Leaf Characteristics at Recovery Stage Affect Seed Oil and Protein Content Under the Interactive Effects of Nitrogen and Waterlogging in Rapeseed
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Cultivation
2.2. Measured Indicators and Methods
2.2.1. Seed Quality
2.2.2. Agronomic Traits
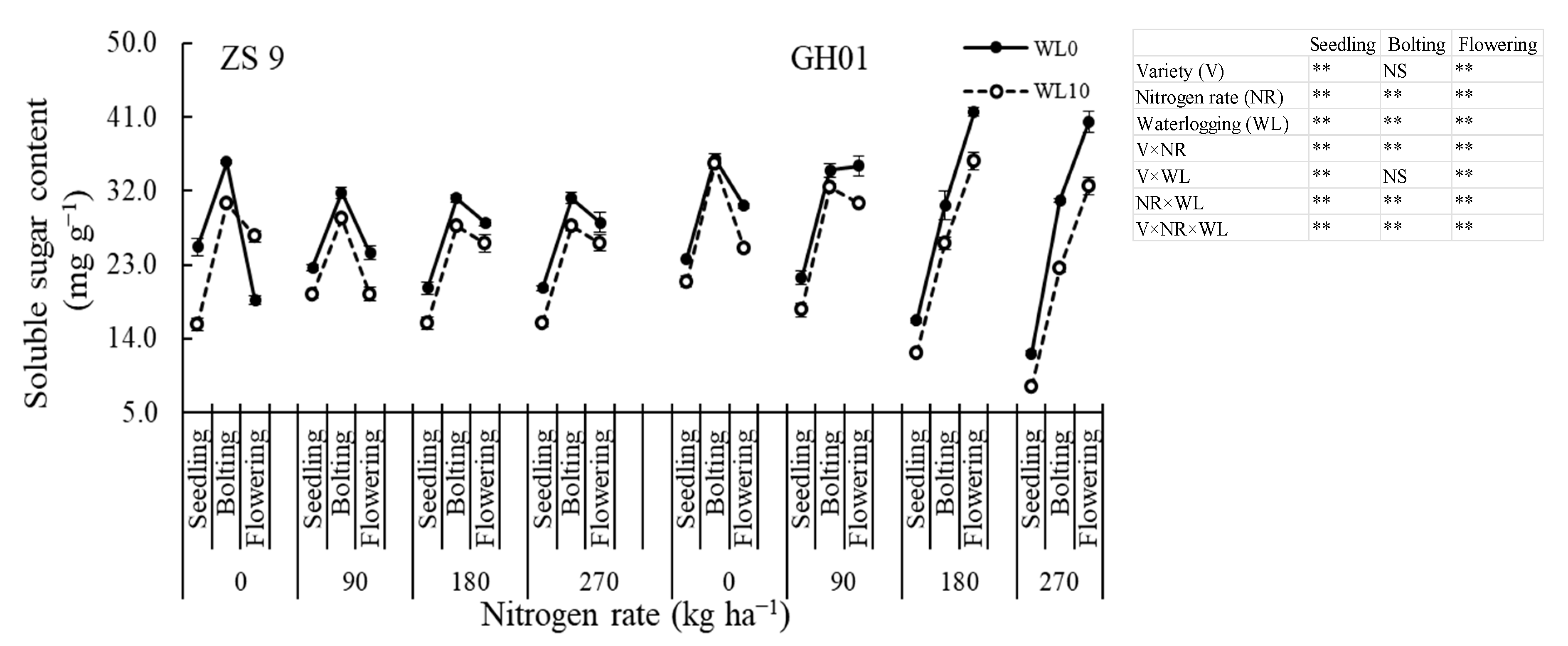
2.2.3. Soluble Sugar Content
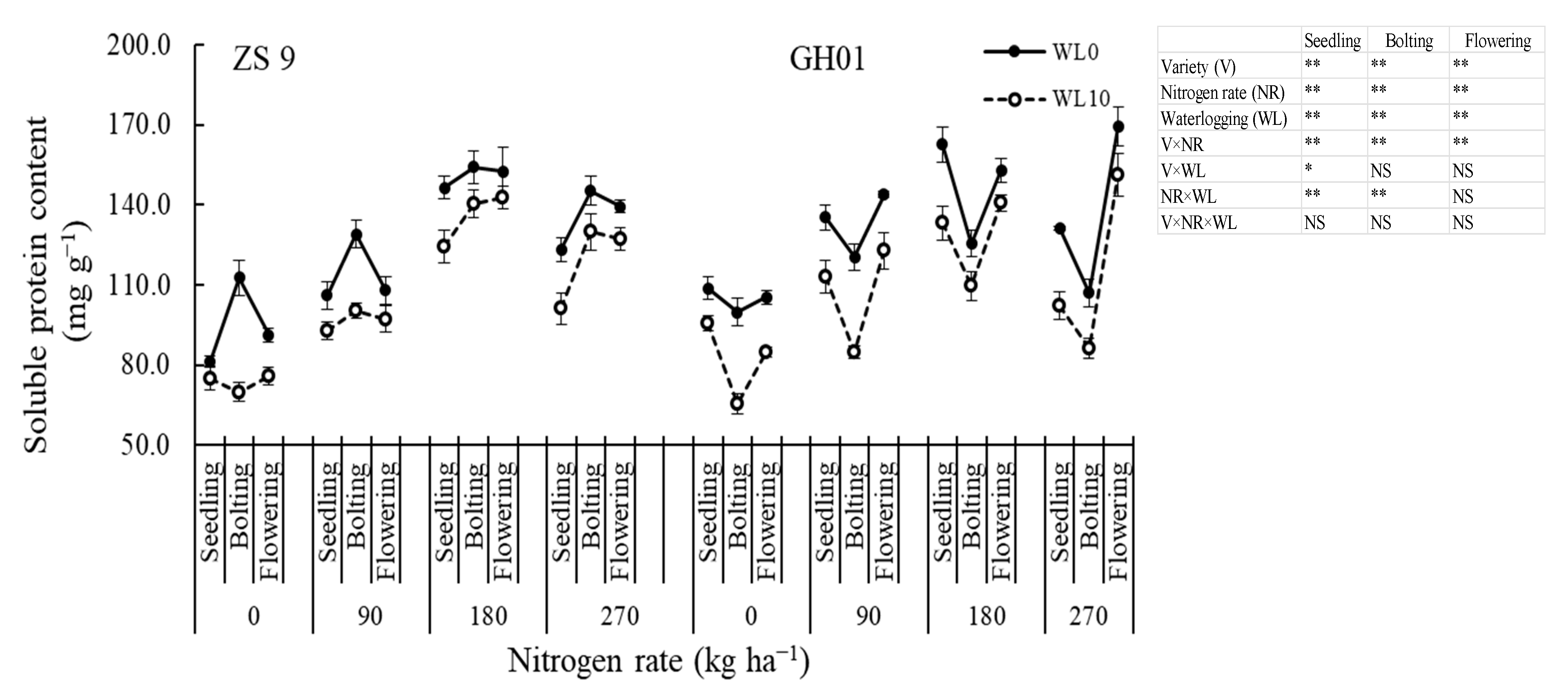
2.2.4. Soluble Protein Content
2.3. Data Analysis
3. Results
3.1. Seed Quality
3.2. Biomass Accumulation and Distribution
3.3. Leaf Characteristics
3.3.1. Number of Leaves, Leaf Area and SPAD
3.3.2. Soluble Sugar Content
3.3.3. Soluble Protein Content
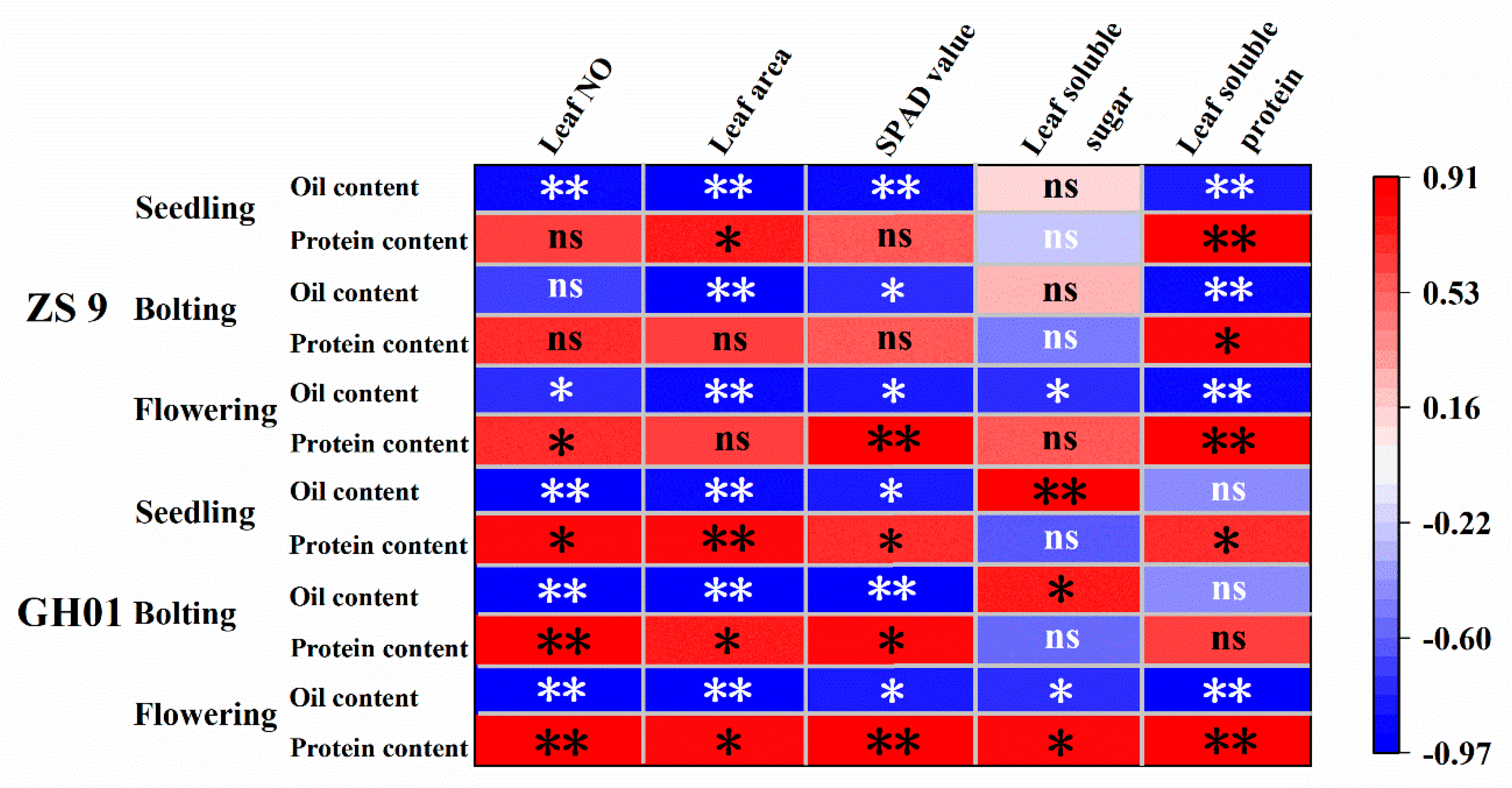
3.4. Relationship Between Leaf Growth and Seed Oil and Protein Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- FAO. Food Outlook–Biannual Report on Global Food Markets. 2016. Available online: http://www.fao.org/3/a-I5703E.pdf (accessed on 20 March 2017).
- Zhou, W.J. Oilseed rape cultivation. In Cultivation of Crops; Ding, Y.S., Ed.; Shanghai Science and Technology Press: Shanghai, China, 1994; pp. 357–380. [Google Scholar]
- Zhou, W.J.; Lin, X.Q. Effects of waterlogging at different growth stages on physiological characteristics and seed yield of winter rape (Brassica napus L). Field Crops Res. 1995, 44, 103–110. [Google Scholar] [CrossRef]
- Zou, X.; Hu, C.; Zeng, L.; Cheng, Y.; Xu, M.; Zhang, X. A comparison of screening methods to identify waterlogging tolerance in the field in Brassica napus L. during plant ontogeny. PLoS ONE 2014, 3, e89731. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Chiariello, N.R.; Gulmon, S.L. Stress effects on plant reproduction. In Response of Plants to Multiple Stresses; Academic Press: San Diego, CA, USA, 1991; pp. 161–188. [Google Scholar]
- Bouchereau, A.; ClossaisBesnard, N.; Bensaoud, A.; Leport, L.; Renard, M. Water stress effects on rapeseed quality. Eur. J. Agron. 1996, 5, 19–30. [Google Scholar] [CrossRef]
- de San Celedonio, R.P.; Abeledo, L.G.; Miralles, D.J. Identifying the critical period for waterlogging on yield and its components in wheat and barley. Plant Soil. 2014, 378, 265–277. [Google Scholar] [CrossRef]
- Arduini, I.; Orlandi, C.; Pampana, S.; Masoni, A. Waterlogging at tillering affects spike and spikelet formation in wheat. Crop Pasture Sci. 2016, 67, 703–711. [Google Scholar] [CrossRef]
- Xu, M.; Ma, H.; Zeng, L.; Cheng, Y.; Lu, G.; Xu, J.; Zhang, X.; Zou, X. The effect of waterlogging on yield and seed quality at the early flowering stage in Brassica napus L. Field Crops Res. 2015, 180, 238–245. [Google Scholar] [CrossRef]
- Boem, F.H.G.; Lavado, R.S.; Porcelli, C.A. Note on the effects of winter and spring waterlogging on growth, chemical composition and yield of rapeseed. Field Crops Res. 1996, 47, 175–179. [Google Scholar] [CrossRef]
- Wollmer, A.C.; Pitann, B.; Muehling, K.H. Waterlogging events during stem elongation or flowering affect yield of oilseed rape (Brassica napus L.) but not seed quality. J. Agron. Crop Sci. 2018, 204, 165–174. [Google Scholar] [CrossRef]
- Striker, G.G. Flooding Stress on Plants: Anatomical, Morphological and Physiological Responses; Mworia, J.K., Ed.; In Tech Open Access Publisher: Rijeka, Croatia, 2012. [Google Scholar]
- Ponnamperuma, F.N. The chemistry of submerged soil. Adv. Agron. 1972, 24, 29–96. [Google Scholar]
- Colmer, T.D.; Voesenek, L.A.C.J. Flooding tolerance: Suites of plant traits in variable environments. Funct. Plant Biol. 2009, 36, 665–681. [Google Scholar] [CrossRef]
- Malik, A.I.; Colmer, T.D.; Lambers, H.; Setter, T.L.; Schortemeyer, M. Short-term waterlogging has long-term effects on the growth and physiology of wheat. New Phytol. 2002, 153, 225–236. [Google Scholar] [CrossRef]
- Araki, H.; Hamada, A.; Hossain, M.A.; Takahashi, T. Waterlogging at jointing and/or after anthesis in wheat induces early leaf senescence and impairs grain filling. Field Crops Res. 2012, 137, 27–36. [Google Scholar] [CrossRef]
- Meyer, W.S.; Barrs, H.D.; Mosier, A.R.; Schaefer, N.L. Response of maize to three short-term periods of waterlogging at high and low nitrogen levels on undisturbed and repacked soil. Irrigation Sci. 1987, 8, 257–272. [Google Scholar] [CrossRef]
- Sigua, G.C.; Williams, M.; Grabowski, J.; Chase, C.; Kongchum, M. Effect of flooding duration and nitrogen fertilization on yield and protein content of three forage species. Agron. J. 2012, 104, 791–798. [Google Scholar] [CrossRef][Green Version]
- Simpson, N.L.; Brennan, R.F.; Anderson, W.K. Grain yield increases in wheat and barley to nitrogen applied after transient waterlogging in the high rainfall cropping zone of western Australia. J. Plant Nutr. 2016, 39, 974–992. [Google Scholar] [CrossRef]
- Zhang, B.G.; Puard, M.; Couchat, P. Effect of hypoxia, acidity and nitrate on inorganic nutrition in rice plants. Plant Physiol. Bioch. 1990, 28, 655–661. [Google Scholar]
- Ashraf, M.; Habibur, R. Interactive effects of nitrate and long-term waterlogging on growth, water relations, and gaseous exchange properties of maize (Zea mays L.). Plant Sci. 1999, 144, 35–43. [Google Scholar] [CrossRef]
- Ponnamperuma, F.N. Effects of flooding on soils. Chapter 2, In Flooding and Plant Growth; Kozlowski, T.T., Ed.; Academic Press: Orlando, FL, USA, 1984; pp. 9–193. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1995. [Google Scholar]
- Wang, C.; Hai, J.; Yang, J.; Tian, J.; Chen, W.; Chen, T.; Luo, H.; Wang, H. Influence of leaf and silique photosynthesis on seeds yield and seeds oil quality of oilseed rape (Brassica napus L.). Eur. J. Agron. 2016, 74, 112–118. [Google Scholar] [CrossRef]
- Paul, M.J.; Foyer, C.H. Sink regulation of photosynthesis. J. Exp. Bot. 2001, 52, 1383–1400. [Google Scholar] [CrossRef]
- Seebauer, J.R.; Singletary, G.W.; Krumpelman, P.M.; Ruffo, M.L.; Below, F.E. Relationship of source and sink in determining kernel composition of maize. J. Exp. Bot. 2010, 61, 511–519. [Google Scholar] [CrossRef]
- Gironde, A.; Etienne, P.; Trouverie, J.; Bouchereau, A.; Le Caherec, F.; Leport, L.; Orsel, M.; Niogret, M.F.; Nesi, N.; Carole, D.; et al. The contrasting N management of two oilseed rape genotypes reveals the mechanisms of proteolysis associated with leaf N remobilization and the respective contributions of leaves and stems to N storage and remobilization during seed filling. BMC Plant Biol. 2015, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhao, D.; Lin, X. Effects of waterlogging on nitrogen accumulation and alleviation of waterlogging damage by application of nitrogen fertilizer and mixtalol in winter rape (Brassica napus L). J. Plant Growth Regul. 1997, 16, 47–53. [Google Scholar] [CrossRef]
- Leul, M.; Zhou, W.J. Alleviation of waterlogging damage in winter rape by application of uniconazole-Effects on morphological characteristics, hormones and photosynthesis. Field Crops Res. 1998, 59, 121–127. [Google Scholar] [CrossRef]
- Zou, X.L.; Zeng, L.; Lu, G.Y.; Cheng, Y.; Xu, J.S.; Zhang, X.K. Comparison of transcriptomes undergoing waterlogging at the seedling stage between tolerant and sensitive varieties of Brassica napus L. J. Integr. Agric. 2015, 14, 1723–1734. [Google Scholar] [CrossRef]
- Lancashire, P.D.; Bleiholder, H.; Boom, T.V.D.; Langelüddeke, P.; Stauss, R.; Weber, E.; Witzenberger, A. A uniform decimal code for growth stages of crops and weeds. Ann. Appl. Biol. 1991, 119, 561–601. [Google Scholar] [CrossRef]
- Rathke, G.W.; Behrens, T.; Diepenbrock, W. Integrated nitrogen management strategies to improve seed yield, oil content and nitrogen efficiency of winter oilseed rape (Brassica napus L.): A review. Agr. Ecosyst. Environ. 2006, 117, 80–108. [Google Scholar] [CrossRef]
- Diepenbrock, W. Yield analysis of winter oilseed rape (Brassica napus L.): A review. Field Crops Res. 2000, 67, 35–49. [Google Scholar] [CrossRef]
- Brandt, S.A.; Malhi, S.S.; Ulrich, D.; Lafond, G.R.; Kutcher, H.R.; Johnston, A.M. Seeding rate, fertilizer level and disease management effects on hybrid versus open pollinated canola (Brassica napus L.). Can J. Plant Sci. 2007, 87, 255–266. [Google Scholar] [CrossRef]
- Arduini, I.; Baldanzi, M.; Pampana, S. Reduced growth and nitrogen uptake during waterlogging at tillering permanently affect yield components in late sown oats. Front Plant Sci. 2019, 10. [Google Scholar]
- Jiang, D.; Fan, X.; Dai, T.; Cao, W. Nitrogen fertilizer rate and post-anthesis waterlogging effects on carbohydrate and nitrogen dynamics in wheat. Plant Soil. 2008, 304, 301–314. [Google Scholar] [CrossRef]
- Araki, H.; Hossain, M.A.; Takahashi, T. Waterlogging and hypoxia have permanent effects on wheat root growth and respiration. J. Agron. Crop Sci. 2012, 198, 264–275. [Google Scholar] [CrossRef]
- Cannell, R.Q.; Belford, R.K.; Blackwell, P.S.; Govi, G.; Thomson, R.J. Effects of waterlogging on soil aeration and on root and shoot growth and yield of winter oats (Avena sativa L.). Plant Soil. 1985, 85, 361–373. [Google Scholar] [CrossRef]
- Masoni, A.; Pampana, S.; Arduini, I. Barley response to waterlogging duration at tillering. Crop Sci. 2016, 56, 2722–2730. [Google Scholar] [CrossRef]
- Iwasa, Y.; Roughgarden, J. Shoot/root balance of plants: Optimal growth of a system with many vegetative organs. Theor. Popul. Biol. 1984, 25, 78–105. [Google Scholar] [CrossRef]
- Huang, B.R.; Johnson, J.W.; Nesmith, S.; Bridges, D.C. Growth, physiological and anatomical responses of two wheat genotypes to waterlogging and nutrient supply. J. Exp. Bot. 1994, 45, 193–202. [Google Scholar] [CrossRef]
- Hua, W.; Li, R.J.; Zhan, G.M.; Liu, J.; Li, J.; Wang, X.F.; Liu, G.H.; Wang, H.Z. Maternal control of seed oil content in Brassica napus: The role of silique wall photosynthesis. Plant J. 2012, 69, 432–444. [Google Scholar] [CrossRef]
- Allen, E.J.; Morgan, D.G.; Ridgman, W.J. A physiological analysis of the growth of oilseed rape. J. Agric. Sci. 1971, 77, 339–341. [Google Scholar] [CrossRef]
- Tayo, T.O.; Morgan, D.G. Factors influencing flower and pod development in oil-seed rape (Brassica napus L.). J. Agr. Sci. 1979, 92, 363–373. [Google Scholar] [CrossRef]
- Papakosta, D.K. Phosphorus accumulation and translocation in wheat as affected by cultivar and nitrogen fertilization. J. Agron. Crop Sci. 1994, 173, 260–270. [Google Scholar] [CrossRef]
- Papakosta, D.K.; Gagianas, A.A. Nitrogen and dry matter accumulation, remobilization, and losses for Mediterranean wheat during grain filling. Agron. J. 1991, 83, 864–870. [Google Scholar] [CrossRef]
- Ehdaie, B.; Alloush, G.A.; Madore, M.A.; Waines, J.G. Genotypic variation for stem reserves and mobilization in wheat: I. postanthesis changes in internode dry matter. Crop Sci. 2006, 46, 735–746. [Google Scholar] [CrossRef]
- Ehdaie, B.; Alloush, G.A.; Madore, M.A.; Waines, J.G. Genotypic variation for stem reserves and mobilization in wheat: II. Postanthesis changes in internode water-soluble carbohydrates. Crop Sci. 2006, 46, 2093–2103. [Google Scholar] [CrossRef]
- Patterson, D.T.; Bunce, J.A.; Volkenburgh, A.E.V. Photosynthesis in relation to leaf characteristics of cotton from controlled and field Environments. Plant Physiol. 1977, 59, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Osaki, M.; Tadano, T. Effect of potassium nutrition on translocation of photosynthesized 14C and carbon-nitrogen metabolism in leaves of various crop plants. In Plant Nutrition for Sustainable Food Production and Environment; Springer: Dordrecht, The Netherlands, 1997. [Google Scholar]
- Santiago, J.P.; Tegeder, M. Implications of nitrogen phloem loading for carbon metabolism and transport during Arabidopsis development. J. Integr. Plant Biol. 2017, 6, 63–75. [Google Scholar]
- Zhang, X.Q.; Li, K.C.; Xing, R.E.; Liu, S.; Li, P.C. Metabolite profiling of wheat seedlings induced by chitosan: Revelation of the enhanced carbon and nitrogen metabolism. Front Plant Sci. 2017, 8, 2017. [Google Scholar] [CrossRef]
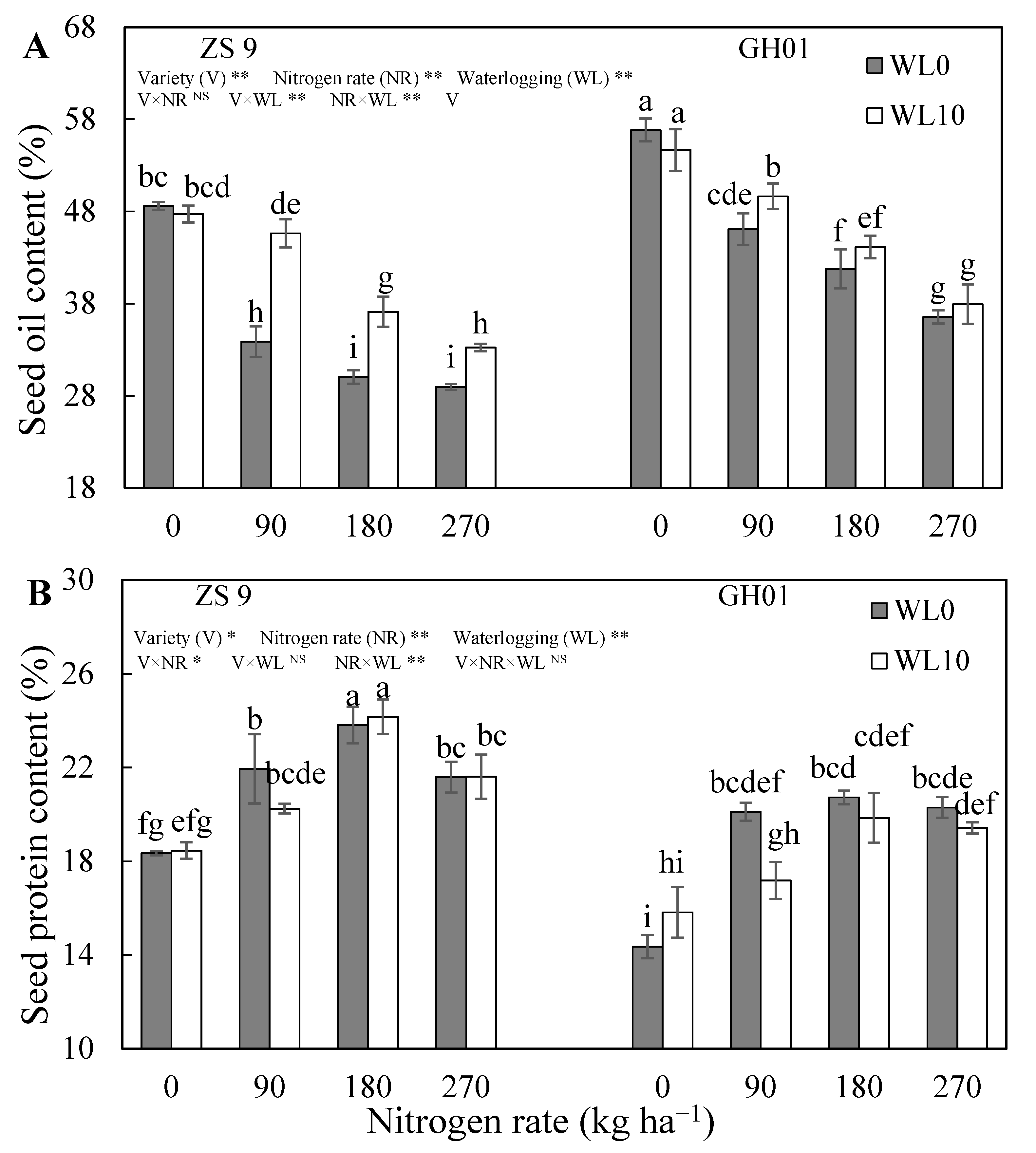
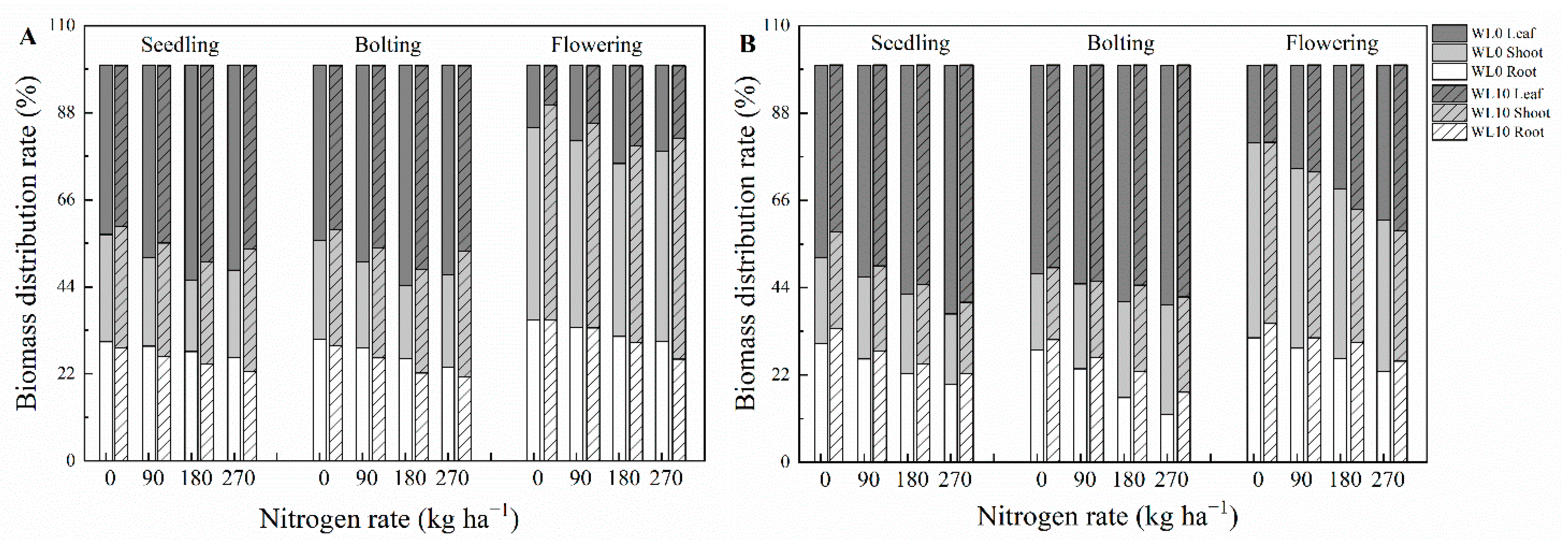
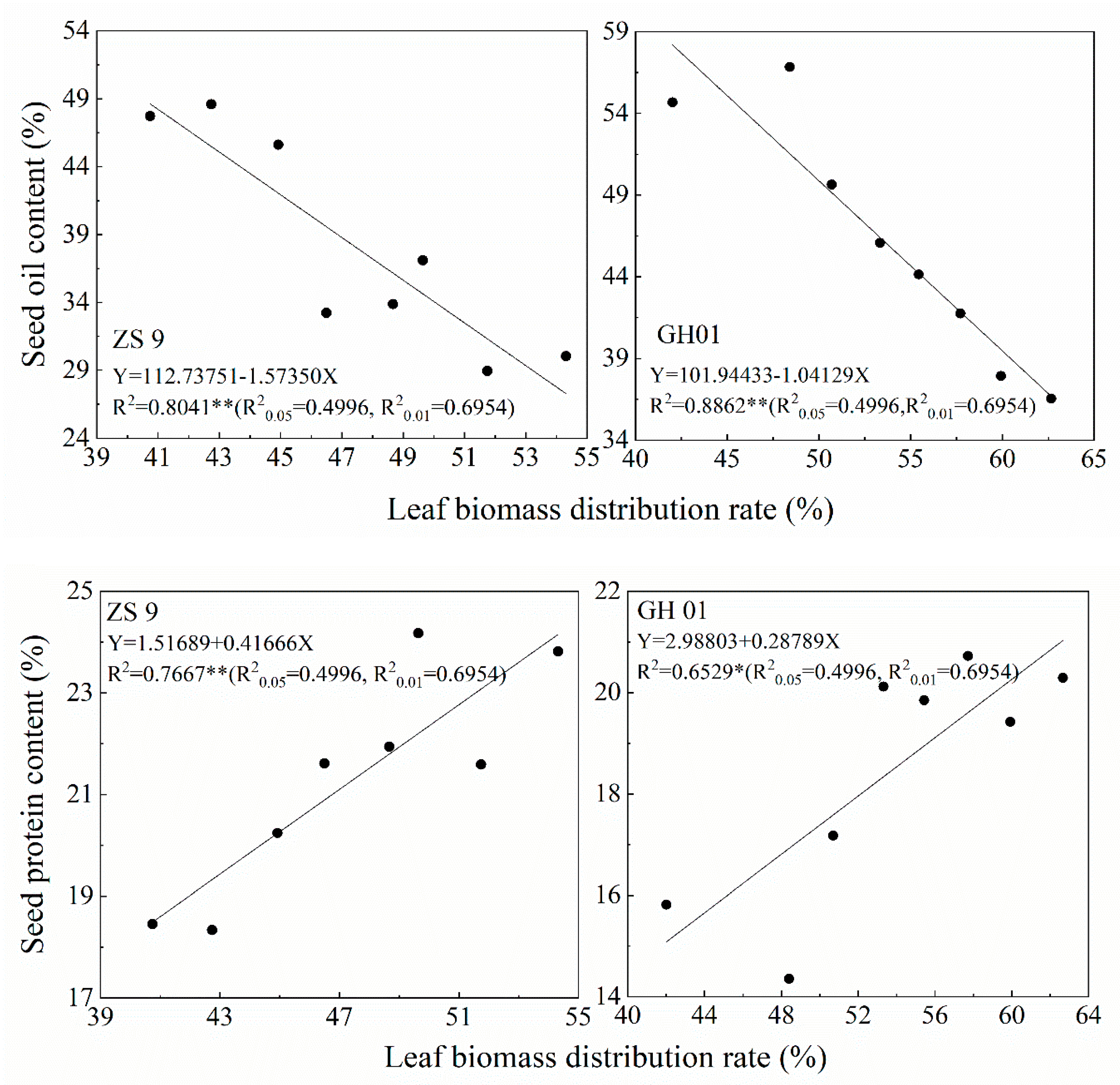
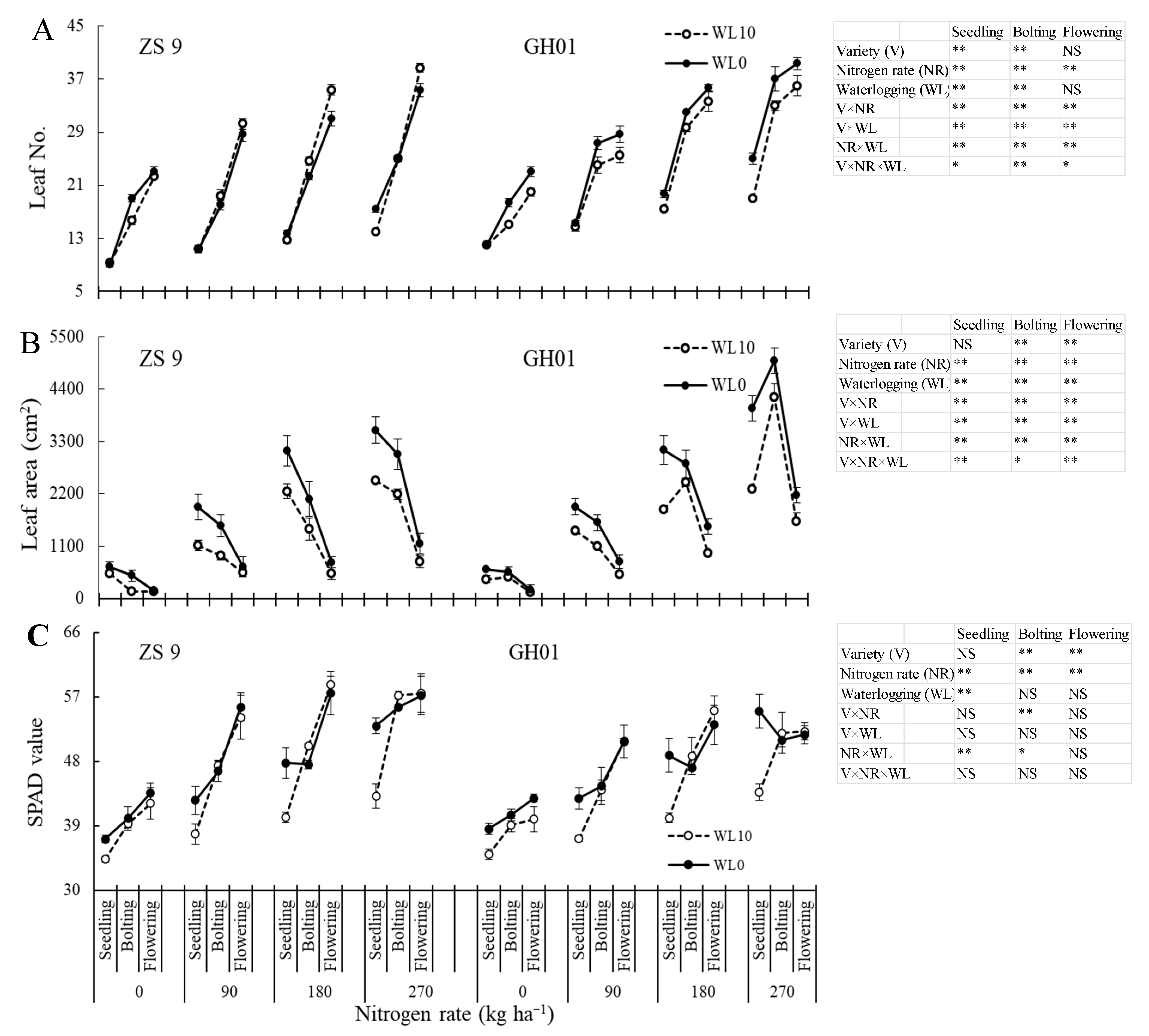
| Nitrogen Rate (kg ha−1) | Days of Waterlogging | ZS 9 | GH01 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Seedling | Bolting | Flowering | Maturity | Seedling | Bolting | Flowering | Maturity | ||
| 0 | WL0 | 9.93d | 13.35f | 14.43f | 15.05g | 5.12f | 7.54f | 9.93f | 15.64g |
| WL10 | 5.82f | 8.05g | 10.93g | 14.30g | 2.40h | 4.18g | 6.78g | 13.77h | |
| 90 | WL0 | 13.47c | 31.95d | 40.72e | 51.74f | 8.59e | 23.50d | 34.10e | 40.33f |
| WL10 | 8.04e | 22.28e | 50.74d | 71.63d | 4.30g | 15.84e | 35.43e | 47.12e | |
| 180 | WL0 | 19.80b | 45.52c | 60.45c | 65.21e | 16.13b | 45.67b | 55.24d | 60.24d |
| WL10 | 13.57c | 31.89d | 80.48a | 89.95b | 10.60d | 30.28c | 72.40b | 83.85b | |
| 270 | WL0 | 26.66a | 55.18a | 66.09b | 73.68c | 20.23a | 54.87a | 63.63c | 70.08c |
| WL10 | 18.92b | 46.85b | 79.15a | 94.72a | 11.90c | 45.71b | 80.43a | 92.74a | |
| Analyses of variance | |||||||||
| Variety (V) | ** | ** | ** | ** | |||||
| Nitrogen rate (NR) | ** | ** | ** | ** | |||||
| Waterlogging (WL) | ** | ** | ** | ** | |||||
| V × NR | ** | ** | ** | ** | |||||
| V × WL | * | NS | ** | ** | |||||
| NR × WL | ** | ** | ** | ** | |||||
| V × NR × WL | NS | ** | ** | ** | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuai, J.; Li, X.; Xie, Y.; Li, Z.; Wang, B.; Zhou, G. Leaf Characteristics at Recovery Stage Affect Seed Oil and Protein Content Under the Interactive Effects of Nitrogen and Waterlogging in Rapeseed. Agriculture 2020, 10, 207. https://doi.org/10.3390/agriculture10060207
Kuai J, Li X, Xie Y, Li Z, Wang B, Zhou G. Leaf Characteristics at Recovery Stage Affect Seed Oil and Protein Content Under the Interactive Effects of Nitrogen and Waterlogging in Rapeseed. Agriculture. 2020; 10(6):207. https://doi.org/10.3390/agriculture10060207
Chicago/Turabian StyleKuai, Jie, Xiaoyong Li, Yan Xie, Zhen Li, Bo Wang, and Guangsheng Zhou. 2020. "Leaf Characteristics at Recovery Stage Affect Seed Oil and Protein Content Under the Interactive Effects of Nitrogen and Waterlogging in Rapeseed" Agriculture 10, no. 6: 207. https://doi.org/10.3390/agriculture10060207
APA StyleKuai, J., Li, X., Xie, Y., Li, Z., Wang, B., & Zhou, G. (2020). Leaf Characteristics at Recovery Stage Affect Seed Oil and Protein Content Under the Interactive Effects of Nitrogen and Waterlogging in Rapeseed. Agriculture, 10(6), 207. https://doi.org/10.3390/agriculture10060207





