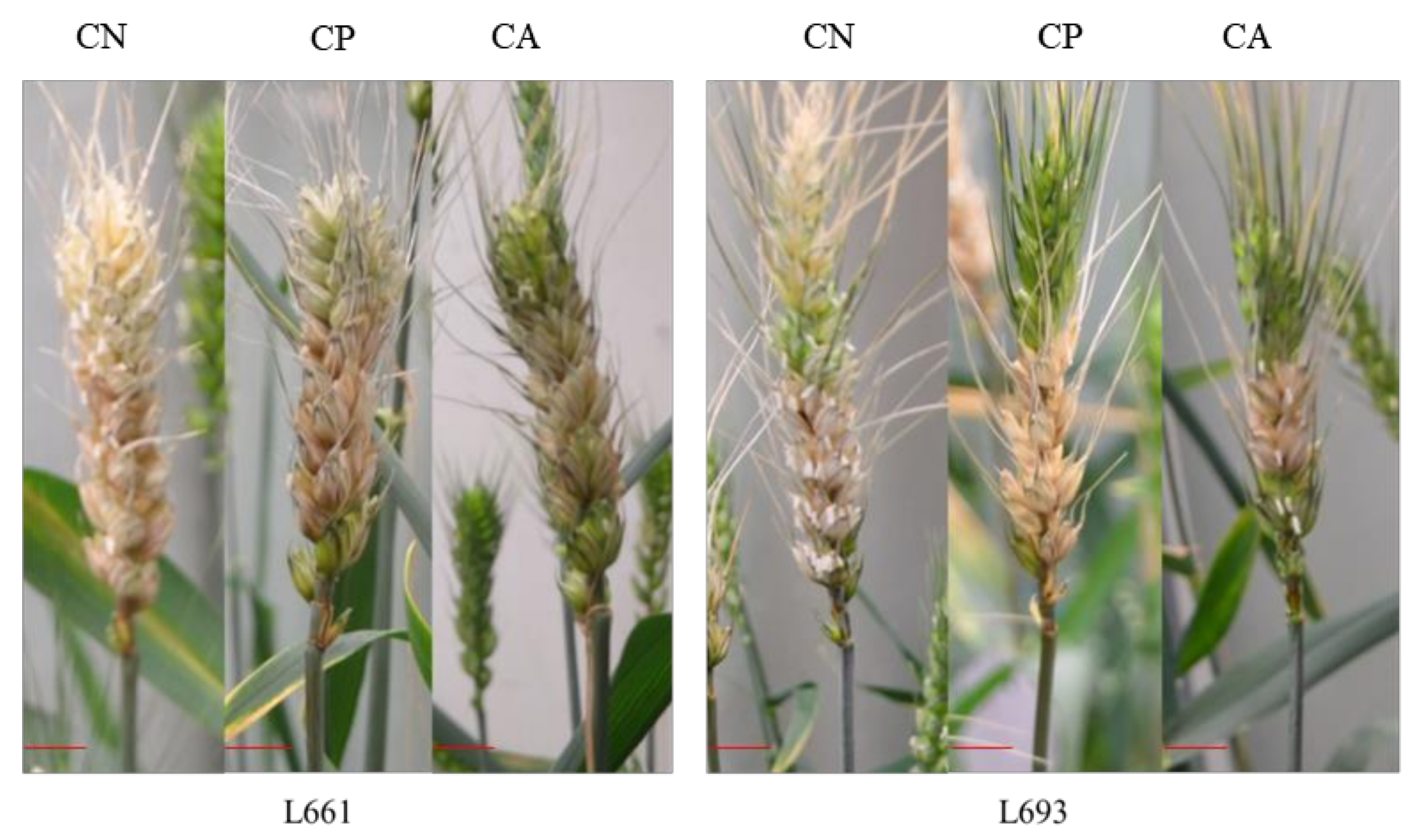
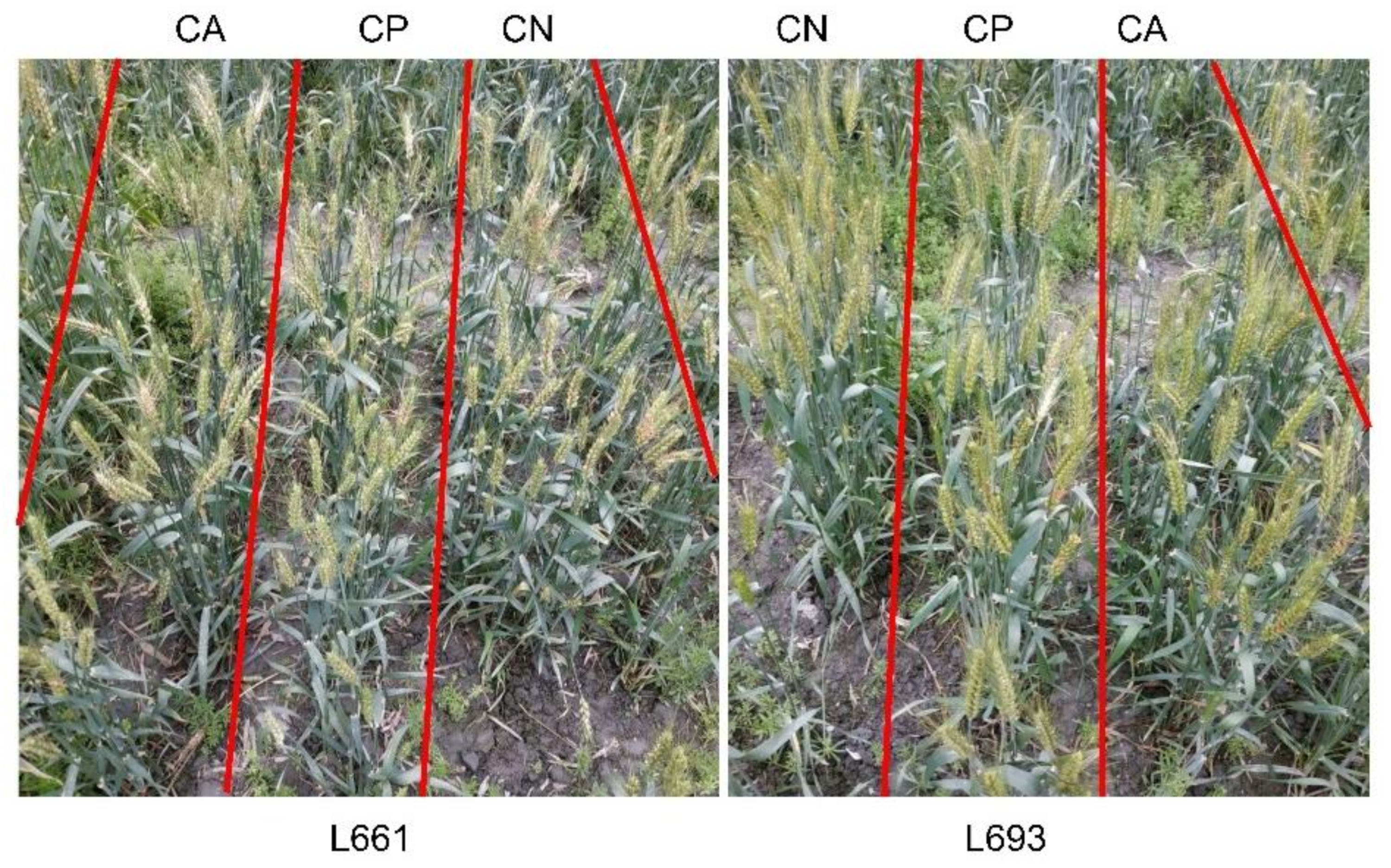

| Year | Genotype | Treatments | N | NDS1 | NDS2 |
|---|---|---|---|---|---|
| 2019 greenhouse | L661 | No leaf cutting | 29 | 6.34 ± 0.35 a A | - |
| Flag leaf remains | 27 | 6.07 ± 0.42 a A | - | ||
| Cutting all leaves | 27 | 4.92 ± 0.34 b A | - | ||
| L693 | No leaf cutting | 22 | 4.00 ± 0.42 b A | - | |
| Flag leaf remains | 24 | 3.42 ± 0.31 b A | - | ||
| Cutting all leaves | 26 | 3.50 ± 0.33 b A | - | ||
| 2020 greenhouse | L661 | No leaf cutting | 29 | 6.24 ± 0.24 a A | 7.93 ± 0.28 a A |
| Flag leaf remains | 28 | 5.61 ± 0.24 a b A | 7.25 ± 0.26 a b A | ||
| Cutting all leaves | 31 | 5.55 ± 0.23 b A | 7.03 ± 0.22 b A | ||
| L693 | No leaf cutting | 27 | 3.56 ± 0.28 c A | 5.96 ± 0.33 c A | |
| Flag leaf remains | 29 | 3.66 ± 0.23 c A | 5.48 ± 0.35 c d A | ||
| Cutting all leaves | 25 | 3.04 ± 0.20 c A | 4.83 ± 0.41 d A | ||
| 2021 field group 1 | L661 | No leaf cutting | 24 | 3.70 ± 0.30 a B | 5.15 ± 0.62 a B |
| Flag leaf remains | 19 | 2.56 ± 0.20 b c B | 3.89 ± 0.42 b B | ||
| Cutting all leaves | 31 | 2.81 ± 0.18 b B | 3.76 ± 0.31 b B | ||
| L693 | No leaf cutting | 24 | 2.17 ± 0.10 c B | 2.24 ± 0.12 c B | |
| Flag leaf remains | 14 | 2.21 ± 0.11 bc B | 2.50 ± 0.18 c B | ||
| Cutting all leaves | 23 | 2.00 ± 0.00 c B | 2.18 ± 0.11 c B | ||
| 2021 field group 2 | L661 | No leaf cutting | 20 | 3.42 ± 0.27 a B | 4.17 ± 0.38 a B |
| Flag leaf remains | 22 | 2.95 ± 0.23 a b B | 3.87 ± 0.35 a B | ||
| Cutting all leaves | 23 | 2.80 ± 0.24 b B | 3.17 ± 0.23 b B | ||
| L693 | No leaf cutting | 26 | 2.19 ± 0.19 c B | 2.16 ± 0.10 c B | |
| Flag leaf remains | 26 | 2.09 ± 0.09 c B | 2.12 ± 0.09 c B | ||
| Cutting all leaves | 16 | 2.03 ± 0.03 c B | 2.06 ± 0.06 c B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Q.; Luo, P. Effects of Leaf Cutting on Fusarium Head Blight Disease Development, Photosynthesis Parameters and Yield of Wheat under F. graminearum Inoculation Condition. Agriculture 2021, 11, 1065. https://doi.org/10.3390/agriculture11111065
Huang Q, Luo P. Effects of Leaf Cutting on Fusarium Head Blight Disease Development, Photosynthesis Parameters and Yield of Wheat under F. graminearum Inoculation Condition. Agriculture. 2021; 11(11):1065. https://doi.org/10.3390/agriculture11111065
Chicago/Turabian StyleHuang, Qianglan, and Peigao Luo. 2021. "Effects of Leaf Cutting on Fusarium Head Blight Disease Development, Photosynthesis Parameters and Yield of Wheat under F. graminearum Inoculation Condition" Agriculture 11, no. 11: 1065. https://doi.org/10.3390/agriculture11111065
APA StyleHuang, Q., & Luo, P. (2021). Effects of Leaf Cutting on Fusarium Head Blight Disease Development, Photosynthesis Parameters and Yield of Wheat under F. graminearum Inoculation Condition. Agriculture, 11(11), 1065. https://doi.org/10.3390/agriculture11111065






