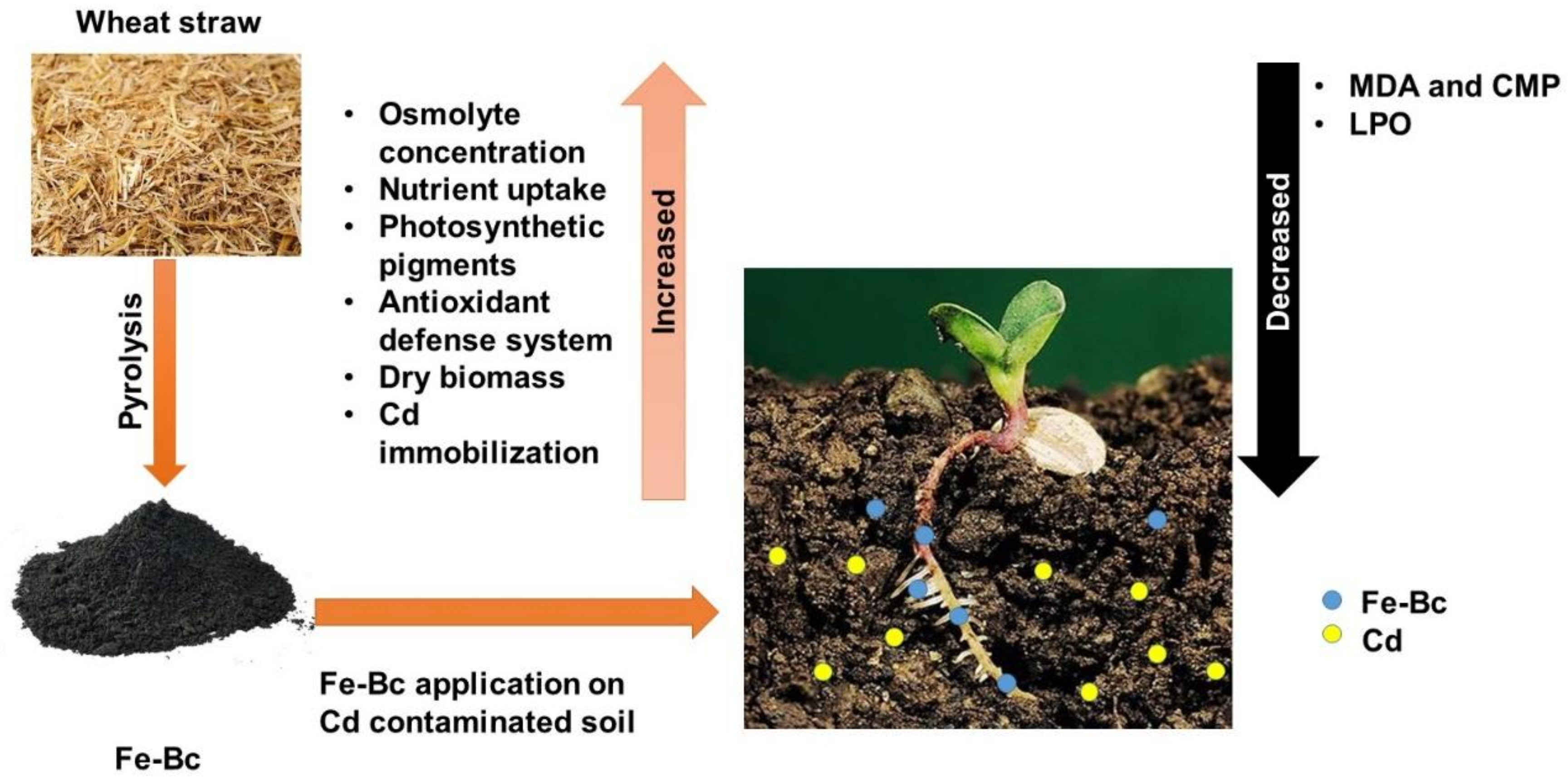
Influence of Iron-Enriched Biochar on Cd Sorption, Its Ionic Concentration and Redox Regulation of Radish under Cadmium Toxicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Iron Enriched Biochar
2.2. Pot Study
2.3. Biochemical Analysis
2.4. Crude Leaf Extract for Antioxidant Enzyme Assays
2.5. Nutrients and Heavy Metal Analysis
2.6. Biosorption Analysis
2.7. Statistical Analysis
3. Results
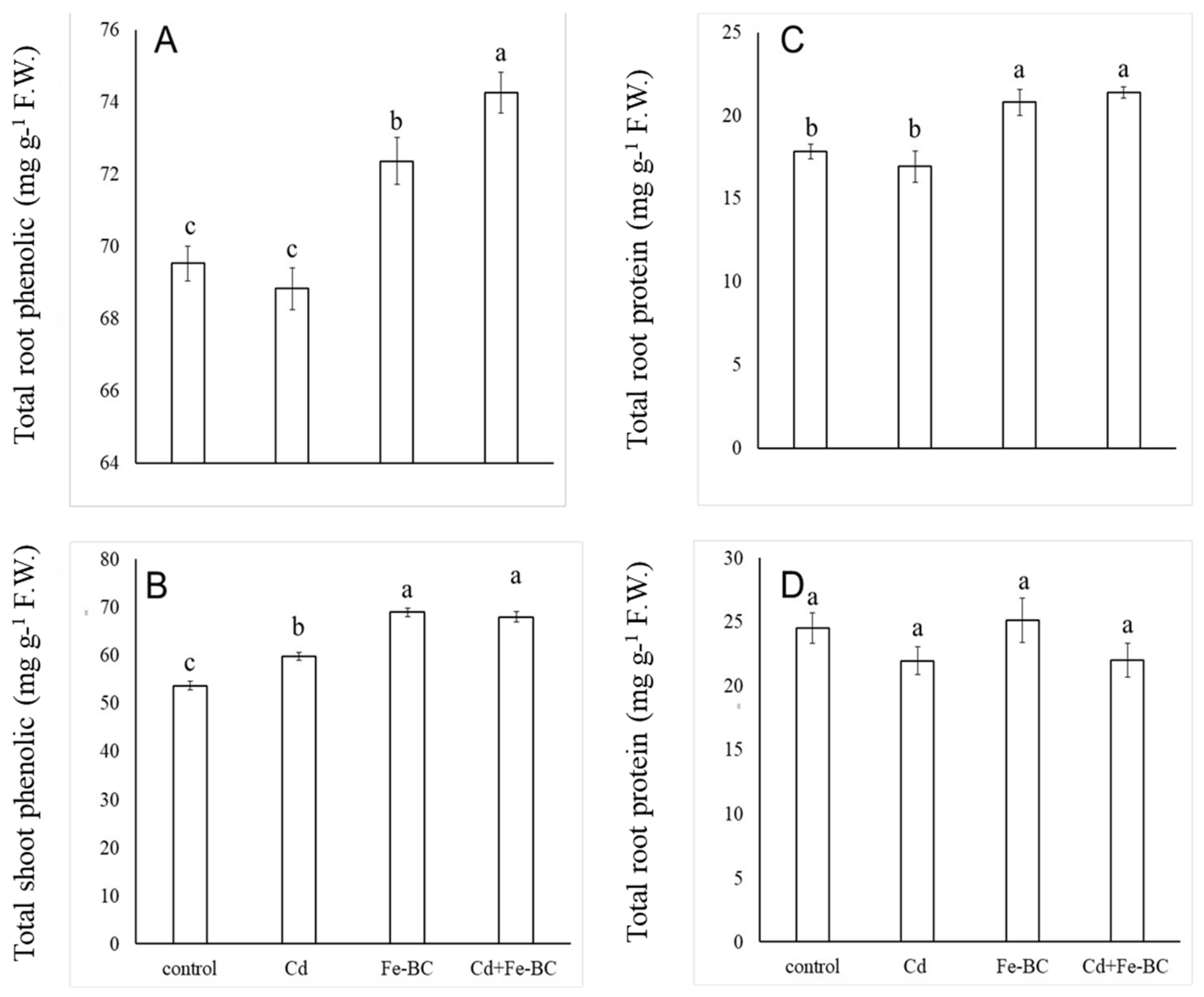
3.1. Fe-Rich Biochar Improves Radish Growth and Nutrient Uptake under Cd Stress
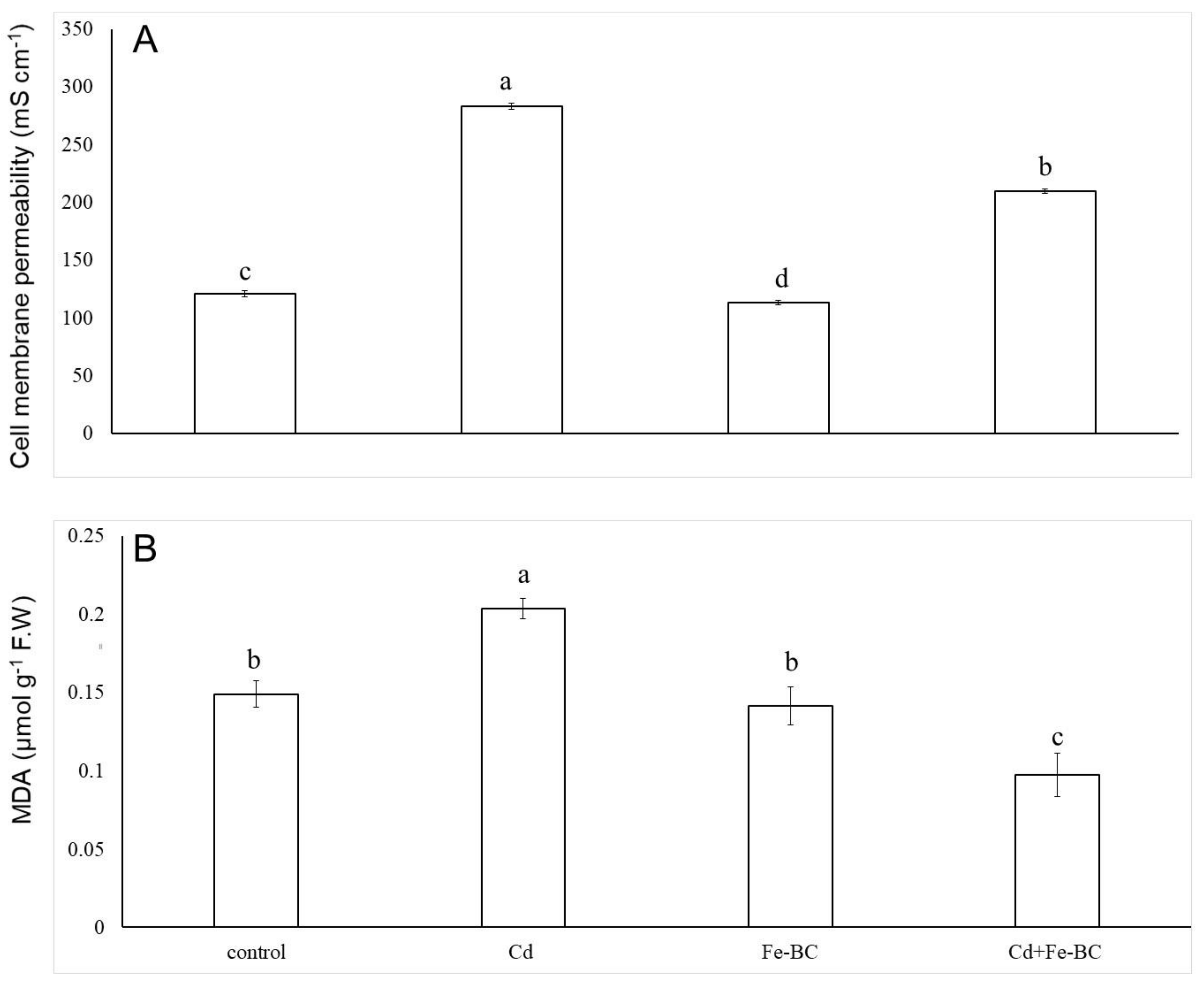
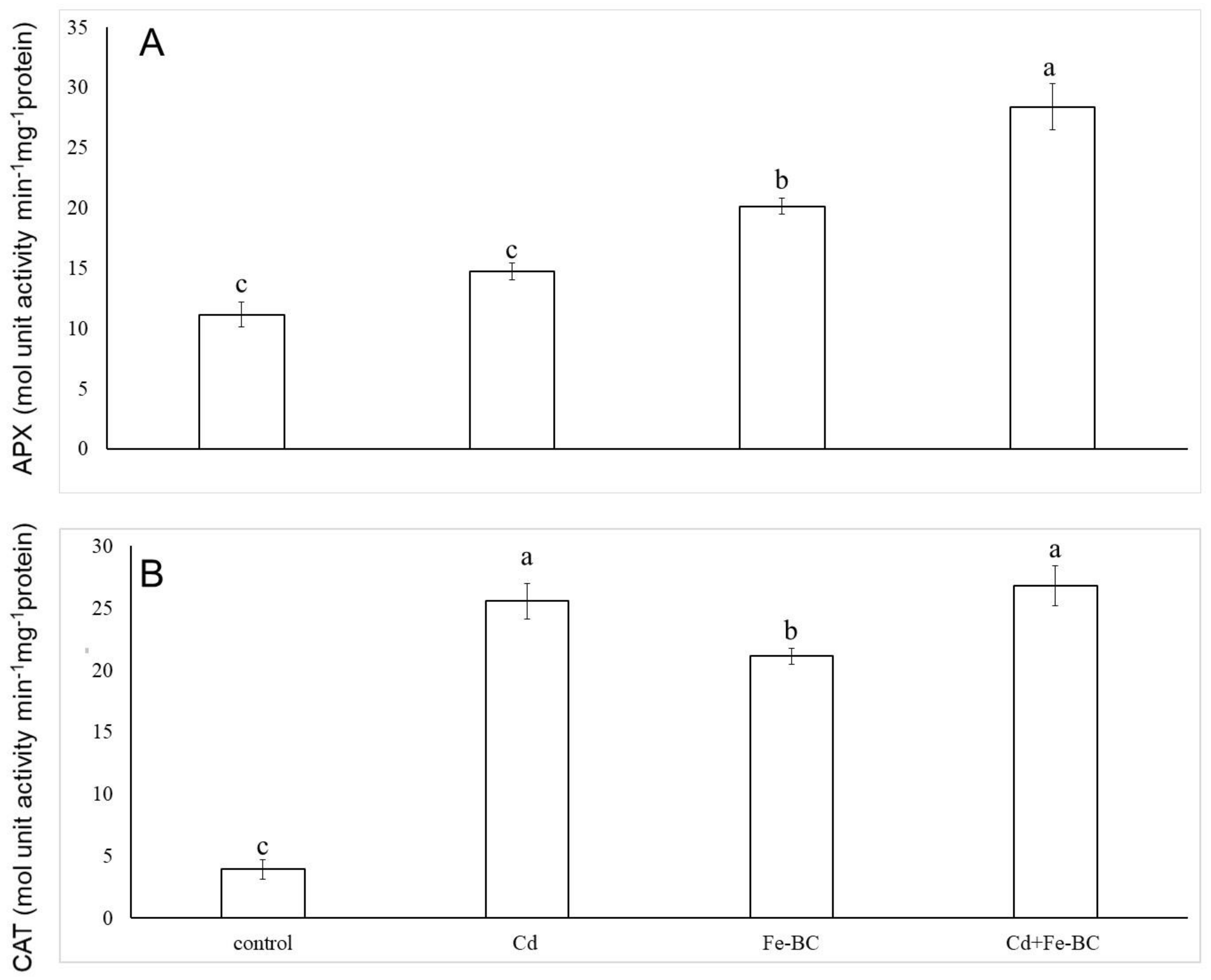
3.2. Fe-Rich Biochar Reduces Membrane Permeability by Improving Antioxidant Defence System
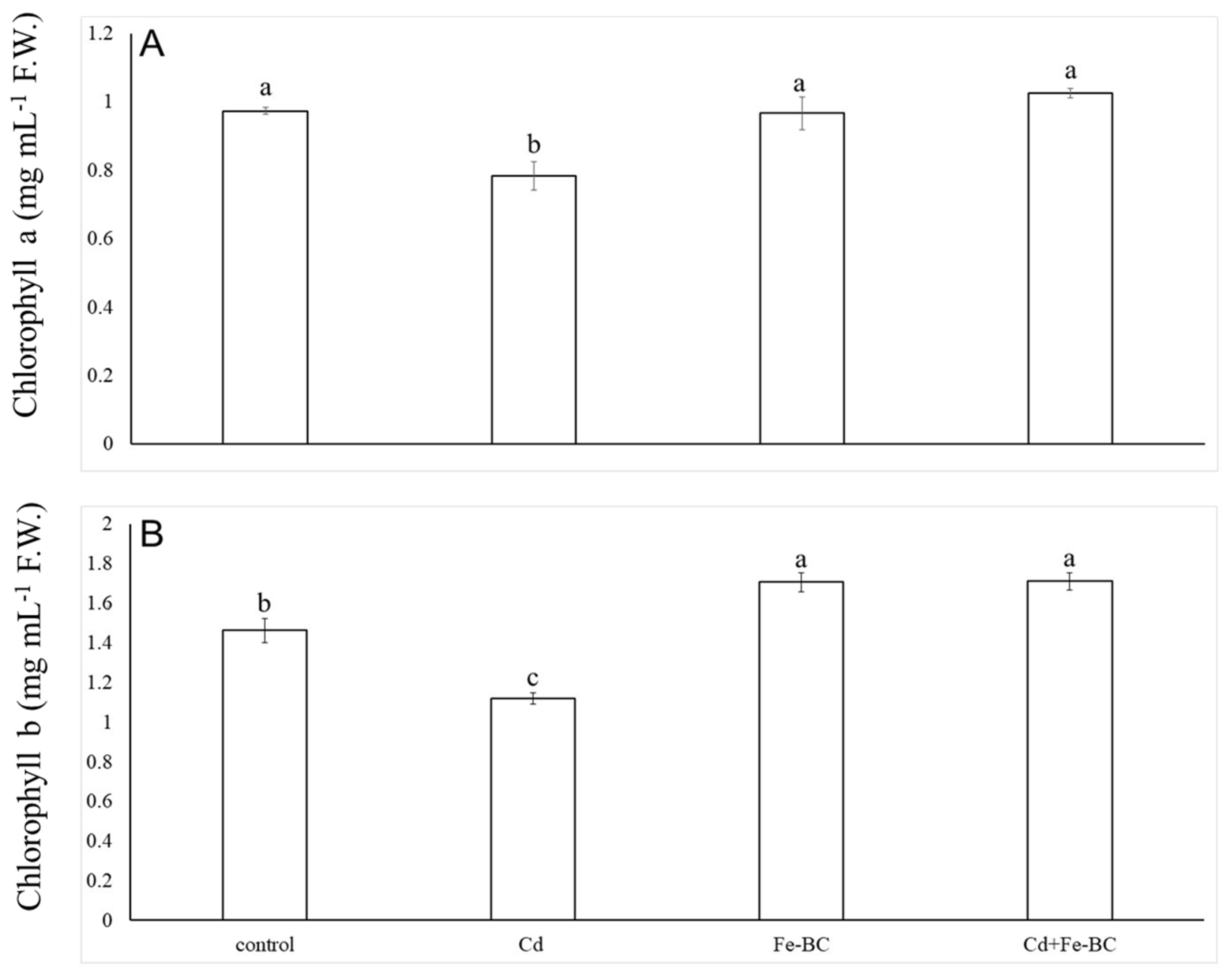
3.3. Fe-Rich Biochar Improves Osmolytes Accumulation and Photosynthetic Pigments under Cd Stress
3.4. Soil Cd, Langmuir and Freundlich Models
4. Discussion
4.1. Iron-Rich Biochar Maintained Cell Membrane Stability by Triggering Antioxidant Defence System and Osmolyte under Cd Stress
4.2. Iron-Rich Biochar-Induced Radish Growth Improvement was Due to Higher Chlorophyll Concentrations under Cd Stress
4.3. Biochar Improves Biomass Accumulation and Plant Nutrition under Cd Stress
4.4. Soil Cd and Langmuir and Freundlich Models
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Anjum, S.A.; Ashraf, U.; Tanveer, M.; Khan, I.; Hussain, S.; Shahzad, B.; Wang, L.C. Drought induced changes in growth, osmolyte accumulation and antioxidant metabolism of three maize hybrids. Front. Plant Sci. 2017, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Tanveer, M.; Han, W.; Tian, C.; Wang, L. High and differential strontium tolerance in germinating dimorphic seeds of Salicornia europaea. Seed Sci. Technol. 2020, 48, 231–239. [Google Scholar] [CrossRef]
- Shah, A.N.; Tanveer, M.; Hussain, S.; Yang, G. Beryllium in the environment: Whether fatal for plant growth? Rev. Environ. Sci. Biotechnol. 2016, 15, 549–561. [Google Scholar] [CrossRef]
- Shahzad, B.; Tanveer, M.; Che, Z.; Rehman, A.; Cheema, S.A.; Sharma, A.; Zhaorong, D. Role of 24-epibrassinolide (EBL) in mediating heavy metal and pesticide induced oxidative stress in plants: A review. Ecotoxicol. Environ. Saf. 2018, 147, 935–944. [Google Scholar] [CrossRef]
- Tanveer, M.; Yousaf, U. Plant single-cell biology and abiotic stress tolerance. In Plant Life under Changing Environment; Academic Press: Cambridge, MA, USA, 2020; pp. 611–626. [Google Scholar]
- Tanveer, M.; Hasanuzzaman, M.; Wang, L. Lithium in Environment and Potential Targets to Reduce Lithium Toxicity in Plants. J. Plant Growth Regul. 2019, 38, 1574–1586. [Google Scholar] [CrossRef]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Ashraf, U.; Khan, I.; Wang, L. Alteration in growth, leaf gas exchange, and photosynthetic pigments of maize plants under combined cadmium and arsenic stress. Water Air Soil Pollut. 2017, 228, 13. [Google Scholar] [CrossRef]
- Rady, M.M.; Hemida, K.A. Modulation of cadmium toxicity and enhancing cadmium-tolerance in wheat seedlings by exogenous application of polyamines. Ecotoxicol. Environ. Saf. 2015, 119, 178–185. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Exogenous selenium pretreatment protects rapeseed seedlings from cadmium-induced oxidative stress by upregulating antioxidant defense and methylglyoxal detoxification systems. Biol. Trace Elem. Res. 2012, 149, 248–261. [Google Scholar] [CrossRef]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Bao, M.; Wang, L.; Khan, I.; Shahzad, B. Cadmium toxicity in Maize (Zea mays L.): Consequences on antioxidative systems, reactive oxygen species and cadmium accumulation. Environ. Sci. Pollut. Res. 2015, 22, 17022–17030. [Google Scholar] [CrossRef]
- Gratão, P.L.; Monteiro, C.C.; Tezotto, T.; Carvalho, R.F.; Alves, L.R.; Peters, L.P.; Azevedo, R.A. Cadmium stress antioxidant responses and root-to-shoot communication in grafted tomato plants. Biometals 2015, 28, 803–816. [Google Scholar] [CrossRef]
- Shanying, H.E.; Xiaoe, Y.A.N.G.; Zhenli, H.E.; Baligar, V.C. Morphological and physiological responses of plants to cadmium toxicity: A review. Pedosphere 2017, 27, 421–438. [Google Scholar]
- Xin, J.; Zhao, X.; Tan, Q.; Sun, X.; Hu, C. Comparison of cadmium absorption, translocation, subcellular distribution and chemical forms between two radish cultivars (Raphanus sativus L.). Ecotoxicol. Environ. Saf. 2017, 145, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Hussain, S.; Tanveer, M.; Khan, S.; Hussain, H.A.; Iqbal, B.; Geng, M. Coordinated effects of lead toxicity and nutrient deprivation on growth, oxidative status, and elemental composition of primed and non-primed rice seedlings. Environ. Sci. Pollut. Res. 2018, 25, 21185–21194. [Google Scholar] [CrossRef] [PubMed]
- Fahad, S.; Hussain, S.; Saud, S.; Tanveer, M.; Bajwa, A.A.; Hassan, S.; Shah, F. A biochar application protects rice pollen from high-temperature stress. Plant Physiol. Biochem. 2015, 96, 281–287. [Google Scholar] [CrossRef]
- Liu, W.; Huo, R.; Xu, J.; Liang, S.; Li, J.; Zhao, T.; Wang, S. Effects of biochar on nitrogen transformation and heavy metals in sludge composting. Bioresour. Technol. 2017, 235, 43–49. [Google Scholar] [CrossRef]
- Lu, K.; Yang, X.; Shen, J.; Robinson, B.; Huang, H.; Liu, D.; Wang, H. Effect of bamboo and rice straw biochars on the bioavailability of Cd, Cu, Pb and Zn to Sedum plumbizincicola. Agric. Ecosyst. Environ. 2014, 191, 124–132. [Google Scholar] [CrossRef]
- Puga, A.P.; Abreu, C.A.; Melo, L.C.A.; Beesley, L. Biochar application to a contaminated soil reduces the availability and plant uptake of zinc, lead and cadmium. J. Environ. Manag. 2015, 159, 86–93. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Jun, M.; Shu, D.; Chen, W.F. Effect of biochar on relieving cadmium stress and reducing accumulation in super japonica rice. J. Integr. Agric. 2014, 13, 547–553. [Google Scholar] [CrossRef]
- Rehman, M.; Liu, L.; Bashir, S.; Saleem, M.H.; Chen, C.; Peng, D.; Siddique, K.H. Influence of rice straw biochar on growth, antioxidant capacity and copper uptake in ramie (Boehmeria nivea L.) grown as forage in aged copper-contaminated soil. Plant Physiol. Biochem. 2019, 138, 121–129. [Google Scholar] [CrossRef]
- Joseph, S.; Anawar, H.M.; Storer, P.; Blackwell, P.; Chee, C.H.I.A.; Yun, L.I.N.; Solaiman, Z.M. Effects of enriched biochars containing magnetic iron nanoparticles on mycorrhizal colonisation, plant growth, nutrient uptake and soil quality improvement. Pedosphere 2015, 25, 749–760. [Google Scholar] [CrossRef]
- Rizwan, M.; Noureen, S.; Ali, S.; Anwar, S.; ur Rehman, M.Z.; Qayyum, M.F.; Hussain, A. Influence of biochar amendment and foliar application of iron oxide nanoparticles on growth, photosynthesis, and cadmium accumulation in rice biomass. J. Soils Sediments 2019, 19, 3749–3759. [Google Scholar] [CrossRef]
- O’Connor, D.; Peng, T.; Li, G.; Wang, S.; Duan, L.; Mulder, J.; Hou, D. Sulfur-modified rice husk biochar: A green method for the remediation of mercury contaminated soil. Sci. Total Environ. 2018, 621, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Xian, Y.; He, Z.; Zhang, Q.; Wu, J.; Yang, G.; Long, L. Adsorption characteristics of Pb (II) using biochar derived from spent mushroom substrate. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Ji, H.Y.; Lyu, H.H.; Liu, Y.X.; He, L.L.; You, L.C.; Yang, S.M. Simultaneous alleviation of Sb and Cd availability in contaminated soil and accumulation in Lolium multiflorum Lam. After amendment with Fe–Mn-Modified biochar. J. Clean. Prod. 2019, 231, 556–564. [Google Scholar] [CrossRef]
- Mandal, S.; Sarkar, B.; Bolan, N.; Ok, Y.S.; Naidu, R. Enhancement of chromate reduction in soils by surface modified biochar. J. Environ. Manag. 2017, 186, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Lucena, J.J.; Hernandez-Apaolaza, L. Iron nutrition in plants: An overview. Plant Soil 2017, 418, 1–4. [Google Scholar] [CrossRef]
- Jia, W.; Sun, X.; Gao, Y.; Yang, Y.; Yang, L. Fe-modified biochar enhances microbial nitrogen removal capability of constructed wetland. Sci. Total Environ. 2020, 740, 139534. [Google Scholar] [CrossRef]
- Qiao, Y.; Wu, J.; Xu, Y.; Fang, Z.; Zheng, L.; Cheng, W.; Zhao, D. Remediation of cadmium in soil by biochar-supported iron phosphate nanoparticles. Ecol. Eng. 2017, 106, 515–522. [Google Scholar] [CrossRef]
- Ahmad, M.; Ok, Y.S.; Rajapaksha, A.U.; Lim, J.E.; Kim, B.Y.; Ahn, J.H.; Lee, S.S. Lead and copper immobilization in a shooting range soil using soybean stover-and pine needle-derived biochars: Chemical, microbial and spectroscopic assessments. J. Hazard. Mater. 2016, 301, 179–186. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.; Hashimoto, Y.; Hou, D.; Ok, Y.S. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef]
- Shen, Q.; Hedley, M.; Camps Arbestain, M.; Kirschbaum, M.U.F. Can biochar increase the bioavailability of phosphorus? J. Soil Sci. Plant Nutr. 2016, 16, 268–286. [Google Scholar] [CrossRef][Green Version]
- Shen, Z.; Tian, D.; Zhang, X.; Tang, L.; Su, M.; Zhang, L.; Hou, D. Mechanisms of biochar assisted immobilization of Pb2+ by bioapatite in aqueous solution. Chemosphere 2018, 190, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Igalavithana, A.D.; Lee, S.E.; Lee, Y.H.; Tsang, D.C.; Rinklebe, J.; Kwon, E.E.; Ok, Y.S. Heavy metal immobilization and microbial community abundance by vegetable waste and pine cone biochar of agricultural soils. Chemosphere 2017, 174, 593–603. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.; Peng, T.; Zhang, J.; Tsang, D.C.; Alessi, D.S.; Shen, Z.; Hou, D. Biochar application for the remediation of heavy metal polluted land: A review of in situ field trials. Sci. Total Environ. 2018, 619, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Matouq, M.; Jildeh, N.; Qtaishat, M.; Hindiyeh, M.; Al Syouf, M.Q. The adsorption kinetics and modeling for heavy metals removal from wastewater by Moringa pods. J. Environ. Chem. Eng. 2015, 3, 775–784. [Google Scholar] [CrossRef]
- Saadi, R.; Saadi, Z.; Fazaeli, R.; Fard, N.E. Monolayer and multilayer adsorption isotherm models for sorption from aqueous media. Korean J. Chem. Eng. 2015, 32, 787–799. [Google Scholar] [CrossRef]
- Ho, S.H.; Wang, D.; Wei, Z.S.; Chang, J.S.; Ren, N.Q. Lead removal by a magnetic biochar derived from persulfate-ZVI treated sludge together with one-pot pyrolysis. Bioresour. Technol. 2018, 247, 463–470. [Google Scholar]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and interpretation of adsorption isotherms. J. Chem. 2017. [Google Scholar] [CrossRef]
- Liu, L.; Luo, X.B.; Ding, L.; Luo, S.L. Application of nanotechnology in the removal of heavy metal from water. In Nanomaterials for the Removal of Pollutants and Resource Reutilization; Elsevier: Amsterdam, The Netherlands, 2019; pp. 83–147. [Google Scholar]
- Mustapha, A.A.; Abdu, N.; Jibrin, J.M. Adsorption of Cadmium, Copper, Lead and Zinc on Organically Amended Soil Fractions Using the Freundlich, Langmuir and Dubinin-Raduskevich Models. Int. J. Soil Sci. 2017, 12, 43–53. [Google Scholar] [CrossRef]
- De Castro Alves, L.; Yáñez-Vilar, S.; Piñeiro-Redondo, Y.; Rivas, J. Novel Magnetic Nanostructured Beads for Cadmium (II) Removal. Nanomaterials 2019, 9, 356. [Google Scholar] [CrossRef]
- Song, Z.; Lian, F.; Yu, Z.; Zhu, L.; Xing, B.; Qiu, W. Synthesis and characterization of a novel MnOx-loaded biochar and its adsorption properties for Cu2+ in aqueous solution. Chem. Eng. J. 2014, 242, 36–42. [Google Scholar] [CrossRef]
- Tan, X.F.; Liu, Y.G.; Gu, Y.L.; Xu, Y.; Zeng, G.M.; Hu, X.J.; Li, J. Biochar-based nano-composites for the decontamination of wastewater: A review. Bioresour. Technol. 2016, 212, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Strain, H.H.; SVEC, W.A. Extraction, separation, estimation, and isolation of the chlorophylls. In The Chlorophylls; Academic Press: Cambridge, MA, USA, 1966; pp. 21–66. [Google Scholar]
- Jambunathan, N. Determination and detection of reactive oxygen species (ROS), lipid peroxidation, and electrolyte leakage in plants. In Plant Stress Tolerance; Humana Press: Totowa, NJ, USA, 2010; pp. 291–297. [Google Scholar]
- Cakmak, I.; Marschner, H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Jones, J.B. Plant tissue analysis in micronutrients. Micronutr. Agric. 1991, 4, 477–521. [Google Scholar]
- Manzoor, Q.; Nadeem, R.; Iqbal, M.; Saeed, R.; Ansari, T.M. Organic acids pretreatment effect on Rosa bourbonia phyto-biomass for removal of Pb (II) and Cu (II) from aqueous media. Bioresour. Technol. 2013, 132, 446–452. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H.; Dickey, D.A. Principles and procedures of statistics. In A Bio-Metrical Approach, 3rd ed.; McGraw Hill: New York, NY, USA, 1997. [Google Scholar]
- Khan, W.D.; Ramzani, P.M.A.; Anjum, S.; Abbas, F.; Iqbal, M.; Yasar, A.; Ihsan, M.Z. Potential of miscanthus biochar to improve sandy soil health, in situ nickel immobilization in soil and nutritional quality of spinach. Chemosphere 2017, 185, 1144–1156. [Google Scholar] [CrossRef]
- Tanveer, M.; Ahmed, H.A.I. ROS Signalling in Modulating Salinity Stress Tolerance in Plants. In Salt and Drought Stress Tolerance in Plants; Springer: Cham, Switzerland, 2020; pp. 299–314. [Google Scholar]
- Tanveer, M.; Wang, L. Potential targets to reduce beryllium toxicity in plants: A review. Plant Physiol. Biochem. 2019, 139, 691–696. [Google Scholar] [CrossRef]
- Hatata, M.M.; Abdel-Aal, E.A. Oxidative stress and antioxidant defense mechanisms in response to cadmium treatments. J. Environ. Agric. Sci. 2008, 4, 655–669. [Google Scholar]
- Mishra, S.; Srivastava, S.; Tripathi, R.D.; Govindarajan, R.; Kuriakose, S.V.; Prasad, M.N.V. Phytochelatin synthesis and response of antioxidants during cadmium stress in Bacopa monnieri L. Plant Physiol. Biochem. 2006, 44, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh, L.; Moghaddam, M.; Lakzian, A. Response of summer savory at two different growth stages to biochar amendment under NaCl stress. Arch. Agron. Soil Sci. 2019, 65, 1120–1133. [Google Scholar] [CrossRef]
- Tanveer, M.; Shabala, S. Targeting redox regulatory mechanisms for salinity stress tolerance in crops. In Salinity Responses and Tolerance in Plants; Springer: Cham, Switzerland, 2018; Volume 1, pp. 213–234. [Google Scholar]
- Mohamed, A.A.; Aly, A.A. Iron deficiency stimulated some enzymes activity, lipid peroxidation and free radicals production in Borage officinalis induced in vitro. Int. J. Agric. Biol. 2004, 6, 179–184. [Google Scholar]
- Fourcroy, P.; Vansuyt, G.; Kushnir, S.; Inzé, D.; Briat, J.F. Iron-regulated expression of a cytosolic ascorbate peroxidase encoded by the APX1 gene in Arabidopsis seedlings. Plant Physiol. 2004, 134, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, A.A.; Ghaderi, N. Grape response to salinity stress and role of iron nanoparticle and potassium silicate to mitigate salt induced damage under in vitro conditions. Physiol. Mol. Biol. Plants 2018, 24, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.A.; Kosobryukhov, A.A. Ecophysiology of Plants under Cadmium Toxicity: Photosynthetic and Physiological Responses. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives I; Springer: Singapore, 2020; pp. 429–484. [Google Scholar]
- Su, Y.; Qin, C.; Begum, N.; Ashraf, M.; Zhang, L. Acetylcholine ameliorates the adverse effects of cadmium stress through mediating growth, photosynthetic activity and subcellular distribution of cadmium in tobacco (Nicotiana benthamiana L.). Ecotoxicol. Environ. Saf. 2020, 198, 110671. [Google Scholar] [CrossRef]
- Liu, H.; Yang, L.; Li, N.; Zhou, C.; Feng, H.; Yang, J.; Han, X. Cadmium toxicity reduction in rice (Oryza sativa L.) through iron addition during primary reaction of photosynthesis. Ecotoxicol. Environ. Saf. 2020, 200, 110746. [Google Scholar] [CrossRef]
- Xu, C.Y.; Hosseini-Bai, S.; Hao, Y.; Rachaputi, R.C.; Wang, H.; Xu, Z.; Wallace, H. Effect of biochar amendment on yield and photosynthesis of peanut on two types of soils. Environ. Sci. Pollut. Res. 2015, 22, 6112–6125. [Google Scholar] [CrossRef]
- Younis, U.; Malik, S.A.; Rizwan, M.; Qayyum, M.F.; Ok, Y.S.; Shah, M.H.R.; Ahmad, N. Biochar enhances the cadmium tolerance in spinach (Spinacia oleracea) through modification of Cd uptake and physiological and biochemical attributes. Environ. Sci. Pollut. Res. 2016, 23, 21385–21394. [Google Scholar] [CrossRef]
- Keshavarz Afshar, R.; Hashemi, M.; DaCosta, M.; Spargo, J.; Sadeghpour, A. Biochar application and drought stress effects on physiological characteristics of Silybum marianum. Commun. Soil Sci. Plant Anal. 2016, 47, 743–752. [Google Scholar] [CrossRef]
- Parmar, P.; Kumari, N.; Sharma, V. Structural and functional alterations in photosynthetic apparatus of plants under cadmium stress. Bot. Sci. 2013, 54, 45. [Google Scholar] [CrossRef] [PubMed]
- Bashir, K.; Rasheed, S.; Kobayashi, T.; Seki, M.; Nishizawa, N.K. Regulating subcellular metal homeostasis: The key to crop improvement. Front. Plant Sci. 2016, 7, 1192. [Google Scholar] [CrossRef]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Shahzad, B.; Ashraf, U.; Fahad, S.; Bajwa, A.A. Osmoregulation and antioxidant production in maize under combined cadmium and arsenic stress. Environ. Sci. Pollut. Res. 2016, 23, 11864–11875. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Ullah, E.; Wang, L.; Khan, I.; Shahzad, B. Morpho-physiological growth and yield responses of two contrasting maize cultivars to cadmium exposure. Clean (Weinh) 2016, 44, 29–36. [Google Scholar] [CrossRef]
- Gratão, P.L.; Monteiro, C.C.; Rossi, M.L.; Martinelli, A.P.; Peres, L.E.; Medici, L.O.; Azevedo, R.A. Differential ultrastructural changes in tomato hormonal mutants exposed to cadmium. Environ. Exp. Bot. 2009, 67, 387–394. [Google Scholar] [CrossRef]
- Farhangi-Abriz, S.; Torabian, S. Antioxidant enzyme and osmotic adjustment changes in bean seedlings as affected by biochar under salt stress. Ecotoxicol. Environ. Saf. 2017, 137, 64–70. [Google Scholar] [CrossRef]
- Gupta, D.K.; Pena, L.B.; Romero-Puertas, M.C.; Hernández, A.; Inouhe, M.; Sandalio, L.M. NADPH oxidases differentially regulate ROS metabolism and nutrient uptake under cadmium toxicity. Plant Cell Environ. 2017, 40, 509–526. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation; Routledge: Abingdon, UK, 2015. [Google Scholar]
- Kizito, S.; Luo, H.; Lu, J.; Bah, H.; Dong, R.; Wu, S. Role of nutrient-enriched biochar as a soil amendment during maize growth: Exploring practical alternatives to recycle agricultural residuals and to reduce chemical fertilizer demand. Sustainability 2019, 11, 3211. [Google Scholar] [CrossRef]
- Xu, G.; Lv, Y.; Sun, J.; Shao, H.; Wei, L. Recent advances in biochar applications in agricultural soils: Benefits and environmental implications. Clean (Weinh) 2012, 40, 1093–1098. [Google Scholar] [CrossRef]
- Biederman, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Song, Y.; Jin, L.; Wang, X. Cadmium absorption and transportation pathways in plants. Int. J. Phytoremediat. 2017, 19, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Uraguchi, S.; Kamiya, T.; Sakamoto, T.; Kasai, K.; Sato, Y.; Nagamura, Y.; Fujiwara, T. Low-affinity cation transporter (OsLCT1) regulates cadmium transport into rice grains. Proc. Natl. Acad. Sci. USA 2011, 108, 20959–20964. [Google Scholar] [CrossRef] [PubMed]
- Bari, M.A.; Akther, M.S.; Reza, M.A.; Kabir, A.H. Cadmium tolerance is associated with the root-driven coordination of cadmium sequestration, iron regulation, and ROS scavenging in rice. Plant Physiol. Biochem. 2019, 136, 22–33. [Google Scholar] [CrossRef]
- Ma, X.; Liu, H.; Cao, H.; Qi, R.; Yang, K.; Zhao, R.; Zhang, Y. Genome-wide analysis of zinc-and iron-regulated transporter-like protein family members in apple and functional validation of ZIP10. Biometals 2019, 32, 657–669. [Google Scholar] [CrossRef]
- Yu, R.; Ma, Y.; Li, Y.; Li, X.; Liu, C.; Du, X.; Shi, G. Comparative transcriptome analysis revealed key factors for differential cadmium transport and retention in roots of two contrasting peanut cultivars. BMC Genom. 2018, 19, 938. [Google Scholar] [CrossRef] [PubMed]
- Bashir, K.; Ishimaru, Y.; Nishizawa, N.K. Iron uptake and loading into rice grains. Rice 2010, 3, 122–130. [Google Scholar] [CrossRef]
- Ishimaru, Y.; Takahashi, R.; Bashir, K.; Shimo, H.; Senoura, T.; Sugimoto, K.; Nakanishi, H. Characterizing the role of rice NRAMP5 in manganese, iron and cadmium transport. Sci. Rep. 2012, 2, 286. [Google Scholar] [CrossRef] [PubMed]
- Satoh-Nagasawa, N.; Mori, M.; Nakazawa, N.; Kawamoto, T.; Nagato, Y.; Sakurai, K.; Akagi, H. Mutations in rice (Oryza sativa) heavy metal ATPase 2 (OsHMA2) restrict the translocation of zinc and cadmium. Plant Cell Physiol. 2012, 53, 213–224. [Google Scholar] [CrossRef]
- Miyadate, H.; Adachi, S.; Hiraizumi, A.; Tezuka, K.; Nakazawa, N.; Kawamoto, T.; Satoh-Nagasawa, N. OsHMA3, a P1B-type of ATPase affects root-to-shoot cadmium translocation in rice by mediating efflux into vacuoles. New Phytol. 2011, 189, 190–199. [Google Scholar] [CrossRef]
- Thomine, S.; Wang, R.; Ward, J.M.; Crawford, N.M.; Schroeder, J.I. Cadmium and iron transport by members of a plant metal transporter family in Arabidopsis with homology to Nramp genes. Proc. Natl. Acad. Sci. USA 2000, 97, 4991–4996. [Google Scholar] [CrossRef]
- Nazar, R.; Iqbal, N.; Masood, A.; Khan, M.I.R.; Syeed, S.; Khan, N.A. Cadmium toxicity in plants and role of mineral nutrients in its alleviation. Am. J. Plant Sci. 2012, 3, 1476–1489. [Google Scholar] [CrossRef]
- Clemens, S.; Deinlein, U.; Ahmadi, H.; Höreth, S.; Uraguchi, S. Nicotianamine is a major player in plant Zn homeostasis. Biometals 2013, 26, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Guerinot, M.L. The ZIP family of metal transporters. Biochim. Biophys. Acta. Biomembr. 2000, 1465, 190–198. [Google Scholar] [CrossRef]
- Ismael, M.; Elyamine, A.; Zhao, Y.; Moussa, M.; Rana, M.; Afzal, J.; Hu, C. Can selenium and molybdenum restrain cadmium toxicity to pollen grains in Brassica napus? Int. J. Mol. Sci. 2018, 19, 2163. [Google Scholar] [CrossRef]
- Tran, T.A.; Popova, L.P. Functions and toxicity of cadmium in plants: Recent advances and future prospects. Turk. J. Bot. 2013, 37, 1–13. [Google Scholar]
- Leupin, O.X.; Hug, S.J. Oxidation and removal of arsenic (III) from aerated groundwater by filtration through sand and zero-valent iron. Water Res. 2005, 39, 1729–1740. [Google Scholar] [CrossRef]
- Ming, L.; Naimei, Y.; Huayong, H. Remediation of available Cd and Pb contamination in acidic soil by ferric chloride and ferric sulfate. Chin. J. Environ. Eng. 2015, 9, 469–476. [Google Scholar]
- Wu, C.; Shi, L.; Xue, S.; Li, W.; Jiang, X.; Rajendran, M.; Qian, Z. Effect of sulfur-iron modified biochar on the available cadmium and bacterial community structure in contaminated soils. Sci. Total Environ. 2019, 647, 1158–1168. [Google Scholar] [CrossRef]
- Lyu, H.; Tang, J.; Cui, M.; Gao, B.; Shen, B. Biochar/iron (BC/Fe) composites for soil and groundwater remediation: Synthesis, applications, and mechanisms. Chemosphere 2020, 246, 125609. [Google Scholar] [CrossRef]
- Iqbal, M.J.; Farhan, C.; Khalil, A.; Munawar, I.; Mushtaq, M.; Naeem, M.A.; Bokhari, T.H. Kinetic study of Cr (III) and Cr (VI) biosorption using Rosa damascena phytomass: A rose waste biomass. Asian J. Chem. 2013, 25, 2099–2103. [Google Scholar] [CrossRef]
- Cope, C.O.; Webster, D.S.; Sabatini, D.A. Arsenate adsorption onto iron oxide amended rice husk char. Sci. Total Environ. 2014, 488, 554–561. [Google Scholar] [CrossRef]
- He, R.; Peng, Z.; Lyu, H.; Huang, H.; Nan, Q.; Tang, J. Synthesis and characterization of an iron-impregnated biochar for aqueous arsenic removal. Sci. Total Environ. 2018, 612, 1177–1186. [Google Scholar] [CrossRef]
- Samsuri, A.W.; Sadegh-Zadeh, F.; Seh-Bardan, B.J. Adsorption of As (III) and As (V) by Fe coated biochars and biochars produced from empty fruit bunch and rice husk. J. Environ. Chem. Eng. 2013, 1, 981–988. [Google Scholar] [CrossRef]
- Lefèvre, E.; Bossa, N.; Gardner, C.M.; Gehrke, G.E.; Cooper, E.M.; Stapleton, H.M.; Gunsch, C.K. Biochar and activated carbon act as promising amendments for promoting the microbial debromination of tetrabromobisphenol A. Water Res. 2018, 128, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, B.; Li, Y.; Creamer, A.E.; He, F. Adsorptive removal of arsenate from aqueous solutions by biochar supported zero-valent iron nanocomposite: Batch and continuous flow tests. J. Hazard. Mater 2017, 322, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Li, B.; Huang, H.; Luo, L.; Zhang, J.; Yang, Y.; Zhou, Y. Biochar-based functional materials in the purification of agricultural wastewater: Fabrication, application and future research needs. Chemosphere 2018, 197, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhang, Y.; Gao, B.; Chen, R.; Wu, F. Removal of sulfamethoxazole (SMX) and sulfapyridine (SPY) from aqueous solutions by biochars derived from anaerobically digested bagasse. Environ. Sci. Pollut. Res. 2018, 25, 25659–25667. [Google Scholar] [CrossRef] [PubMed]
| Treatment | Root | Shoot | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DW | Ca | Mg | K | Na | Fe | DW | Ca | Mg | K | Na | Fe | |
| g | mg g−1 | mg kg−1 | g | mg g−1 | mg kg−1 | |||||||
| Control | 0.07 ± 0.01b | 0.59 ± 0.11c | 0.22 ± 0.03c | 0.03 ± 0.01b | 0.48 ± 0.01b | 37.9 ± 11.1b | 0.1 ± 0.02ab | 1.94 ± 0.06a | 0.5 ± 0.03a | 0.18 ± 0.07a | 1.28 ± 0.07b | 37.9 ± 11.1a |
| Cd | 0.01 ± 0.03c | 0.42 ± 0.13d | 0.19 ± 0.06c | 0.01 ± 0.03b | 0.49 ± 0.11b | 71.4 ± 22.3b | 0.07 ± 0.01ab | 0.99 ± 0.001c | 0.2 ± 0.04b | 0.14 ± 0.07b | 0.92 ± 0.09c | 49.1 ± 22.3a |
| Fe-BC | 0.2 ± 0.01a | 2.39 ± 0.09a | 0.47 ± 0.03a | 0.08 ± 0.07b | 0.60 ± 0.02b | 82.5 ± 11.1b | 0.27 ± 0.01a | 1.40 ± 0.13b | 0.3 ± 0.03b | 0.49 ± 0.03c | 1.72 ± 0.02a | 60.2 ± 11.1a |
| Cd+Fe-BC | 0.22 ± 0.07a | 2.17 ± 0.57b | 0.49 ± 0.15b | 0.14 ± 0.05a | 0.93 ± 0.09a | 171.8 ± 11.1a | 0.20 ± 0.04a | 2.10 ± 0.08a | 0.6 ± 0.08a | 0.35 ± 0.02d | 0.72 ± 0.09d | 71.4 ± 22.3a |
| LSD0.05 | 0.03 | 0.15 | 0.06 | 0.09 | 0.16 | 46.48 | 0.17 | 0.2 | 0.06 | 0.04 | 0.17 | 26.83 |
| Parameters | Root Cd mg g−1 | Shoot Cd mg g−1 | DTPA-Extractable Cd in Soil mg kg−1 |
|---|---|---|---|
| Control | n.d. * | n.d. * | 1.21 ± 0.30 |
| Cd | 0.0257 ± 0 | 0.005 ± 0.04 | 1.86 ± 0.06 |
| Fe-BC | n.d. * | n.d. * | 0.78 ± 0.30 |
| Cd+Fe-BC | 0.002 ± 0.02 | n.d. * | 1.21 ± 0.30 |
| Biosorbent | Freundlich | Langmuir | |||||
|---|---|---|---|---|---|---|---|
| KF mg kg−1 | 1/n | R2 | qe mg kg−1 | Xm qmax mg kg−1 | KL mg kg−1 | R2 | |
| Control | 0.1194 | 0.437 | 0.9951 | 2.6422 | 0.6608 | 3.6616 | 0.9993 |
| Cd | 0.788 | 0.0276 | 0.9983 | 0.3358 | 2.1786 | 8.470 | 0.9982 |
| Fe-BC | 0.1254 | 0.3962 | 0.9989 | 2.6621 | 4.9904 | 1.00001 | 0.9998 |
| Cd+Fe-BC | 0.9059 | 0.2879 | 0.9517 | 1.4707 | 9.9786 | 9.7276 | 0.9991 |
| CMP | MDA | APX | CAT | Total Root Phenolic | TRP | TSP | Chl a | Chl b | Total Shoot Phenolic | RDW | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MDA | 0.2773 | ||||||||||
| APX | 0.0811 | −0.7376 ** | |||||||||
| CAT | −0.5720 * | −0.1369 | 0.6464 * | ||||||||
| Total root phenolic | −0.2106 | −0.7745 ** | 0.8945 ** | 0.3861 | |||||||
| TRP | −0.3777 | −0.7020 ** | 0.6899 ** | 0.2769 | 0.7753 ** | ||||||
| TSP | −0.5423 | 0.1428 | −0.4083 | −0.4057 | −0.2044 | 0.2045 | |||||
| Chl a | −0.4664 | −0.9051 ** | 0.5930 * | −0.0510 | 0.6835 ** | 0.5642 * | 0.0128 | ||||
| Chl b | −0.6177 * | −0.7853 ** | 0.6744 ** | 0.0926 | 0.7725 ** | 0.8740 ** | 0.1791 | 0.7359 ** | |||
| Total shoot phenolic | −0.2479 | −0.5678 * | 0.7377 ** | 0.6069* | 0.6948 ** | 0.7633 ** | 0.0428 | 0.5120 | 0.7325 ** | ||
| RDW | −0.5150 | −0.8375 ** | 0.7602 ** | 0.2434 | 0.8772 ** | 0.8833 ** | 0.1127 | 0.7857 ** | 0.9459 ** | 0.8256 ** | |
| SDW | −0.7988 ** | −0.3926 | 0.2593 | −0.0329 | 0.4545 | 0.5952 * | 0.3900 | 0.4585 | 0.7324 ** | 0.6767 ** | 0.7501 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dad, F.P.; Khan, W.-u.-D.; Tanveer, M.; Ramzani, P.M.A.; Shaukat, R.; Muktadir, A. Influence of Iron-Enriched Biochar on Cd Sorption, Its Ionic Concentration and Redox Regulation of Radish under Cadmium Toxicity. Agriculture 2021, 11, 1. https://doi.org/10.3390/agriculture11010001
Dad FP, Khan W-u-D, Tanveer M, Ramzani PMA, Shaukat R, Muktadir A. Influence of Iron-Enriched Biochar on Cd Sorption, Its Ionic Concentration and Redox Regulation of Radish under Cadmium Toxicity. Agriculture. 2021; 11(1):1. https://doi.org/10.3390/agriculture11010001
Chicago/Turabian StyleDad, Fiza Pir, Waqas-ud-Din Khan, Mohsin Tanveer, Pia Muhammad Adnan Ramzani, Rabia Shaukat, and Abdul Muktadir. 2021. "Influence of Iron-Enriched Biochar on Cd Sorption, Its Ionic Concentration and Redox Regulation of Radish under Cadmium Toxicity" Agriculture 11, no. 1: 1. https://doi.org/10.3390/agriculture11010001
APA StyleDad, F. P., Khan, W.-u.-D., Tanveer, M., Ramzani, P. M. A., Shaukat, R., & Muktadir, A. (2021). Influence of Iron-Enriched Biochar on Cd Sorption, Its Ionic Concentration and Redox Regulation of Radish under Cadmium Toxicity. Agriculture, 11(1), 1. https://doi.org/10.3390/agriculture11010001







