Effects of the Fertilizer Added with DMPP on Soil Nitrous Oxide Emissions and Microbial Functional Diversity
Abstract
1. Introduction
2. Materials and Methods
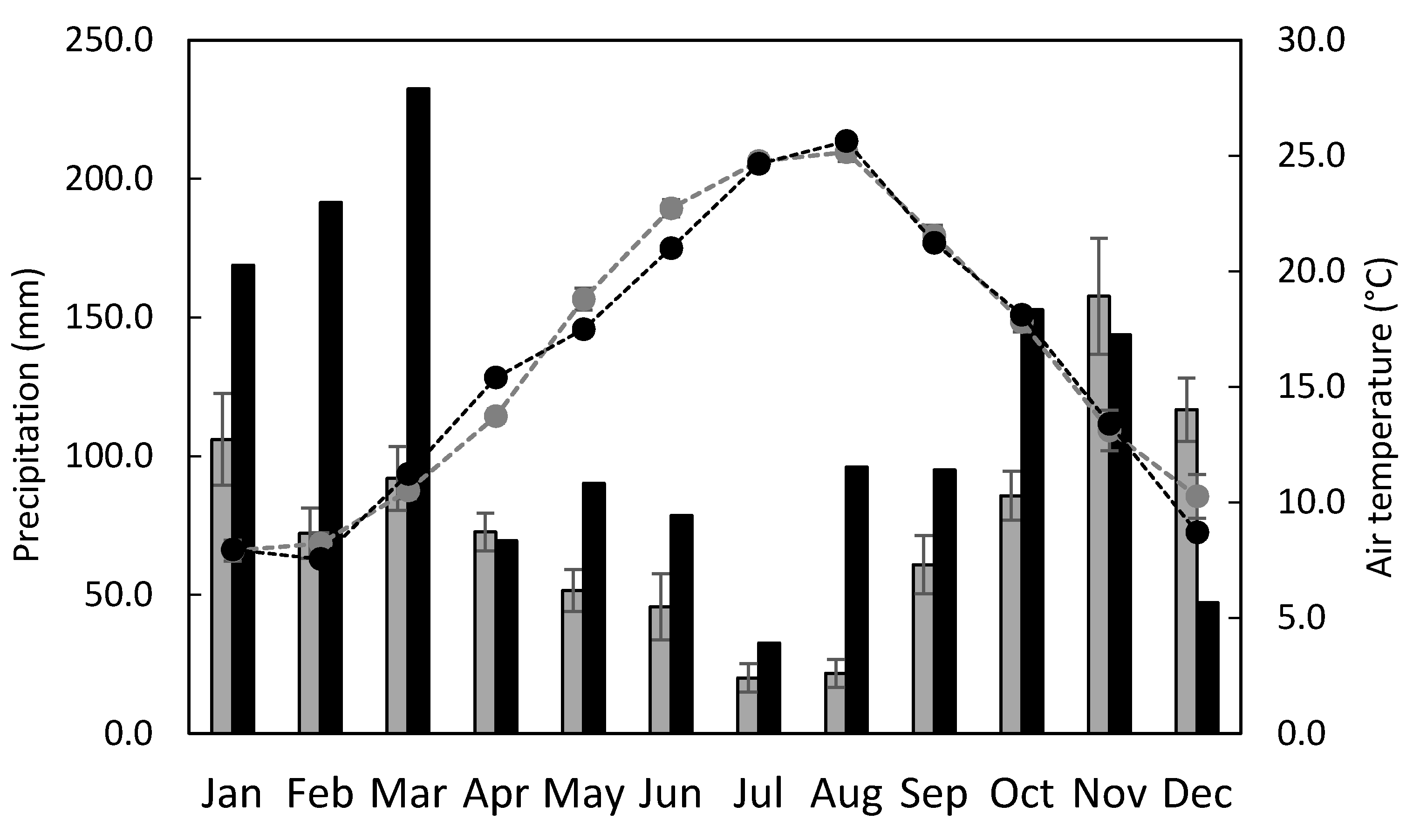
2.1. Experimental Site
2.2. Biometrical and Physiological Determinations
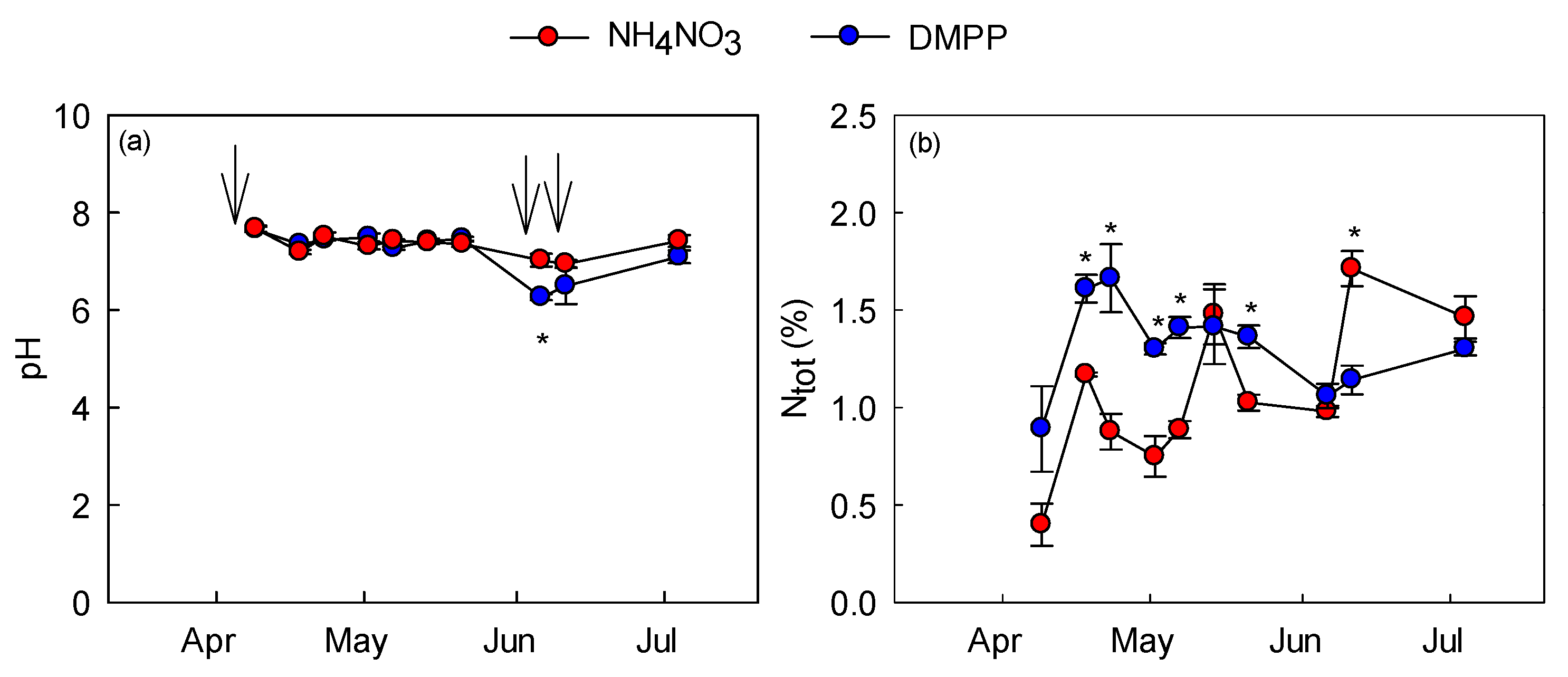
2.3. Soil Temperature, Humidity, and Nitrogen Content
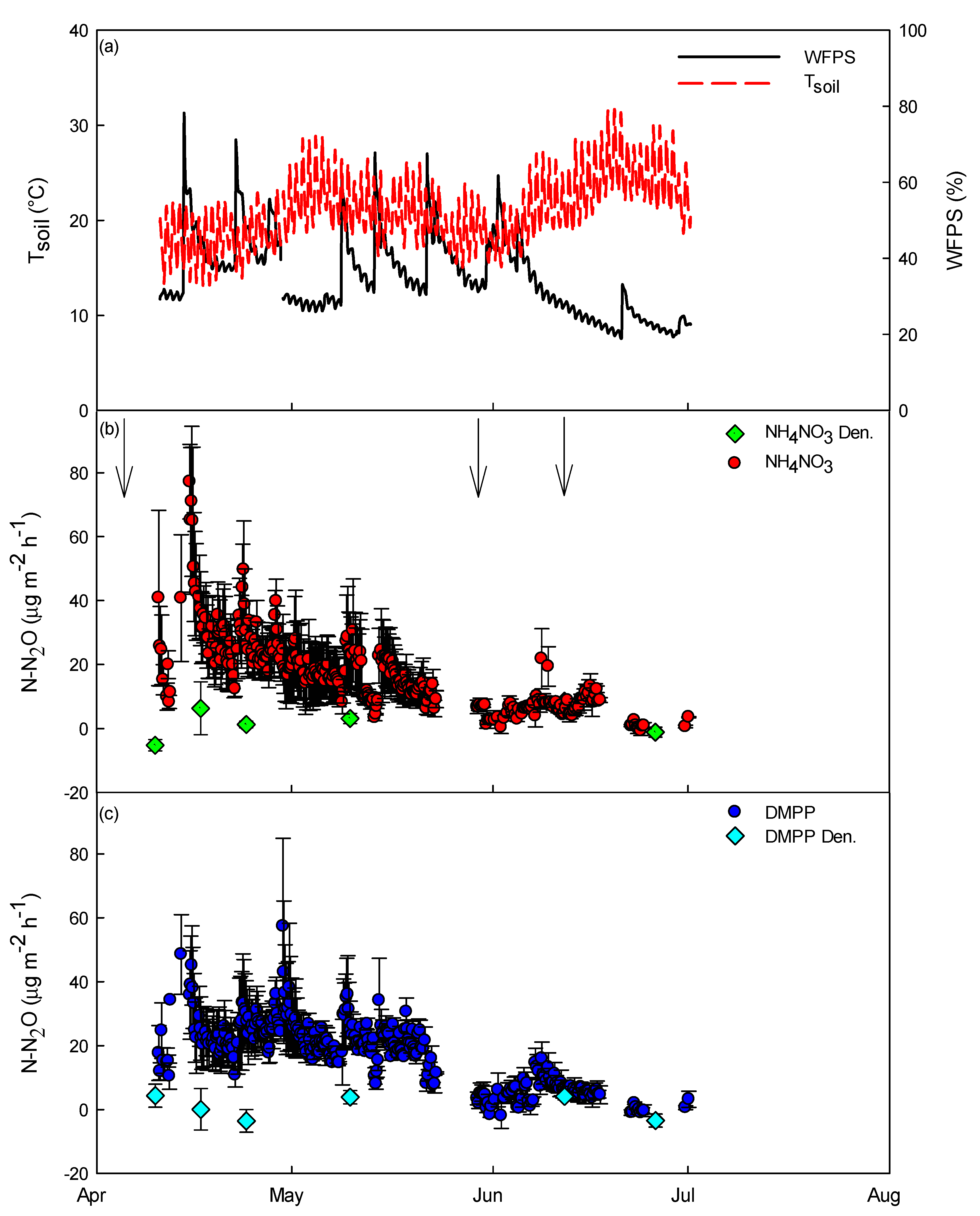
2.4. Soil N2O Emissions and Treatments with Acetylene (C2H2)
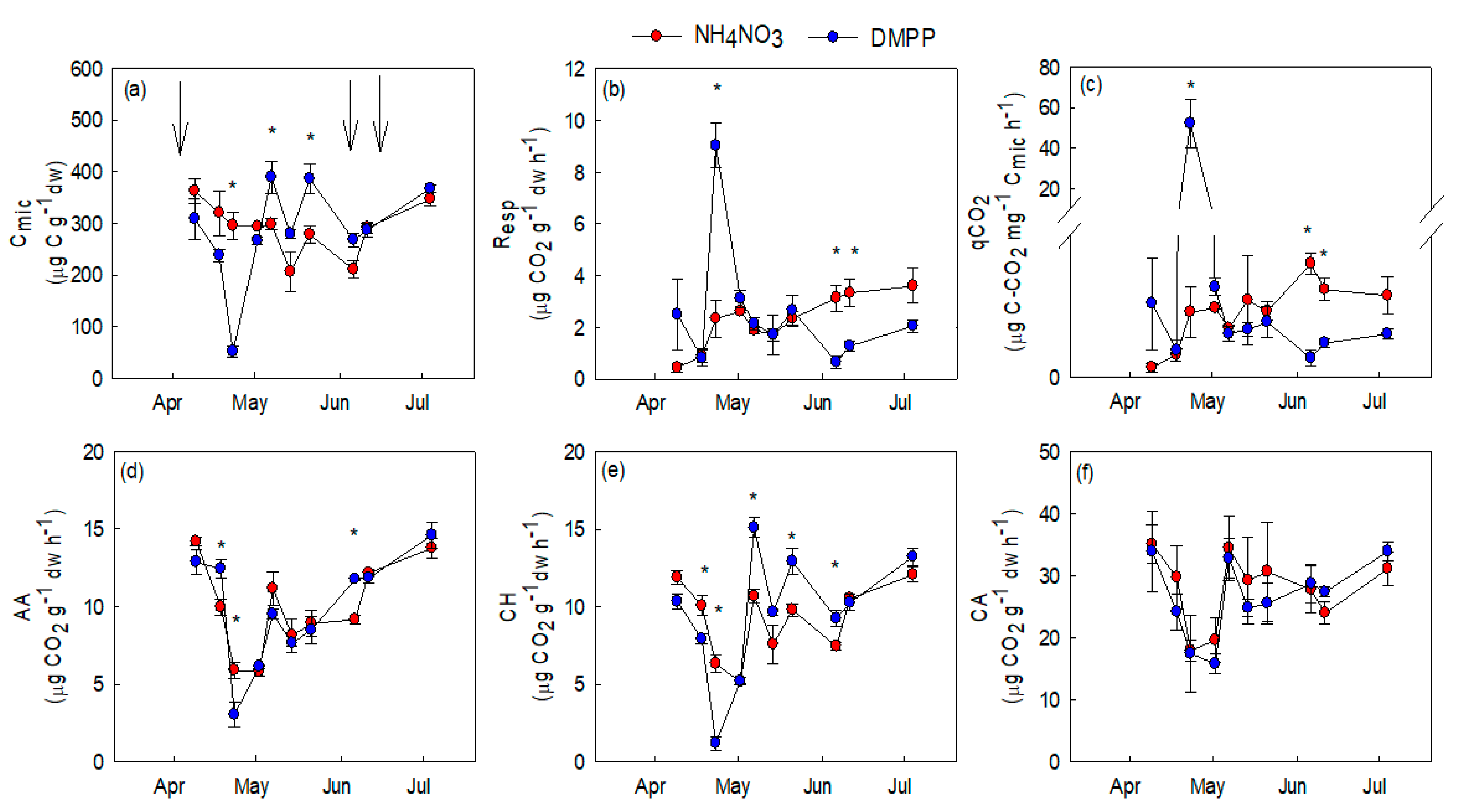
2.5. Soil Biological Analisys
2.6. Statistical Analysis
3. Results
3.1. Plant Greenness, Biomass, and Yield
3.2. Soil-Related Measurements and N2O Emission
3.3. Soil Microbial Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Climate Smart Agriculture Sourcebook. Available online: http://www.fao.org/climate-smart-agriculture-sourcebook/production-resources/module-b1-crops/chapter-b1-1 (accessed on 26 December 2020).
- Vitale, L.; Polimeno, F.; Ottaiano, L.; Maglione, G.; Tedeschi, A.; De Marco, A.; Di Tommasi, P.; Mori, M.; Magliulo, V. Fertilizer type influences tomato yield and soil N2O emissions. Plant. Soil Environ. 2017, 63, 105–110. [Google Scholar]
- Vitale, L.; Tedeschi, A.; Polimeno, F.; Ottaiano, L.; Maglione, G.; Arena, C.; De Marco, A.; Magliulo, V. Water regime affects soil N2O emission and tomato yield grown under different types of fertilisers. Ital. J. Agron. 2018, 13, 74–79. [Google Scholar] [CrossRef]
- Norton, J.; Ouyang, Y. Controls and adaptive management of nitrification in agricultural soils. Front. Microbiol. 2019, 10, 1931. [Google Scholar] [CrossRef]
- Forte, A.; Fierro, A. Denitrification rate and its potential to predict biogenic N2O field emissions in a Mediterranean maize-cropped soil in Southern Italy. Land 2019, 8, 97. [Google Scholar] [CrossRef]
- Tedeschi, A.; Volpe, M.G.; Polimeno, F.; Siano, F.; Maglione, G.; Di Tommasi, P.; Vasca, E.; Magliulo, V.; Vitale, L. Soil fertilization with urea has little effect on seed quality but reduces soil N2O emissions from a hemp cultivation. Agriculture 2020, 10, 240. [Google Scholar] [CrossRef]
- Smith, P.; Martino, D.; Cai, Z.; Gwary, D.; Janzen, H.; Kumar, P.; McCarl, B.; Ogle, S.; O’Mara, F.; Rice, C.; et al. Agriculture. In Climate Change 2007: Mitigation. Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Metz, B., Davidson, O.R., Bosch, P.R., Dave, R., Meyer, L.A., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007; pp. 497–540. [Google Scholar]
- Cassman, K.G.; Dobermann, A.R.; Walters, D. Agroecosystems, nitrogen-use efficiency, and nitrogen management. Ambio 2002, 31, 132–140. [Google Scholar] [CrossRef]
- Herr, C.; Mannheim, T.; Müller, T.; Ruser, R. Effect of nitrification inhibitors on N2O emissions after cattle slurry application. Agronomy 2020, 10, 1174. [Google Scholar] [CrossRef]
- Zerulla, W.; Barth, T.; Dressel, J.; Erhardt, K.; von Locquenghien, K.H.; Pasda, G.; Rädle, M.; Wissemeier, A. 3,4-dimethylpyrazole phosphate (DMPP)—A new nitrification inhibitor for agriculture and horticulture. Biol. Fertil. Soils 2001, 34, 79–84. [Google Scholar] [CrossRef]
- Weiske, A.; Benckiser, G.; Herbert, T.; Ottow, J. Influence of the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) in comparison to dicyandiamide (DCD) on nitrous oxide emissions, carbon dioxide fluxes and methane oxidation during 3 years of repeated application in field experiments. Biol. Fertil. Soils 2001, 34, 109–117. [Google Scholar]
- Zerulla, W.; Pasda, G.; Hähndel, R.; Wissemeier, A. The new nitrification inhibitor DMPP (ENTEC®) for use in agricultural and horticultural crops—An overview. Plant Nutr. 2001, 92, 754–755. [Google Scholar]
- Recio, J.; Vallejo, A.; Le-Noë, J.; Garnier, J.; García-Marco, S.; Álvarez, J.M.; Sanz-Cobena, A. The effect of nitrification inhibitors on NH3 and N2O emissions in highly N fertilized irrigated Mediterranean cropping systems. Sci. Total Environ. 2018, 636, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Friedl, J.; Scheer, C.; Rowlings, D.W.; Deltedesco, E.; Gorfer, M.; De Rosa, D.; Grace, P.R.; Müller, C.; Keiblinge, K.M. Efect of the nitrifcation inhibitor 3,4-dimethylpyrazole phosphate (DMPP) on N-turnover, the N2O reductase-gene nosZ and N2O:N2 partitioning from agricultural soils. Sci. Rep. 2020, 10, 2399. [Google Scholar] [CrossRef] [PubMed]
- Fuertes-Mendizábal, T.; Huérfano, X.; Vega-Mas, I.; Torralbo, F.; Menéndez, S.; Ippolito, J.A.; Kammann, C.; Wrage-Mönnig, N.; Cayuela, M.L.; Borchard, N.; et al. Biochar reduces the efficiency of nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) mitigating N2O emissions. Sci. Rep. 2019, 9, 2346. [Google Scholar] [CrossRef]
- Duan, Y.F.; Kong, X.W.; Schramm, A.; Labouriau, R.; Eriksen, J.; Petersen, S.O. Microbial N Transformations and N2O Emission after Simulated Grassland Cultivation: Effects of the Nitrification Inhibitor 3,4-Dimethylpyrazole Phosphate (DMPP). Appl. Environ. Microbiol. 2017, 83, e02019-16. [Google Scholar] [CrossRef]
- Shi, X.; Hu, H.W.; Müller, C.; He, J.Z.; Chen, D.; Suter, H.C. Effects of the nitrification inhibitor 3,4-dimethylpyrazole phosphate on nitrification and nitrifiers in two contrasting agricultural soils. Appl. Environ. Microbiol. 2016, 82, 5236–5248. [Google Scholar] [CrossRef]
- Benckiser, G.; Christ, E.; Herbert, T.; Weiske, A.; Blome, J.; Hardt, M. The nitrification inhibitor 3,4 dimethylpyrazole-phosphat (DMPP)—Quantification and effects on soil metabolism. Plant Soil 2013, 371, 257–266. [Google Scholar] [CrossRef]
- Dong, X.X.; Zhang, L.L.; Wu, Z.J.; Zhang, H.W.; Gong, P. The response of nitrifier, N-fixer and denitrifier gene copy numbers to the nitrification inhibitor 3,4-dimethylpyrazole phosphate. Plant Soil Environ. 2013, 59, 398–403. [Google Scholar]
- Liu, R.; Hayden, H.; Suter, H.; He, J.; Chen, D. The effect of nitrification inhibitors in reducing nitrification and the ammonia oxidizer population in three contrasting soils. J. Soils Sediments 2015, 15, 1113–1118. [Google Scholar] [CrossRef]
- White, D.C.; MacNaughton, S.J. Chemical and molecular approaches for rapid assessment of the biological status of soils. In Biological Indicators of Soil Health; Pankhurst, C.E., Doube, B.M., Gupta, V.V.S.R., Eds.; CAB: Wallingford, CO, USA, 1997; pp. 371–396. [Google Scholar]
- Degens, B.P.; Schipper, L.A.; Sparling, G.P.; Duncan, L.C. Is the microbial community in a soil with reduced catabolic diversity less resistant to stress or disturbance? Soil Biol. Biochem. 2001, 33, 1143–1153. [Google Scholar] [CrossRef]
- Ventorino, V.; De Marco, A.; Pepe, O.; Virzo De Santo, A.; Moschetti, G. Impact of innovative agricultural practices of carbon sequestration on soil microbial community. In Carbon Sequestration in Agricultural Soils. A Multidisciplinary Approach to Innovative Methods; Piccolo, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 145–177. [Google Scholar]
- Irigoyen, I.; Muro, J.; Azpilikueta, M.; Aparicio-Tejo, P.; Lamsfus, C. Ammonium oxidation kinetics in the presence of nitrification inhibitors DCD and DMPP at various temperatures. Aust. J. Soil Res. 2003, 41, 1177–1183. [Google Scholar] [CrossRef]
- Machefert, S.E.; Dise, N.B. Hydrological controls on denitrification in riparian ecosystems. Hydrol. Earth Syst. Sci. 2004, 8, 686–694. [Google Scholar] [CrossRef][Green Version]
- Shen, J.P.; Zhang, L.M.; Hong, J.; Di, H.J.; He, J.Z. A review of ammonia-oxidizing bacteria and archaea in Chinese soils. Front. Microbiol. 2012, 3, 296. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, W.D.; Gu, J.D. Community structure and abundance of ammonia-oxidizing archaea and bacteria after conversion from soybean to rice paddy in albic soils of Northeast China. Appl. Microbiol. Biotechnol. 2014, 98, 2765–2778. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.Y.; Luo, J.; Wang, J.Q.; Huang, R.; Guo, K.; Li, Y.; Wu, Q.L. The influence of land use on the abundance and diversity of ammonia oxidizers. Curr. Microbiol. 2015, 70, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Barrena, I.; Menendez, S.; Correa-Galeote, D.; Vega-Mas, I.; Bedmar, E.J.; Gonzalez-Murua, C.; Estavillo, J.M. Soil water content modulates the effect of the nitrification inhibitor 3,4-Dimethylpyrazole Phosphate (DMPP) on nitrifying and denitrifying bacteria. Geoderma 2017, 303, 1–8. [Google Scholar] [CrossRef]
- He, Y.; Qi, Y.; Dong, Y.; Xiao, S.; Peng, Q.; Liu, X.; Sun, L. Effects of nitrogenf on soil microbial biomass and community functional diversity in temperate grassland in inner Mongolia, China. Clean Soil Air Water 2013, 41, 1216–1221. [Google Scholar] [CrossRef]
- Wang, C.; Liu, D.; Bai, E. Decreasing soil microbial diversity is associated with decreasing microbial biomass under nitrogen addition. Soil Biol. Biochem. 2018, 120, 126–133. [Google Scholar] [CrossRef]
- Tindaon, F.; Benckiser, G.; Ottow, J.C.G. Evaluation of ecological doses of the nitrification inhibitors 3,4-dimethylpyrazole phosphate (DMPP) and 4-chloromethylpyrazole (ClMP) in comparison to dicyandiamide (DCD) in their effects on dehydrogenase and dimethyl sulfoxide reductase activity in soils. Biol. Fertil. Soils 2012, 48, 643–650. [Google Scholar] [CrossRef]
- Kong, X.; Duan, Y.; Schramm, A.; Eriksen, J.; Petersen, S.O. 3,4-Dimethylpyrazole phosphate (DMPP) reduces activity of ammonia oxidizers without adverse effects on non-target soil microorganisms and functions. Appl. Soil Ecol. 2016, 105, 67–75. [Google Scholar] [CrossRef]

| Plot | SPAD | Aboveground Biomass (kg m−2) | Tuber Yield (kg m−2) | ||
|---|---|---|---|---|---|
| 06 June | 20 June | 09 July | |||
| NH4NO3 | 47.64 ± 1.48 a | 47.35 ± 1.68 a | 43.429 ± 0.89 b | 0.68 ± 0.068a | 6.39 ± 0.26 a |
| DMPP | 49.27 ± 1.76 a | 51.64 ± 0.92 a | 45.18 ± 1.94 b | 0.59 ± 0.035a | 5.78 ± 0.13 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tedeschi, A.; De Marco, A.; Polimeno, F.; Di Tommasi, P.; Maglione, G.; Ottaiano, L.; Arena, C.; Magliulo, V.; Vitale, L. Effects of the Fertilizer Added with DMPP on Soil Nitrous Oxide Emissions and Microbial Functional Diversity. Agriculture 2021, 11, 12. https://doi.org/10.3390/agriculture11010012
Tedeschi A, De Marco A, Polimeno F, Di Tommasi P, Maglione G, Ottaiano L, Arena C, Magliulo V, Vitale L. Effects of the Fertilizer Added with DMPP on Soil Nitrous Oxide Emissions and Microbial Functional Diversity. Agriculture. 2021; 11(1):12. https://doi.org/10.3390/agriculture11010012
Chicago/Turabian StyleTedeschi, Anna, Anna De Marco, Franca Polimeno, Paul Di Tommasi, Giuseppe Maglione, Lucia Ottaiano, Carmen Arena, Vincenzo Magliulo, and Luca Vitale. 2021. "Effects of the Fertilizer Added with DMPP on Soil Nitrous Oxide Emissions and Microbial Functional Diversity" Agriculture 11, no. 1: 12. https://doi.org/10.3390/agriculture11010012
APA StyleTedeschi, A., De Marco, A., Polimeno, F., Di Tommasi, P., Maglione, G., Ottaiano, L., Arena, C., Magliulo, V., & Vitale, L. (2021). Effects of the Fertilizer Added with DMPP on Soil Nitrous Oxide Emissions and Microbial Functional Diversity. Agriculture, 11(1), 12. https://doi.org/10.3390/agriculture11010012









