Root Distribution of Brassica napus and Vicia faba within the Sheath of Root or Earthworm Biopore
Abstract
1. Introduction
- (i)
- The RLD and the share of fine roots (root diameter ≤0.2 mm) is higher in the biopore sheath (0–8 mm) of root type than of worm type biopore,
- (ii)
- there is a higher RLD of oilseed rape in the biopore sheath than of faba bean, and
- (iii)
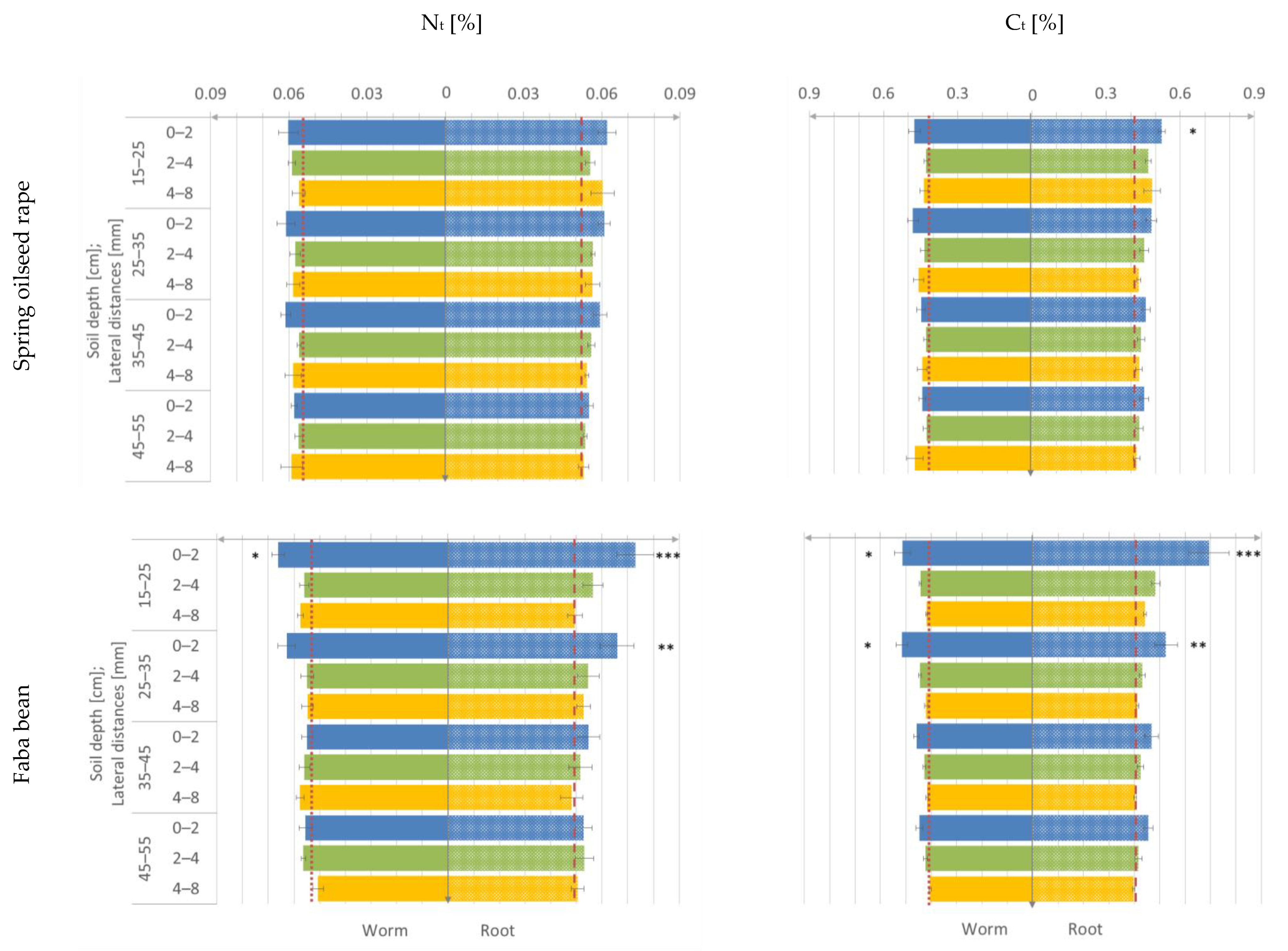
- the decrease of the Nt- and Ct-content is sharper in the worm type than in the root type biopore sheath from biopore surface until 8 mm distance from macropore.
2. Materials and Methods
2.1. Experimental Setup
2.2. Statistical Data Analysis
3. Results
3.1. Root Distribution
3.2. Nt- and Ct-Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Springett, J.; Gray, R. The Interaction between Plant Roots and Earthworm Burrows in Pasture. Soil Biol. Biochem. 1997, 29, 621–625. [Google Scholar] [CrossRef]
- Zangerlé, A.; Pando, A.; Lavelle, P. Do Earthworms and Roots Cooperate to Build Soil Macroaggregates? A Microcosm Experiment. Geoderma 2011, 167–168, 303–309. [Google Scholar] [CrossRef]
- Tiunov, A.V.; Bonkowski, M.; Tiunov, J.A.; Scheu, S. Microflora, Protozoa and Nematoda in Lumbricus terrestris Burrow Walls: A Laboratory Experiment. Pedobiology 2001, 45, 46–60. [Google Scholar] [CrossRef]
- Stewart, J.; Moran, C.; Wood, J. Macropore Sheath: Quantification of Plant Root and Soil Macropore Association. Plant Soil 1999, 211, 59–67. [Google Scholar] [CrossRef]
- Schrader, S.; Rogasik, H.; Onasch, I.; Jégou, D. Assessment of Soil Structural Differentiation around Earthworm Burrows by Means of X-ray Computed Tomography and Scan-Ning Electron Microscopy. Geoderma 2007, 137, 378–387. [Google Scholar] [CrossRef]
- Tiunov, A.V.; Scheu, S. Microbial Respiration, Biomass, Biovolume and Nutrient Status in Burrow Walls of Lumbricus terrestris L. (Lumbricidae). Soil Biol. Biochem. 1999, 31, 2039–2048. [Google Scholar] [CrossRef]
- Pankhurst, C.; Pierret, A.; Hawke, B.; Kirby, J. Microbiological and Chemical Properties of Soil Associated with Macropores at Different Depths in a Red-Duplex Soil in NSW Australia. Plant Soil 2002, 238, 11–20. [Google Scholar] [CrossRef]
- Athmann, M.; Kautz, T.; Banfield, C.; Bauke, S.; Hoang, D.T.T.; Lüsebrink, M.; Pausch, J.; Amelung, W.; Kuzyakov, Y.; Köpke, U. Six Months of L. Terrestris L. Activity in Root-Formed Biopores Increases Nutrient Availability, Microbial Biomass and Enzyme Activity. Appl. Soil Ecol. 2017, 120, 135–142. [Google Scholar] [CrossRef]
- Lavelle, P. Earthworm Activities and the Soil System. Biol. Fertil. Soils 1988, 6, 237–251. [Google Scholar] [CrossRef]
- Jégou, D.; Cluzeau, D.; Hallaire, V.; Balesdent, J.; Tréhen, P. Burrowing Activity of the Earthworms Lumbricus terrestris and Aporrectodea Giardi and Consequences on C Transfers in Soil. Eur. J. Soil Biol. 2000, 36, 27–34. [Google Scholar] [CrossRef]
- Don, A.; Steinberg, B.; Schöning, I.; Pritsch, K.; Joschko, M.; Gleixner, G.; Schulze, E.-D. Organic Carbon Sequestration in Earthworm Burrows. Soil Biol. Biochem. 2008, 40, 1803–1812. [Google Scholar] [CrossRef]
- Andriuzzi, W.S.; Bolger, T.; Schmidt, O. The Drilosphere Concept: Fine-Scale Incorporation of Surface Residue-Derived N and C around Natural Lumbricus terrestris Burrows. Soil Biol. Biochem. 2013, 64, 136–138. [Google Scholar] [CrossRef]
- Uteau, D.; Pagenkemper, S.K.; Peth, S.; Horn, R. Root and Time Dependent Soil Structure Formation and Its Influence on Gas Transport in the Subsoil. Soil Tillage Res. 2013, 132, 69–76. [Google Scholar] [CrossRef]
- Ehlers, W.; Köpke, U.; Hesse, F.; Böhm, W. Penetration Resistance and Root Growth of Oats in Tilled and Untilled Loess Soil. Soil Tillage Res. 1983, 3, 261–275. [Google Scholar] [CrossRef]
- Gaiser, T.; Perkons, U.; Küpper, P.M.; Puschmann, D.U.; Peth, S.; Kautz, T.; Pfeifer, J.; Ewert, F.; Horn, R.; Köpke, U. Evidence of Improved Water Uptake from Subsoil by Spring Wheat Following Lucerne in a Temperate Humid Climate. Field Crop. Res. 2012, 126, 56–62. [Google Scholar] [CrossRef]
- Perkons, U. Bioporengenese durch homo-und allorhize Kulturpflanzen: Einfluss auf das Wurzelwachstum der Nachfrüchte. Dissertation. Ph.D. Thesis, University of Bonn, Bonn, Germany, 2018. [Google Scholar]
- Fleige, H.; Grimme, H.; Renger, M.; Strebel, O. Zur Erfassung der Nährstoffanlieferung durch Diffusion im Effektiven Wurzelraum. Mitt. Dtsch. Bodenkd. Ges. 1983, 38, 381–386. [Google Scholar]
- Kuhlmann, H.; Baumgärtel, G. Potential Importance of the Subsoil for the P and Mg Nutrition of Wheat. Plant Soil 1991, 137, 259–266. [Google Scholar] [CrossRef]
- Pires, L.F.; Auler, A.C.; Roque, W.L.; Mooney, S.J. X-ray Microtomography Analysis of Soil Pore Structure Dynamics under Wetting and Drying Cycles. Geoderma 2020, 362, 114103. [Google Scholar] [CrossRef]
- Stirzaker, R.J.; Passioura, J.B.; Wilms, Y. Soil Structure and Plant Growth: Impact of Bulk Density and Biopores. Plant Soil 1996, 185, 151–162. [Google Scholar] [CrossRef]
- Pierret, A.; Moran, C.J.; Doussan, C. Conventional Detection Methodology Is Limiting Our Ability to Understand the Roles and Functions of Fine Roots. New Phytol. 2005, 166, 967–980. [Google Scholar] [CrossRef] [PubMed]
- Bodner, G.; Leitner, D.; Kaul, H.-P. Coarse and Fine Root Plants Affect Pore Size Distributions Differently. Plant Soil 2014, 380, 133–151. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.; Ellsworth, T.; Meek, B.D. Effect of Root Systems on Preferential Flow in Swelling Soil. Commun. Soil Sci. Plant Anal. 1995, 26, 2655–2666. [Google Scholar] [CrossRef]
- Han, E.; Kautz, T.; Perkons, U.; Lüsebrink, M.; Pude, R.; Köpke, U. Quantification of Soil Biopore Density after Perennial Fodder Cropping. Plant Soil 2015, 394, 73–85. [Google Scholar] [CrossRef]
- Perkons, U.; Kautz, T.; Uteau, D.; Peth, S.; Geier, V.; Thomas, K.; Holz, K.L.; Athmann, M.; Pude, R.; Köpke, U. Root-Length Densities of Various Annual Crops Following Crops with Contrasting Root Systems. Soil Tillage Res. 2014, 137, 50–57. [Google Scholar] [CrossRef]
- Han, E.; Kautz, T.; Köpke, U. Precrop Root System Determines Root Diameter of Subsequent Crop. Biol. Fertil. Soils 2015, 52, 113–118. [Google Scholar] [CrossRef]
- Athmann, M.; Kautz, T.; Pude, R.; Köpke, U. Root Growth in Biopores—Evaluation with in Situ Endoscopy. Plant Soil 2013, 371, 179–190. [Google Scholar] [CrossRef]
- Petzoldt, L.; Athmann, M.; Buechse, A.; Kautz, T. Root Growth of Hordeum Vulgare and Vicia Faba in the Biopore Sheath. Agriculture 2020, 10, 650. [Google Scholar] [CrossRef]
- Jégou, D.; Schrader, S.; Diestel, H.; Cluzeau, D. Morphological, Physical and Biochemical Characteristics of Burrow Walls Formed by Earthworms. Appl. Soil Ecol. 2001, 17, 165–174. [Google Scholar] [CrossRef]
- Pagenkemper, S.K.; Athmann, M.; Uteau, D.; Kautz, T.; Peth, S.; Horn, R. The Effect of Earthworm Activity on Soil Bioporosity—Investigated with X-ray Computed Tomography and Endoscopy. Soil Tillage Res. 2015, 146, 79–88. [Google Scholar] [CrossRef]
- Kolb, E.; Legué, V.; Bogeat-Triboulot, M.-B. Physical Root–Soil Interactions. Phys. Biol. 2017, 14, 065004. [Google Scholar] [CrossRef]
- Parkin, T.B.; Berry, E.C. Microbial Nitrogen Transformations in Earthworm Burrows. Soil Biol. Biochem. 1999, 31, 1765–1771. [Google Scholar] [CrossRef]
- Agapit, C.; Gigon, A.; Puga-Freitas, R.; Zeller, B.; Blouin, M. Plant-Earthworm Interactions: Influence of Age and Proportion of Casts in the Soil on Plant Growth, Morphology and Nitrogen Uptake. Plant Soil 2017, 424, 49–61. [Google Scholar] [CrossRef]
- Athmann, M.; Sondermann, J.; Kautz, T.; Köpke, U. Comparing Macropore Exploration by Faba Bean, Wheat, Barley and Oilseed Rape Roots Using in Situ Endoscopy. J. soil Sci. Plant Nutr. 2019, 19, 689–700. [Google Scholar] [CrossRef]
- Pierret, A.; Moran, C.; Pankhurst, C. Differentiation of Soil Properties Related to the Spatial Association of Wheat Roots and Soil Macropores. Plant Soil 1999, 211, 51–58. [Google Scholar] [CrossRef]
- White, R.; Kirkegaard, J.A. The Distribution and Abundance of Wheat Roots in a Dense, Structured Subsoil — Implications for Water Uptake. Plant. Cell Environ. 2010, 33, 133–148. [Google Scholar] [CrossRef]
- Arvidsson, J.; Håkansson, I. Response of Different Crops to Soil Compaction—Short-Term Effects in Swedish Field Experiments. Soil Tillage Res. 2014, 138, 56–63. [Google Scholar] [CrossRef]
- Lipiec, J.; Simota, C. Role of Soil and Climate Factors in Influencing Crop Responses to Soil Compaction in Central and Eastern Europe. In Developments in Agricultural Engineering; Elsevier BV: Amsterdam, The Netherlands, 1994; Volume 11, pp. 365–390. [Google Scholar]
- Li, H.B.; Ma, Q.H.; Li, H.G.; Zhan, F.S.; Rengel, Z.; Shen, J.B. Root Morphological Responses to Localized Nutrient Supply Differ among Crop Species with Contrasting Root Traits. Plant Soil 2014, 376, 151–163. [Google Scholar] [CrossRef]
- Pätzold, S.; Vetterlein, D.; Jahn, R. DFG Research Unit 1320 Crop Sequence and the Nutrient Acquisition from the Subsoil. Description of the Reference Soil Profile. Available online: https://www.cka.uni-bonn.de/standort/bodenprofilbeschreibung-cka (accessed on 28 November 2020).
- Meier, U.; Bleiholder, B.; Buhr, L.; Feller, C.; Hack, H.; Heß, M.; Lancashire, P.D.; Schnock, U.; Stauß, R.; van den Boom, T.; et al. The BBCH System to Coding the Phenological Growth Stages of Plants—History and Publications. J. Kult. 2009, 61, 41–52. [Google Scholar]
- Littell, R.C.; Milliken, G.A.; Stroup, W.W.; Wolfinger, R.D.; Schabenberger, O. SAS for Mixed Model, 2nd ed.; SAS Institute Inc.: Cary, NC, USA, 2006. [Google Scholar]
- Patterson, H.D.; Thompson, R. Recovery of Inter-Block Information when Block Sizes are Unequal. Biometrika 1971, 58, 545–554. [Google Scholar] [CrossRef]
- Piepho, H. A SAS Macro for Generating Letter Displays of Pairwise Mean Comparisons. Commun. Biometry Crop Sci. 2012, 1, 4–13. [Google Scholar]
- Hsu, J.C. The Factor Analytic Approach to Simultaneous Inference in the General Linear Model. J. Comput. Graph. Stat. 1992, 1, 151. [Google Scholar] [CrossRef]
- Materechera, S.A.; Dexter, A.R.; Alston, A.M. Penetration of Very Strong Soils by Seedling Roots of Different Plant Species. Plant Soil 1991, 135, 31–41. [Google Scholar] [CrossRef]
- Materechera, S.A.; Alston, A.M.; Kirby, J.M.; Dexter, A.R. Influence of Root Diameter on the Penetration of Seminal Roots into a Compacted Subsoil. Plant Soil 1992, 144, 297–303. [Google Scholar] [CrossRef]
- Zobel, R.W.; Kinraide, T.B.; Baligar, V.C. Fine Root Diameters Can Change in Response to Changes in Nutrient Concentrations. Plant Soil 2007, 297, 243–254. [Google Scholar] [CrossRef]
- McCormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.; Helmisaari, H.; Hobbie, E.A.; Iversen, C.M.; Jackson, R.B.; et al. Redefining Fine Roots Improves Understanding of Below-Ground Contributions to Terrestrial Biosphere Processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef]
- Bengough, A.G. Root Elongation Is Restricted by Axial but Not by Radial Pressures: So What Happens in Field Soil? Plant Soil 2012, 360, 15–18. [Google Scholar] [CrossRef]
- Lucas, M.; Schlüter, S.; Vogel, H.-J.; Vetterlein, D. Soil Structure Formation along an Agricultural Chronosequence. Geoderma 2019, 350, 61–72. [Google Scholar] [CrossRef]

 ) or worm type (
) or worm type ( ) treatment.
) treatment.
 ) or worm type (
) or worm type ( ) treatment.
) treatment.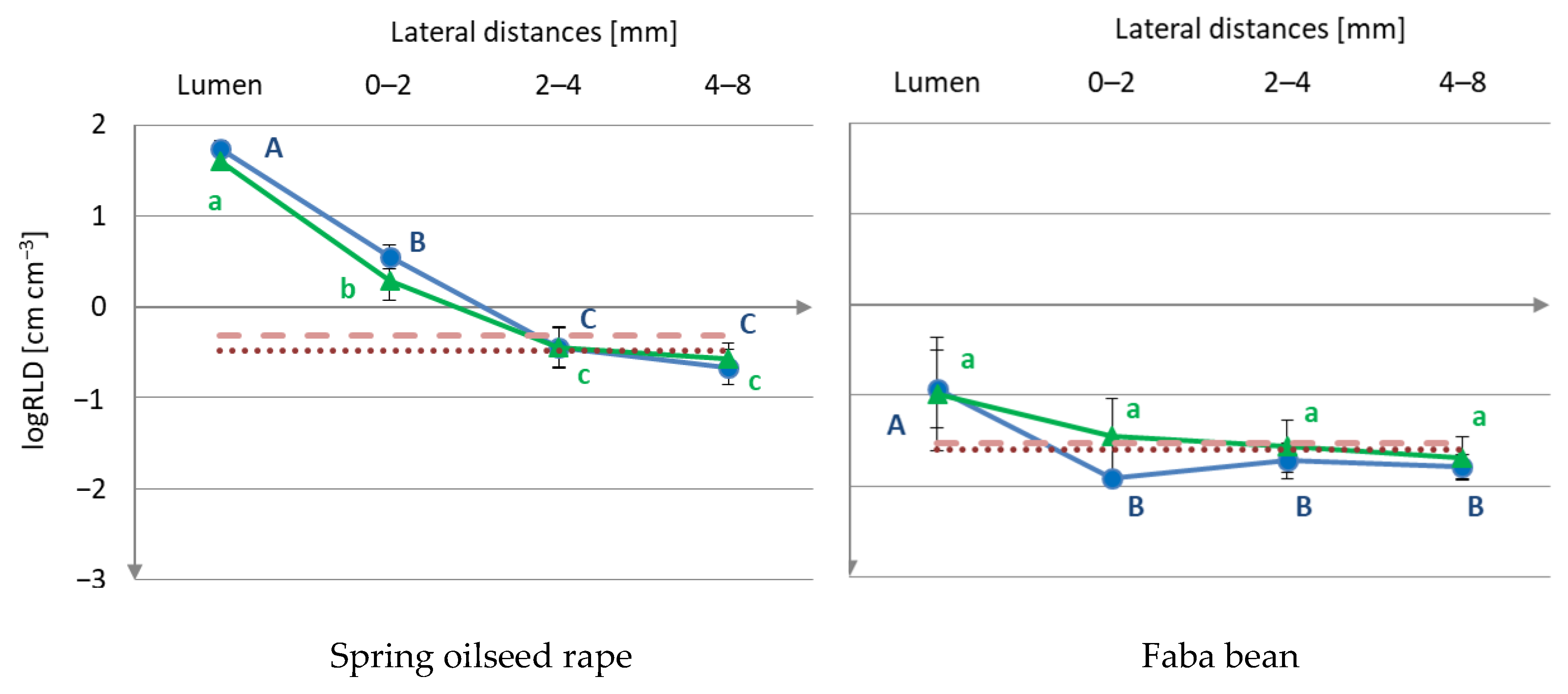
| Year | 2018 | 2019 | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Month | J | F | M | A | M | J | J | A | S | O | N | D | J | F | M | A | M | J | J | A | S | O | N | D | |
| I. | Cultivation chicory | ||||||||||||||||||||||||
| II. | Decay chicory | ||||||||||||||||||||||||
| III. | Earthworm incubation | ||||||||||||||||||||||||
| IV. | Cultivation oilseed rape | ||||||||||||||||||||||||
| V. | Cultivation faba bean | ||||||||||||||||||||||||
| Fine | Small | Medium | Coarse | ||||||||||||||
| Lateral | 0–0.2 mm | 0.2–0.4 mm | 0.4–0.6 mm | >0.6 mm | |||||||||||||
| Pore Type | Distance | [%] | [%] | [%] | [%] | ||||||||||||
| [mm] | Mean | SE | Mean | SE | Mean | SE | Mean | SE | |||||||||
| Worm | Lumen | 80.0 | ab | ± | 2.71 | 15.1 | b | ± | 2.12 | 2.75 | ns | ± | 1.02 | 2.18 | a | ± | 0.97 |
| 0–2 | 83.8 | a | ± | 4.18 | 15.1 | b | ± | 3.42 | 1.04 | ns | ± | 0.58 | 0.10 | b | ± | 0.08 | |
| 2–4 | 73.5 | bc | ± | 8.11 | 25.0 | ab | ± | 7.31 | 1.05 | ns | ± | 0.86 | 0.50 | b | ± | 0.50 | |
| 4–8 | 70.2 | bc | ± | 7.24 | 26.6 | a | ± | 5.90 | 3.18 | ns | ± | 1.91 | 0.07 | b | ± | 0.07 | |
| bulk | 62.0 | c | ± | 5.65 | 33.6 | a | ± | 5.04 | 4.18 | ns | ± | 1.30 | 0.26 | b | ± | 0.17 | |
| Root | Lumen | 79.6 | b | ± | 3.63 | 16.7 | b | ± | 2.04 | 2.25 | ns | ± | 1.00 | 1.44 | ns | ± | 1.11 |
| 0–2 | 89.4 | a | ± | 4.88 | 10.1 | b | ± | 3.50 | 0.44 | ns | ± | 0.38 | 0.01 | ns | ± | 0.01 | |
| 2–4 | 74.9 | abc | ± | 6.64 | 23.4 | ab | ± | 8.36 | 1.70 | ns | ± | 1.49 | 0.01 | ns | ± | 0.01 | |
| 4–8 | 79.6 | ab | ± | 5.10 | 19.4 | ab | ± | 5.46 | 1.00 | ns | ± | 0.75 | 0.03 | ns | ± | 0.03 | |
| bulk | 62.6 | c | ± | 7.87 | 33.9 | a | ± | 8.02 | 3.48 | ns | ± | 2.48 | 0.05 | ns | ± | 0.05 | |
| Lumen | 0–2 mm | 2–4 mm | 4–8 mm | Bulk Soil | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Worm | Root | Worm | Root | Worm | Root | Worm | Root | Worm | Root | ||
| Share of subsoil volume [%] | 0.63 | 0.78 | 1.10 | 3.14 | 94.35 | ||||||
| Spring oilseed rape | Total RL [cm] | 1148.66 | 627.88 | 52.42 | 35.42 | 10.06 | 10.25 | 14.85 | 18.78 | 990.12 | 593.02 |
| Share RL [%] * | 51.83 | 48.84 | 2.37 | 2.76 | 0.45 | 0.80 | 0.67 | 1.46 | 44.69 | 46.14 | |
| Faba bean | Total RL [cm] | 9.77 | 25.84 | 0.06 | 1.51 | 0.54 | 1.10 | 1.32 | 0.89 | 150.82 | 95.60 |
| Share RL [%] * | 6.03 | 20.61 | 0.04 | 1.20 | 0.33 | 0.87 | 0.55 | 1.05 | 93.05 | 76.26 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petzoldt, L.; Kautz, T. Root Distribution of Brassica napus and Vicia faba within the Sheath of Root or Earthworm Biopore. Agriculture 2021, 11, 61. https://doi.org/10.3390/agriculture11010061
Petzoldt L, Kautz T. Root Distribution of Brassica napus and Vicia faba within the Sheath of Root or Earthworm Biopore. Agriculture. 2021; 11(1):61. https://doi.org/10.3390/agriculture11010061
Chicago/Turabian StylePetzoldt, Lisa, and Timo Kautz. 2021. "Root Distribution of Brassica napus and Vicia faba within the Sheath of Root or Earthworm Biopore" Agriculture 11, no. 1: 61. https://doi.org/10.3390/agriculture11010061
APA StylePetzoldt, L., & Kautz, T. (2021). Root Distribution of Brassica napus and Vicia faba within the Sheath of Root or Earthworm Biopore. Agriculture, 11(1), 61. https://doi.org/10.3390/agriculture11010061





