Sugarcane Plant Growth and Physiological Responses to Soil Salinity during Tillering and Stalk Elongation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Culture and Treatments
2.2. Measurements
2.3. Experimental Design and Data Analyses
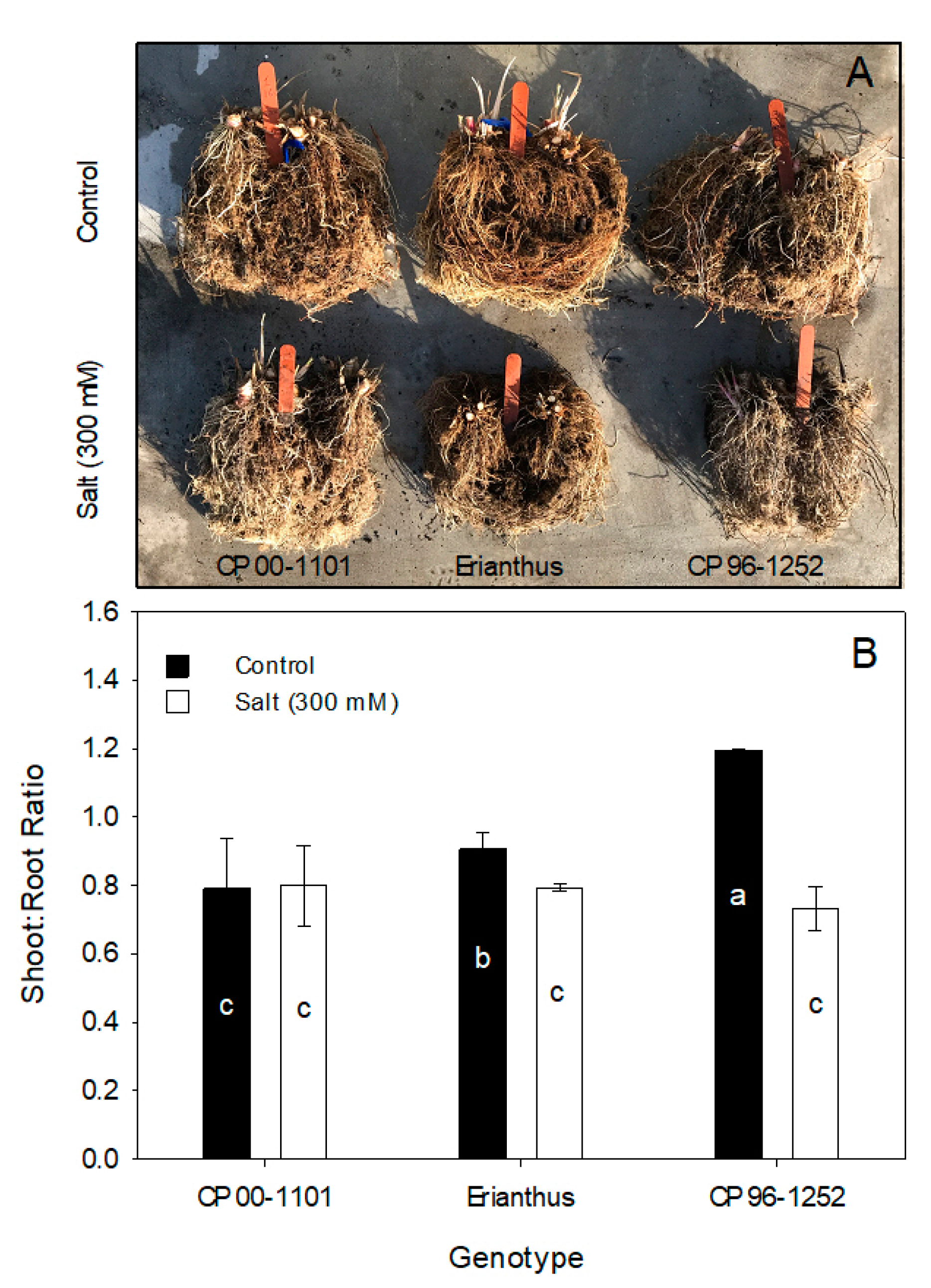
3. Results and Discussions
3.1. Plant Height, Nodes, and Tillers
3.2. Leaf SPAD Readings and Chlorophyll QY
3.3. Leaf Photosynthesis Characters
3.4. Leaf Nonstructural Carbohydrates
3.5. Plant Tissue Dry Weights
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wahid, A.; Rao, A.; Rasul, E. Identification of salt tolerance traits in sugarcane lines. Field Crops Res. 1997, 54, 9–17. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Shomeili, M.; Nabipour, M.; Meskarbashee, M.; Memari, H.R. Evaluation of sugarcane (Saccharum officinarum L.) somaclonals tolerance to salinity via in vitro and in vivo. HAYATI J. Biosci. 2011, 18, 91–96. [Google Scholar] [CrossRef][Green Version]
- Simões, W.L.; Calgaro, M.; Coelho, D.S.; dos Santos, D.B.; de Souza, M.A. Growth of sugar cane varieties under salinity. Rev. Ceres 2017, 63, 265–271. [Google Scholar] [CrossRef]
- Bernstein, L. Effects of salinity and sodality on plant growth. Ann. Rev. Phytopathol. 1975, 13, 295–312. [Google Scholar] [CrossRef]
- Bernstein, L. Salt tolerance of plants. U. S. Dep. Agric. Agric. Inf. Bull. 1964, 283, 23. [Google Scholar]
- Brenes, M.; Pérez, J.; González-Orenga, S.; Solana, A.; Boscaiu, M.; Prohens, J.; Plazas, M.; Fita, A.; Vicente, O. Comparative studies on the physiological and biochemical responses to salt stress of eggplant (Solanum melongena) and its rootstock S. torvum. Agriculture 2020, 10, 328. [Google Scholar] [CrossRef]
- Courtney, P.L.; Cousins, A.B.; Offermann, S.; Okita, T.W.; Edwards, G.E. The effects of salinity on photosynthesis and growth of the single-cell C4 species Bienertia sinuspersici (Chenopodiaceae). Photosynth. Res. 2010, 106, 201–214. [Google Scholar]
- Bliss, M.B.; Smart, C.M.; Maricle, K.L.; Maricle, B.R. Effects of increasing salinity on photosynthesis and plant water potential in Kansas salt marsh species. Trans. Kans. Acad. Sci. 2019, 122, 49–58. [Google Scholar]
- Munns, R.; James, R.A.; Lauchil, A. Approaches to increasing the salt tolerance of wheat and other cereals. J. Exp. Biol. 2006, 57, 1025. [Google Scholar] [CrossRef]
- Sudhir, P.; Murthy, S.D.S. Effects of salt stress on basic processes of photosynthesis. Photosynthetica 2004, 42, 481–486. [Google Scholar] [CrossRef]
- Hare, P.D.; Cress, W.A.; Staden, J.V. Dissecting the roles of osmolyte accumulation during stress. Plant Cell Environ. 1998, 21, 535. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Ann. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef] [PubMed]
- Huaser, M.T.; Aufsatz, W.; Jonak, C.; Luschnig, C. Transgenerational epigenetic inheritance in plants. Biochim. Biophys. Acta 2011, 1809, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, L.; Clark, R.A.; Francois, L.E.; Derderian, M.D. Salt tolerance of N. Co. varieties of sugar cane. II. Effects of soil salinity and sprinkling on chemical composition. Agron. J. 1966, 58, 503–507. [Google Scholar] [CrossRef]
- Hussain, A.; Khan, Z.I.; Ashraf, M.; Rashid, M.H.; Akhtar, M.S. Effect of salt stress on some growth attributes of sugarcane cultivars CP-77-400 and COJ-84. Int. J. Agric. Biol. 2004, 6, 188–191. [Google Scholar]
- Christy, P.M.; Preetha, R.D.; Vasantha, S.; Divya, D. Biochemical and molecular analysis of sugarcane genotypes response to salinity and drought. Int. J. Appl. Biol. Pharmaceut. Technol. 2013, 4, 210–218. [Google Scholar]
- de Almeida Moreira, B.R.; da Silva Viana, R.; de Figueiredo, P.A.M.; Lisboa, L.A.M.; Miasaki, C.T.; Magahães, A.C.; Ramos, S.B.; de Almeida Viana, C.R.; Trindade, V.D.R.; May, A. Glyphosate plus carboxylic compounds boost activity of free radical-scavenging enzymes in sugarcane. Agriculture 2020, 10, 106. [Google Scholar] [CrossRef]
- Lingle, S.E.; Wiegand, C.L. Soil salinity and sugarcane juice quality. Field Crops Res. 1997, 54, 259–268. [Google Scholar] [CrossRef]
- Wiedenfeld, B. Effects of irrigation water salinity and electrostatic water treatment for sugarcane production. Agric. Water Manag. 2008, 95, 85–88. [Google Scholar] [CrossRef]
- Zhao, D. The USDA-ARS Sugarcane Field Station in Canal Point, Florida: 100 years of scientific research and sugarcane cultivar development. Sugar J. 2020, 82, 13–21. [Google Scholar]
- McCord, P.H.; (USDA-ARS Sugarcane Field Station, Canal Point, FL, USA). Unpublished work. 2015.
- Gandonou, C.B.; Gnancadja, L.S.; Abrini, J.; Skali-Senhaji, N. Salinity tolerance of some sugarcane (Saccharum sp.) cultivars in hydroponic medium. Int. Sugar J. 2012, 114, 190–196. [Google Scholar]
- Padmathilake, K.R.E.; Wickramaarachchi, V.N.; Anver, M.A.M.S.; Bandara, D.C. Biological and economical feasibility of growing mint (Mentha sylvestris), mustard (Brassica integrifolia) and asamodagam (Trachyspermum involucratum) under hydroponics. Trop. Agric. Res. 2007, 19, 193–201. [Google Scholar]
- Hussain, A.; Khan, Z.I.; Rashid, M.H.; Ashraf, M.; Akhtar, M.S. Soil salinity effects on sugarcane productivity, biochemical characteristics, and invertase activity. Pak. J. Life Social Sci. 2003, 1, 114–121. [Google Scholar]
- Berding, N.; Roach, B.T. Germplasm collection, maintenance, and use. In Sugarcane Improvement through Breeding; Heinz, D.J., Ed.; Elsevier: Amsterdam, The Netherlands, 1987; pp. 143–210. [Google Scholar]
- Edmé, S.J.; Tai, P.Y.P.; Glaz, B.; Gilbert, R.A.; Miller, J.D.; Davidson, J.O.; Dunckelman, J.W.; Comstock, J.C. Registration of ‘CP 96-1252′ sugarcane. Crop Sci. 2005, 45, 423. [Google Scholar] [CrossRef]
- Gilbert, R.A.; Comstock, J.C.; Glaz, B.; Edmé, S.J.; Davidson, R.W.; Glynn, N.C.; Miller, J.D.; Tai, P.Y.P. Registration of ‘CP 00-1101′ sugarcane. J. Plant Reg. 2008, 2, 95–101. [Google Scholar] [CrossRef]
- VanWeelden, M.; Rice, R.; Davidson, R.W.; Swanson, S. Sugarcane variety census: Florida 2016. Sugar J. 2017, 80, 12–24. [Google Scholar]
- Zhao, D.; Glaz, B.; Irey, M.S.; Hu, C.J. Sugarcane genotype variation in leaf photosynthesis properties and yield as affected by mill mud application. Agron. J. 2015, 107, 506–514. [Google Scholar] [CrossRef]
- Zhao, D.; Oosterhuis, D.M. Cotton responses to shade at different growth stages: Nonstructural carbohydrate composition. Crop Sci. 1998, 38, 1196–1203. [Google Scholar] [CrossRef]
- Zhao, D.; MacKown, C.T.; Starks, P.J.; Kindiger, B.K. Rapid analysis of nonstructural carbohydrate components in grass forage using microplate enzymatic assays. Crop Sci. 2010, 50, 1537–1545. [Google Scholar] [CrossRef]
- Zhao, D.; Glaz, B.; Comstock, J.C. Sugarcane leaf photosynthesis and growth characters during development of water-deficit stress. Crop Sci. 2013, 53, 1066–1075. [Google Scholar] [CrossRef]
- SAS Institute. The SAS system for Windows. In Release 9.0; SAS Inst.: Cary, NC, USA, 2002. [Google Scholar]
- Netondo, G.W.; Onyango, J.C.; Beck, E. Sorghum and salinity: II. Gas exchange and chlorophyll fluorescence of sorghum under salt stress. Crop Sci. 2004, 44, 806–811. [Google Scholar] [CrossRef]
- Mehta, P.; Jajoo, A.; Mathur, S.; Bharti, S. Chlorophyll a fluorescence study revealing effects of high salt stress on Photosystem II in wheat leaves. Plant Physiol. Biochem. 2010, 48, 16–20. [Google Scholar] [CrossRef]
- Sun, Z.W.; Ren, L.K.; Fan, J.W.; Li, Q.; Wang, K.J.; Guo, M.M.; Wang, L.; Li, J.; Zhang, G.X.; Yang, Z.Y.; et al. Salt response of photosynthetic electron transport system in wheat cultivars with contrasting tolerance. Plant Soil Environ. 2016, 62, 515–521. [Google Scholar] [CrossRef]
- Belkhodja, R.; Morales, F.; Abadia, A.; Gomezaparisi, J.; Abadia, J. Chlorophyll fluorescence as a possible tool for salinity tolerance screening in barley (Hordeum vulgare L.). Plant Physiol. 1994, 104, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Vasantha, S.; Venkataramana, S.; Rao, P.N.G.; Gomathi, R. Long term salinity effect on growth, photosynthesis and osmotic characteristics in sugarcane. Sugar Tech. 2010, 12, 5–8. [Google Scholar] [CrossRef]
- Tahjib-UI-Arif, M.; Sohag, A.A.M.; Afrin, S.; Bashar, K.K.; Afrin, T.; Mahamud, A.S.U.; Polash, M.A.S.; Hossain, M.T.; Sohel, M.A.T.; Brestic, M.; et al. Differential response of sugar beet to long-term mild to severe salinity in a soil–pot culture. Agriculture 2019, 9, 223. [Google Scholar] [CrossRef]
- Sharwood, R.E.; Sonawane, B.V.; Ghannoum, O. Photosynthetic flexibility in maize exposed to salinity and shade. J. Exp. Bot. 2014, 65, 3715–3724. [Google Scholar] [CrossRef]
- Yan, K.; Shao, H.; Shao, C.; Chen, P.; Zhao, S.; Brestic, M.; Chen, X. Physiological adaptive mechanisms of plants grown in saline soil and implications for sustainable saline agriculture in coastal zone. Acta Physiol. Plant. 2013, 35, 2867–2878. [Google Scholar] [CrossRef]
- Yeo, A.R.; Caporn, S.J.M.; Floers, T.J. The Effect of salinity upon photosynthesis in rice (Oryza sativa L.): Gas exchange by individual leaves in relation to their salt content. J. Exp. Bot. 1985, 36, 1240–1248. [Google Scholar] [CrossRef]
- Peng, J.; Liu, J.R.; Zhang, L.; Luo, J.Y.; Dong, H.L.; Ma, Y.; Zhao, X.H.; Chen, B.L.; Sui, N.; Zhou, Z.G.; et al. Effects of soil salinity on sucrose metabolism in cotton leaves. PLoS ONE 2016, 11, e0156241. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Wattana, P.; Thitisaksakul, M. Effects of salinity stress on growth and carbohydrate metabolism in three rice (Oryza sativa L.) cultivars differing in salinity tolerance. Indian J. Exp. Biol. 2008, 46, 736–742. [Google Scholar]
- Yin, Y.G.; Kobayashi, Y.; Sanuki, A.; Kondo, S.; Fukuda, N.; Ezura, H.; Sugaya, S.; Matsukura, C. Salinity induces carbohydrate accumulation and sugar regulated starch biosynthetic genes in tomato (Solanum lycopersicum L. cv. ‘Micro-Tom’) fruits in an ABA- and osmotic stress-independent manner. J. Exp. Bot. 2010, 61, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Gomathi, R.; Thandapani, P.V. Sugar metabolism and carbon partitioning of sugarcane genotypes under salinity stress condition. Sugar Tech. 2004, 6, 151–158. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 701596. [Google Scholar] [CrossRef] [PubMed]
- Munns, R. Physiological process limiting plant growth in saline soils: Some dogmas and hypotheses. Plant Cell Environ. 1993, 16, 15–24. [Google Scholar] [CrossRef]
- Vikas, Y.P.; Bhargava, S.; Suprasanna, P. Salt and drought tolerance of sugarcane under iso-osmotic salt and water stress: Growth, osmolytes accumulation, and antioxidant defense. J. Plant Interact. 2011, 6, 275–282. [Google Scholar]
- Sultana, N.; Ikeda, T.; Itoh, R. Effect of NaCl salinity on photosynthesis and dry matter accumulation in developing rice grains. Environ. Exp. Bot. 1999, 42, 211–220. [Google Scholar] [CrossRef]
- Liu, L.J. Salinity effects on sugarcane germination, growth, and root development. J. Agric. Univ. Puerto Rico. 1967, 51, 201–209. [Google Scholar] [CrossRef]
- Akthar, S.; Wahid, A.; Akram, M.; Rahul, V.E. Some growth, photosynthetic and anatomical attributes of sugarcane genotypes under NaCl salinity. Int. J. Agric. Biol. 2001, 3, 439–443. [Google Scholar]



| Pn | gs | Ci | E | ||
|---|---|---|---|---|---|
| (µmol m−2 s−1) | (mol m−2 s−1) | (ppm) | (mmol m−2 s−1) | ||
| Genotype | CP 00-1101 | 25.85 | 0.181 | 144.6 b | 3.014 |
| CP 96-1252 | 23.56 | 0.174 | 157.8 a | 2.873 | |
| Erianthus | 26.67 | 0.178 | 127.8 c | 2.990 | |
| Salt treatment | 0 (Control) | 29.55 a ‡ | 0.209 a | 141.6 | 3.396 a |
| 38 | 28.29 ab | 0.196 ab | 141.7 | 3.221 ab | |
| 75 | 25.79 bc | 0.175 b | 138.9 | 2.949 bc | |
| 150 | 24.02 c | 0.166 bc | 147.2 | 2.808 c | |
| 300 | 19.13 d | 0.144 c | 147.7 | 2.421 d | |
| ANOVA § | Genotype (G) | ns | ns | **** | ns |
| Salt (S) | **** | **** | ns | **** | |
| G × S | ns | ns | ns | ns | |
| Genotype | Salt Conc. | Reducing Sugar | Sucrose | Total Sugar | |||
|---|---|---|---|---|---|---|---|
| 8:30 a.m. | 3:30 p.m. | 8:30 a.m. | 3:30 p.m. | 8:30 a.m. | 3:30 p.m. | ||
| -------------------------------- (µg cm−2) ---------------------------------- | |||||||
| CP 00-1101 | 0 (Control) | 77.8 b ‡ | 68.4 ab | 143.4 b | 306.7 b | 221.2 c | 375.1 |
| 38 | 72.4 b | 89.1 ab | 153.6 b | 347.0 ab | 226.0 c | 436.2 | |
| 75 | 119.3 a | 94.7 a | 163.6 b | 357.8 ab | 282.9 bc | 452.5 | |
| 150 | 122.8 a | 75.2 ab | 190.2 b | 329.8 ab | 313.0 b | 405.1 | |
| 300 | 87.5 b | 53.9 b | 302.6 a | 411.6 a | 390.1 a | 465.4 | |
| Mean | 95.9 B | 76.3 A | 190.7 B | 350.6 B | 286.6 B | 426.9 B | |
| CP 96-1252 | 0 (Control) | 67.7 | 56.8 ab | 287.7 b | 530.0 ab | 355.4 b | 586.8 ab |
| 38 | 68.3 | 45.2 bc | 165.8 c | 473.9 b | 234.2 c | 519.1 b | |
| 75 | 68.1 | 66.8 ab | 197.9 c | 587.3 a | 265.9 c | 654.2 a | |
| 150 | 65.5 | 49.8 bc | 213.0 c | 555.1 ab | 278.5 c | 604.9 ab | |
| 300 | 68.6 | 91.3 a | 406.3 a | 522.4 ab | 474.9 a | 613.7 ab | |
| Mean | 67.6 C | 62.0 A | 254.1 A | 533.7 A | 321.8 A | 597.7 A | |
| Erianthus | 0 (Control) | 65.9 c | 33.3 c | 104.6 b | 194.6 | 170.5 c | 227.9 b |
| 38 | 154.4 a | 76.5 b | 96.3 b | 184.1 | 250.7 b | 260.6 b | |
| 75 | 116.8 b | 61.7 bc | 123.3 b | 220.2 | 240.1 b | 281.9 b | |
| 150 | 72.8 c | 55.1 bc | 130.4 b | 238.1 | 203.2 bc | 293.2 b | |
| 300 | 152.7 a | 116.6 a | 214.1 a | 246.7 | 366.9 a | 363.3 a | |
| Mean | 112.5 A | 68.7 A | 133.7 C | 216.7 C | 246.2 C | 285.4 C | |
| Salt main effect | 0 (Control) | 70.5 b | 52.8 b | 178.6 b | 343.8 b | 249.1 b | 396.6 b |
| 38 | 98.4 a | 70.3 ab | 138.6 c | 335.0 b | 237.0 b | 405.3 b | |
| 75 | 101.4 a | 74.4 a | 161.6 bc | 388.4 a | 263.0 b | 462.9 a | |
| 150 | 87.0 a | 60.0 b | 177.9 b | 374.3 ab | 264.9 b | 434.4 ab | |
| 300 | 102.9 a | 87.3 a | 307.7 a | 393.5 a | 410.6 a | 480.8 a | |
| ANOVA § | Genotype (G) | **** | ns | **** | **** | **** | **** |
| Salt (S) | ** | * | **** | ns | **** | * | |
| G × S | **** | ** | ** | ns | *** | ns | |
| Treatment | Brown Leaf DW | Green Leaf DW | Stalk DW | Total Shoot DW | |
|---|---|---|---|---|---|
| --------------------------- g plant−1 ----------------------------- | |||||
| Genotype | CP 00-1101 | 54.5 a ‡ | 56.1 | 150.1 a | 260.7 a |
| CP 96-1252 | 54.1 a | 59.8 | 119.9 ab | 233.7 a | |
| Erianthus | 36.9 b | 49.0 | 98.2 b | 184.1 b | |
| Salt treatment | 0 (Control) | 58.1 a | 70.6 a | 184.4 a | 313.1 a |
| 38 | 47.8 ab | 59.7 ab | 134.7 b | 242.1 b | |
| 75 | 49.8 ab | 60.7 ab | 143.1 b | 253.6 b | |
| 150 | 45.9 b | 50.0 b | 112.2 b | 208.1 b | |
| 300 | 40.9 b | 33.7 c | 39.4 c | 113.9 c | |
| ANOVA § | Genotype (G) | * | ns | * | * |
| Salt (S) | ** | ** | ** | ** | |
| G × S | ns | ns | ns | ns | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Zhu, K.; Momotaz, A.; Gao, X. Sugarcane Plant Growth and Physiological Responses to Soil Salinity during Tillering and Stalk Elongation. Agriculture 2020, 10, 608. https://doi.org/10.3390/agriculture10120608
Zhao D, Zhu K, Momotaz A, Gao X. Sugarcane Plant Growth and Physiological Responses to Soil Salinity during Tillering and Stalk Elongation. Agriculture. 2020; 10(12):608. https://doi.org/10.3390/agriculture10120608
Chicago/Turabian StyleZhao, Duli, Kai Zhu, Aliya Momotaz, and Xinxin Gao. 2020. "Sugarcane Plant Growth and Physiological Responses to Soil Salinity during Tillering and Stalk Elongation" Agriculture 10, no. 12: 608. https://doi.org/10.3390/agriculture10120608
APA StyleZhao, D., Zhu, K., Momotaz, A., & Gao, X. (2020). Sugarcane Plant Growth and Physiological Responses to Soil Salinity during Tillering and Stalk Elongation. Agriculture, 10(12), 608. https://doi.org/10.3390/agriculture10120608





