The Effects of Different Levels of Sunflower Hulls on Reproductive Performance of Yearly Ewes Fed with Pelleted Complete Diets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals Management
2.2. Experimental Design and Diets
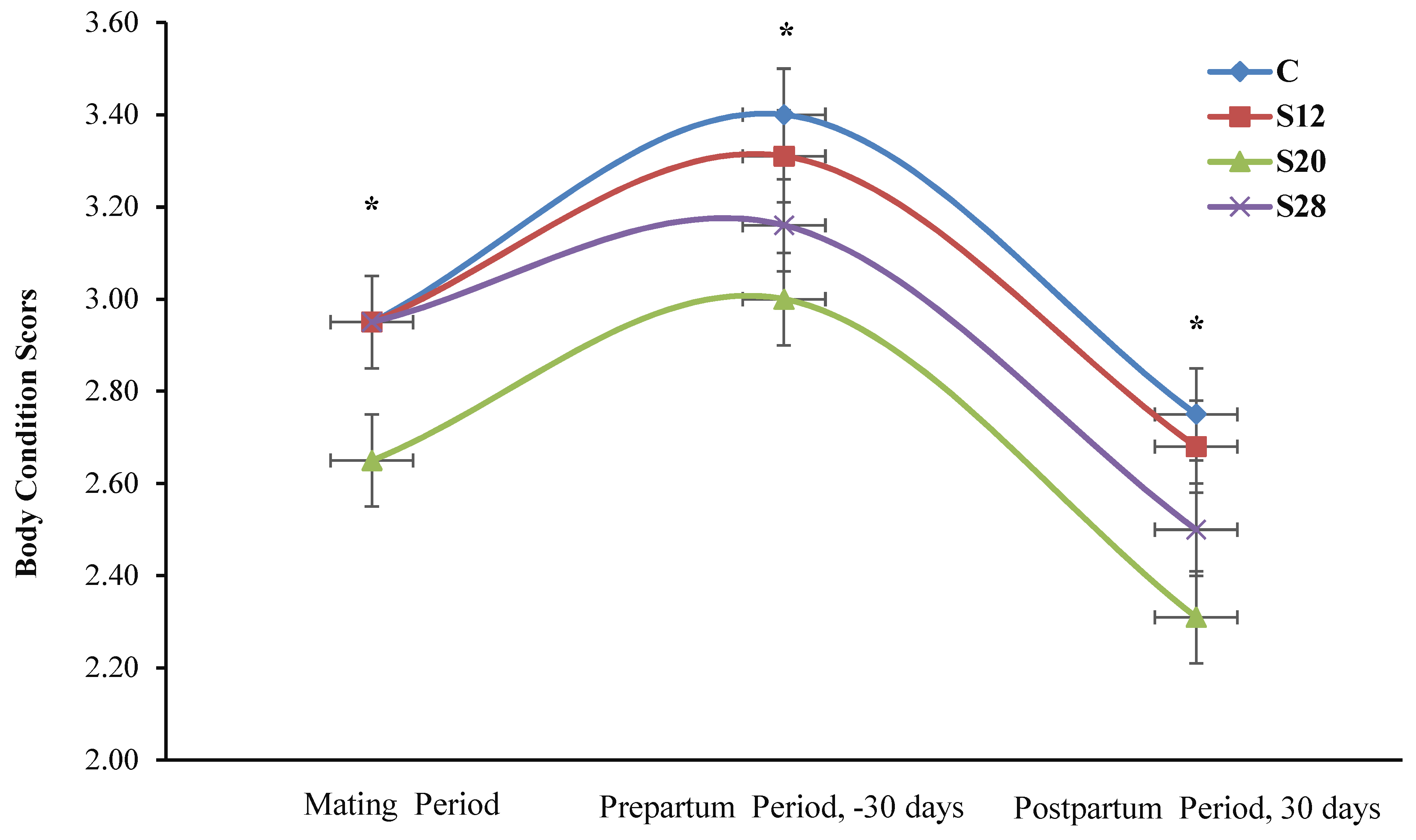
2.3. Feed Intake, Body Weight and Body Condition Score
2.4. Reproductive Performance
2.5. Blood Samples Collection and Analysis
2.6. Statistical Analysis
3. Results
3.1. Diet Composition
3.2. The Effects of Treatment on the General Performance of Ewes
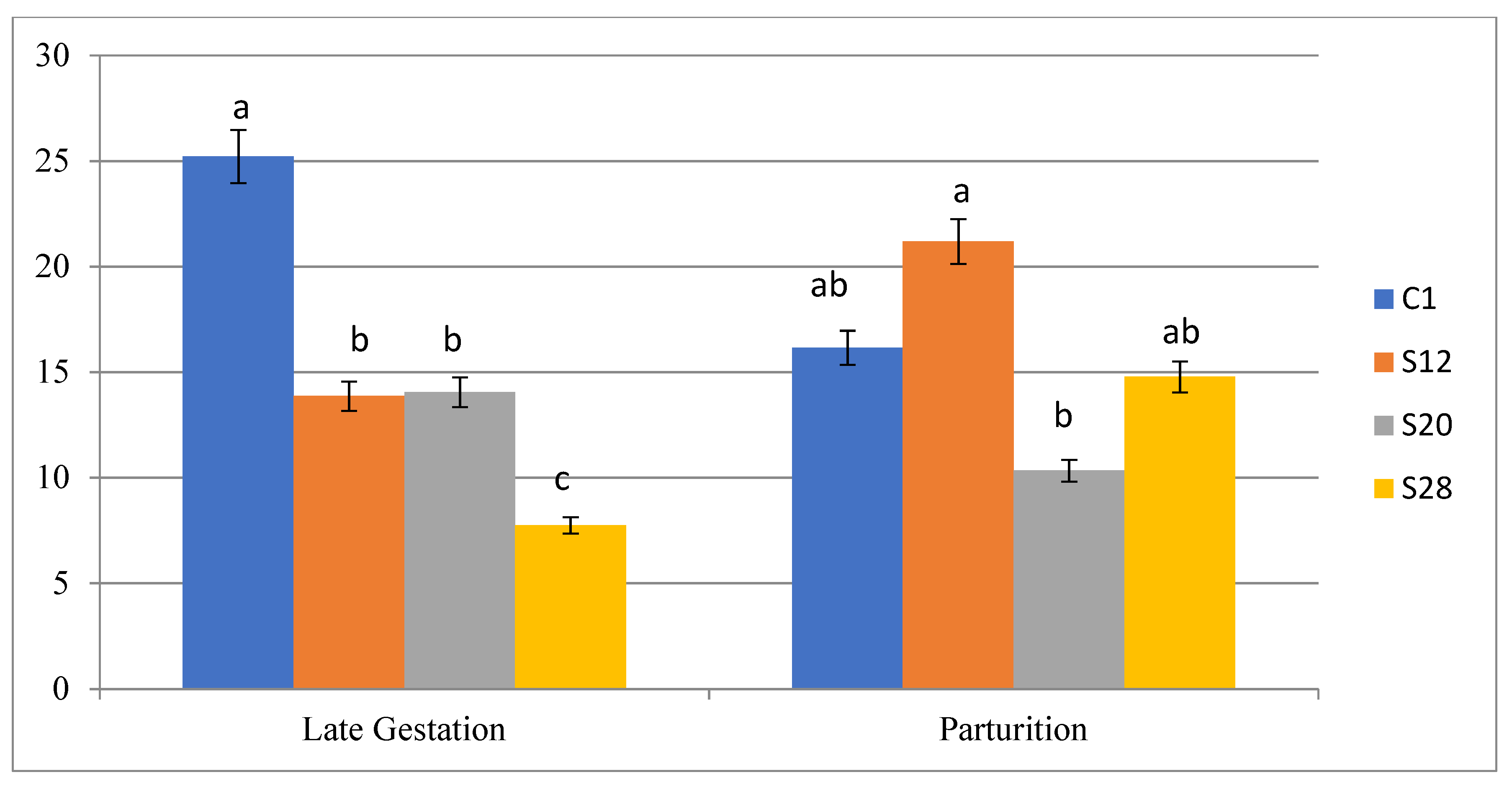
3.3. The Effects of Treatment on Some Blood Parameters of Ewes
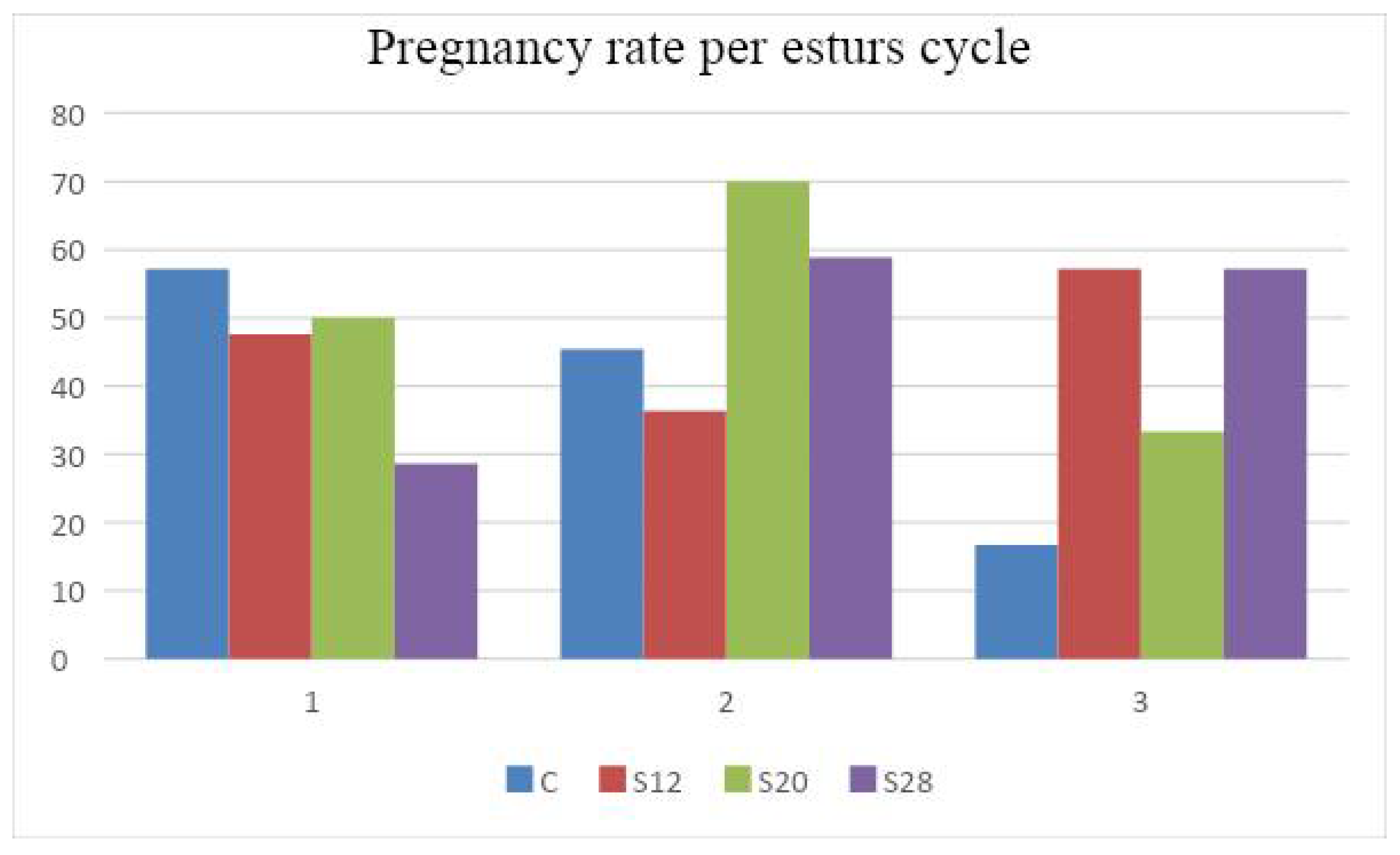
3.4. The Effects of Treatments on the Reproductive Performance of Pregnant Awassi Ewe
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wirsenius, S.; Azar, C.; Berndes, G. How much land is needed for global food production under scenarios of dietary changes and livestock productivity increases in 2030? Agric. Syst. 2010, 103, 621–638. [Google Scholar] [CrossRef]
- Islam, R.; Redoy, M.; Shuvo, A.; Sarker, M.; Akbar, M.; Al-Mamun, M. Effect of pellet from total mixed ration on growth performance, blood metabolomics, carcass and meat characteristics of Bangladeshi garole sheep. Progress. Agric. 2017, 28, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Alhidary, I.A.; Abdelrahman, M.M.; Alyemni, A.H.; Khan, R.U.; Al-Saiady, M.Y.; Amran, R.A.; Alshamiry, F.A. Effect of alfalfa hay on growth performance, carcass characteristics, and meat quality of growing lambs with ad libitum access to total mixed rations. Rev. Bras. Zootec. 2016, 45, 302–308. [Google Scholar] [CrossRef]
- FAO. Statistical Yearbook; FAO: Rome, Italy, 2014; Volume 66. [Google Scholar]
- Rumball, C.W.; Harding, J.E.; Oliver, M.H.; Bloomfield, F.H. Effects of twin pregnancy and periconceptional undernutrition on maternal metabolism, fetal growth and glucose-insulin axis function in ovine pregnancy. J. Physiol. 2008, 586, 1399–1411. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, M.M.; Alhidary, I.; Alyemni, A.H.; Khan, R.U.; Bello, A.R.; Al-Saiady, M.Y.; Amran, R.A. Effect of alfalfa hay on rumen fermentation patterns and serum biochemical profile of growing Awassi lambs with ad libitum access to total mixed rations. Pak. J. Zool. 2017, 49, 1519–1522. [Google Scholar] [CrossRef]
- Sewalt, V.J.; Oliveira, W.D.; Glasser, W.G.; Fontenot, J.P. Lignin impact on fiber degradation: 2. A Model Study Using Cellulosic Hydrogels. J. Sci. Food Agric. 1997, 71, 204–208. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids; National Research Council: Ottawa, ON, Canada, 2007. [Google Scholar]
- Santucci, P.M.; Maestrini, O. Body conditions of dairy goats in extensive systems of production: Method of estimation. In Annales de Zootechnie; Institut National de la Recherche Agronomique: Paris, France, 1985; pp. 473–474. [Google Scholar]
- Donovan, J.; Brown, P. Removal of blood from laboratory mammals and birds: First report of the BVA/FRAME/RSPCA/UFAW Joint working group on refinement. Lab Anim. 1993, 27, 1–22. [Google Scholar]
- Holtgrew-Bohling, K. Large Animal Clinical Procedures for Veterinary Technicians, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- SAS Institute. SAS User’s Guide, 8th ed.; SAS Institute: Cary, NC, USA, 2003. [Google Scholar]
- Reed, K.F.M. Fertility of herbivores consuming phytoestrogen-containing Medicago and Trifolium species. Agriculture 2016, 6, 35. [Google Scholar] [CrossRef] [Green Version]
- Mostrom, M.; Evans, T.J. Phytoestrogens. In Reproductive and Developmental Toxicology; Academic Press: Cambridge, MA, USA, 2011; pp. 707–722. [Google Scholar]
- Chaturvedi, O.H.; Bhatt, R.S.; Sahoo, A. Nutrient utilization in grazing ewes supplemented with complete feed blocks during scarcity in semi-arid region. Indian J. Small Rumin. 2014, 20, 114–117. [Google Scholar]
- Allen, M.; Oba, M. Fiber digestibility of forages. In Proceedings of the 57th Minnesota Nutrition Conference, St. Paul, MN, USA, 23–25 September 1996; p. 151. [Google Scholar]
- Bonawitz, N.D.; Chapple, C. The genetics of lignin biosynthesis: Connecting genotype to phenotype. In Annual Review of Genetics; Annual Reviews: San Mateo, CA, USA, 2010; Volume 44, pp. 337–363. [Google Scholar]
- Li, S.; Chen, G. Thermogravimetric, thermochemical, and infrared spectral characterization of feedstocks and biochar derived at different pyrolysis temperatures. Waste Manag. 2018, 78, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.G.; Mertens, D.R.; Payne, A.J. Correlation of acid detergent lignin and Klason lignin with digestibility of forage dry matter and neutral detergent fiber. J. Dairy Sci. 1997, 80, 1622–1628. [Google Scholar] [CrossRef]
- Oliver, A.L.; Grant, R.J.; Pedersen, J.F.; O’Rear, J. Comparison of brown midrib-6 and -18 forage sorghum with conventional sorghum and corn silage in diets of lactating dairy cows. J. Dairy Sci. 2004, 87, 637–644. [Google Scholar] [CrossRef] [Green Version]
- Vargas-Bello-Pérez, E.; Mustafa, A.F.; Seguin, P. Effects of feeding forage soybean silage on milk production, nutrient digestion, and ruminal fermentation of lactating dairy cows. J. Dairy Sci. 2008, 91, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Harper, K.J.; Barber, D.G.; Callow, M.; McNeill, D.M.; Poppi, D.P. Assessment of the Indf of Subtropical Pastures. Anim. Prod. Aust. 2014, 30, 320. [Google Scholar]
- Ingvartsen, K.L.; Munksgaard, L.; Nielsen, V.K.M.; Pedersen, L.J. Responses to repeated deprivation of lying down on feed intake, performance and blood hormone concentration in geoting bulls. Acta Agric. Scan. 2006, 49, 260–265. [Google Scholar]
| Ingredient (%) | Treatment * | |||
|---|---|---|---|---|
| C | S12 | S20 | S28 | |
| Barley grain | 32.56 | 20.41 | 22.21 | 29.20 |
| Palm kernel meal | 20.00 | 20.00 | 20.00 | 9.25 |
| Wheat straw | 16.30 | 16.33 | 6.51 | |
| Sunflower meal | 13.80 | 13.84 | 13.71 | 16.03 |
| Sunflower hulls | 0.00 | 12.00 | 20.00 | 28.00 |
| Wheat bran | 10.00 | 10.00 | 10.00 | 10.00 |
| Molasses | 5.00 | 5.00 | 5.00 | 5.00 |
| Acid buffer | 0.8 | 0.8 | 0.8 | 0.8 |
| Limestone | 0.72 | 0.74 | 0.87 | 0.90 |
| Salt | 0.52 | 0.56 | 0.57 | 0.49 |
| Vitamin and mineral premix | 0.30 | 0.30 | 0.30 | 0.30 |
| Chemical composition | ||||
| Dry matter (%) | 90.39 | 88.47 | 88.74 | 88.54 |
| Protein (%) | 14.6 | 15.7 | 13.3 | 14.1 |
| Fiber (%) | 18.26 | 20.78 | 21.81 | 22.16 |
| Fat (%) | 4.02 | 4.35 | 4.00 | 4.35 |
| Cellulose (%) | 19.77 | 21.35 | 18.51 | 21.06 |
| Hemicellulose (%) | 6.96 | 8.49 | 9.13 | 10.87 |
| Gross energy (kcal/kg) | 3641 | 3613 | 3744 | 3710 |
| Acid detergent fiber (%) | 28.46 | 30.25 | 29.55 | 30.66 |
| Neutral detergent fiber (%) | 37.59 | 38.74 | 36.50 | 41.52 |
| Lignin (%) | 7.37 | 6.99 | 8.88 | 9.07 |
| Reproduction Stages | |||||||
|---|---|---|---|---|---|---|---|
| Treatments | Mating Period | Late Gestation | Parturition Day | 30 Days Postpartum | 60 Days Postpartum | SEM | p-Value |
| C | 1.29 B | 1.13 aC | 1.91 aA | 1.75 A | 1.46 AB | 0.18 | 0.01 |
| S12 | 1.35 B | 0.53 bC | 1.57 bAB | 1.43 A | 1.38 AB | 0.17 | 0.03 |
| S20 | 1.38 | 0.90 b | 1.56 b | 1.66 | 1.17 | 0.14 | 0.62 |
| S28 | 1.42 | 1.22 a | 1.84 a | 1.75 | 1.41 | 0.13 | 0.44 |
| SEM | 0.04 | 0.86 | 0.06 | 0.06 | 0.21 | ||
| p-value | 0.91 | 0.05 | 0.05 | 0.94 | 0.90 | ||
| Reproduction Stages | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatments | Mating Period | Late Gestation | Parturition Day | 30-Day Postpartum | 60-Day Postpartum | SEM | p-Value | |
| C | 41.23 C | 66.67 A | 61.04 AB | 62.05 AB | 60.90 B | 1.31 | 0.020 | |
| S12 | 40.71 C | 68.85 A | 63.00 AB | 59.11 B | 59.28 B | 1.27 | 0.008 | |
| S20 | 41.47 C | 65.56 A | 60.07 AB | 59.75 AB | 57.71 B | 1.11 | 0.015 | |
| S28 | 41.04 C | 68.025 A | 62.46 B | 59.615 B | 58.68 B | 1.25 | 0.05 | |
| SEM | 0.36 | 1.09 | 1.05 | 1.35 | 1.40 | |||
| p-value | 0.92 | 0.73 | 0.76 | 0.88 | 0.90 | |||
| Reproductive Stages | |||||||
|---|---|---|---|---|---|---|---|
| Treatment | Mating Period | Late Gestation | Parturition Day | 30 Days Postpartum | 60 Days Postpartum | SEM | p-Value |
| C | 35.27 | 39.10 | 48.76 | 38.84 b | 43.01 | 3.58 | 0.19 |
| S12 | 48.12 | 43.85 | 55.02 | 46.99 b | 53.57 | 3.57 | 0.24 |
| S20 | 35.94 C | 47.35 BC | 68.74 A | 74.08 cB | 59.49 B | 4.58 | <0.0001 |
| S28 | 43.24 B | 55.17 B | 83.60 A | 52.12 bB | 55.65 B | 7.15 | 0.0003 |
| SEM | 2.98 | 2.31 | 16.50 | 4.48 | 4.11 | ||
| p-value | 0.27 | 0.10 | 0.11 | 0.02 | 0.55 | ||
| TreatmentItem | C | S12 | S20 | S28 | X2 | p-Value |
|---|---|---|---|---|---|---|
| Number of services per conception | 1.27 ± 0.21 b | 1.60 ± 0.22 ab | 1.50 ± 0.18 ab | 2.00 ± 0.19 a | 0.05 | |
| Pregnancy rate% | 94.44 (17/18) | 94.73 (18/19) | 90.00 (18/20) | 95.23 (20/21) | 0.59 | 0.90 |
| Twining rate% | 5.88 (1/17) | 11.11 (2/18) | 5.56 (1/18) | 5.00 (1/20) | 0.69 | 0.87 |
| Lambing rate% | 65.71 (12/17) | 61.07 (11/18) | 66.63 (12/18) | 69.81 (14/20) | 0.46 | 0.92 |
| Fecundity rate | 83.33 (15/18) | 52.63 (10/19) | 44.82 (13/20) | 80.00 (16/20) | 5.45 | 0.14 |
| Prolificacy rate | 88.23 (15/17) | 55.55 (10/18) | 72.22 (13/18) | 80.00 (16/20) | 5.37 | 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharthi, A.S.; Alobre, M.M.; Abdelrahman, M.M.; Al-Baadani, H.H.; Swelum, A.A.; Khan, R.U.; Alhidary, I.A. The Effects of Different Levels of Sunflower Hulls on Reproductive Performance of Yearly Ewes Fed with Pelleted Complete Diets. Agriculture 2021, 11, 959. https://doi.org/10.3390/agriculture11100959
Alharthi AS, Alobre MM, Abdelrahman MM, Al-Baadani HH, Swelum AA, Khan RU, Alhidary IA. The Effects of Different Levels of Sunflower Hulls on Reproductive Performance of Yearly Ewes Fed with Pelleted Complete Diets. Agriculture. 2021; 11(10):959. https://doi.org/10.3390/agriculture11100959
Chicago/Turabian StyleAlharthi, Abdualrahman S., Mohsen M. Alobre, Mutassim M. Abdelrahman, Hani H. Al-Baadani, Ayman A. Swelum, Rifat Ullah Khan, and Ibrahim A. Alhidary. 2021. "The Effects of Different Levels of Sunflower Hulls on Reproductive Performance of Yearly Ewes Fed with Pelleted Complete Diets" Agriculture 11, no. 10: 959. https://doi.org/10.3390/agriculture11100959
APA StyleAlharthi, A. S., Alobre, M. M., Abdelrahman, M. M., Al-Baadani, H. H., Swelum, A. A., Khan, R. U., & Alhidary, I. A. (2021). The Effects of Different Levels of Sunflower Hulls on Reproductive Performance of Yearly Ewes Fed with Pelleted Complete Diets. Agriculture, 11(10), 959. https://doi.org/10.3390/agriculture11100959









