Intensive Rain Hampers the Effectiveness of Nitrification Inhibition in Controlling N2O Emissions from Dairy Slurry-Fertilized Soils
Abstract
1. Introduction
2. Materials and Methods
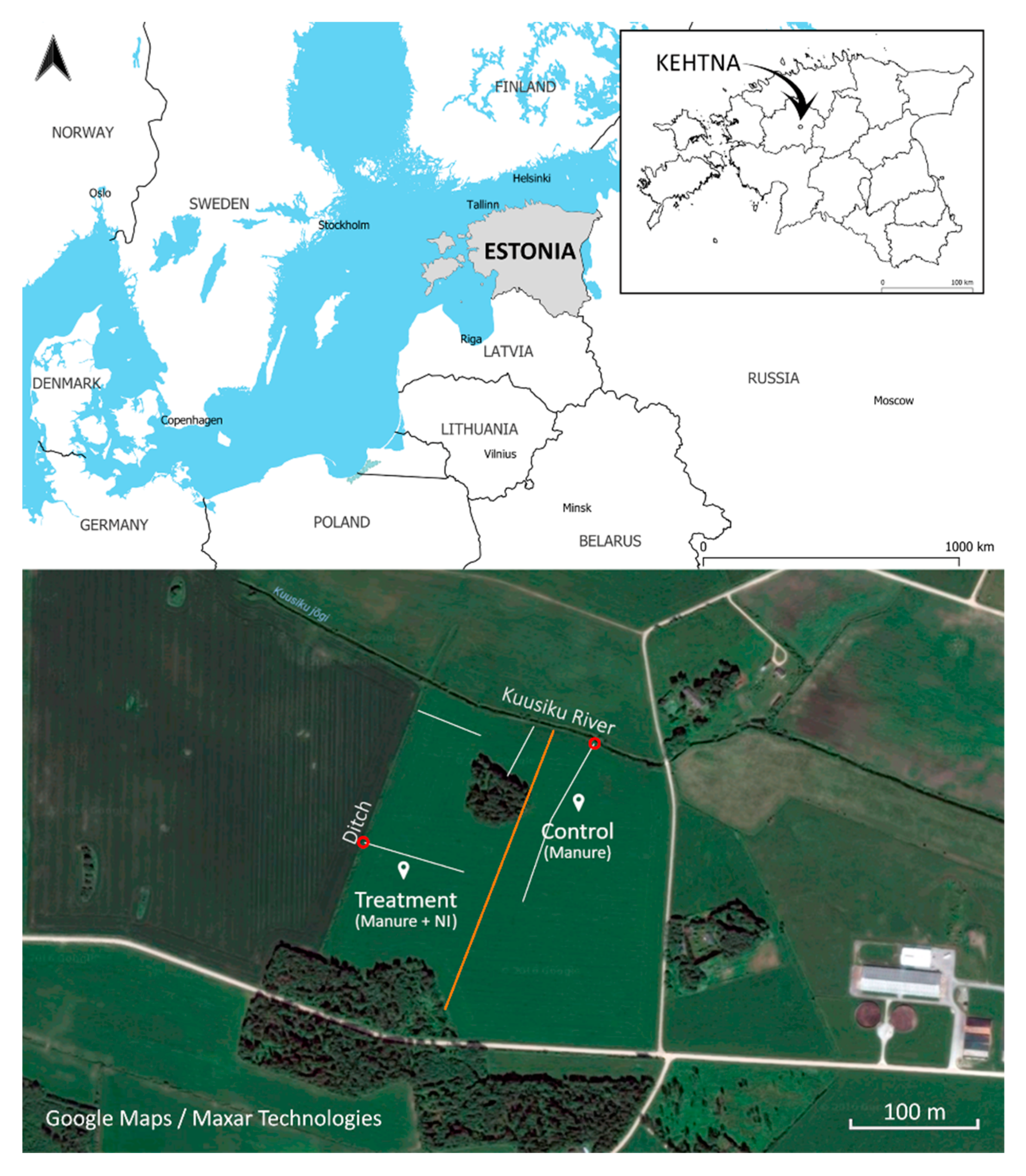
2.1. Study Site and Experiment Set-Up
2.2. Environmental Parameters
2.3. Soil and Leachate Sampling and Analysis
2.4. Flux Measurements
2.5. Statistical Analysis
3. Results
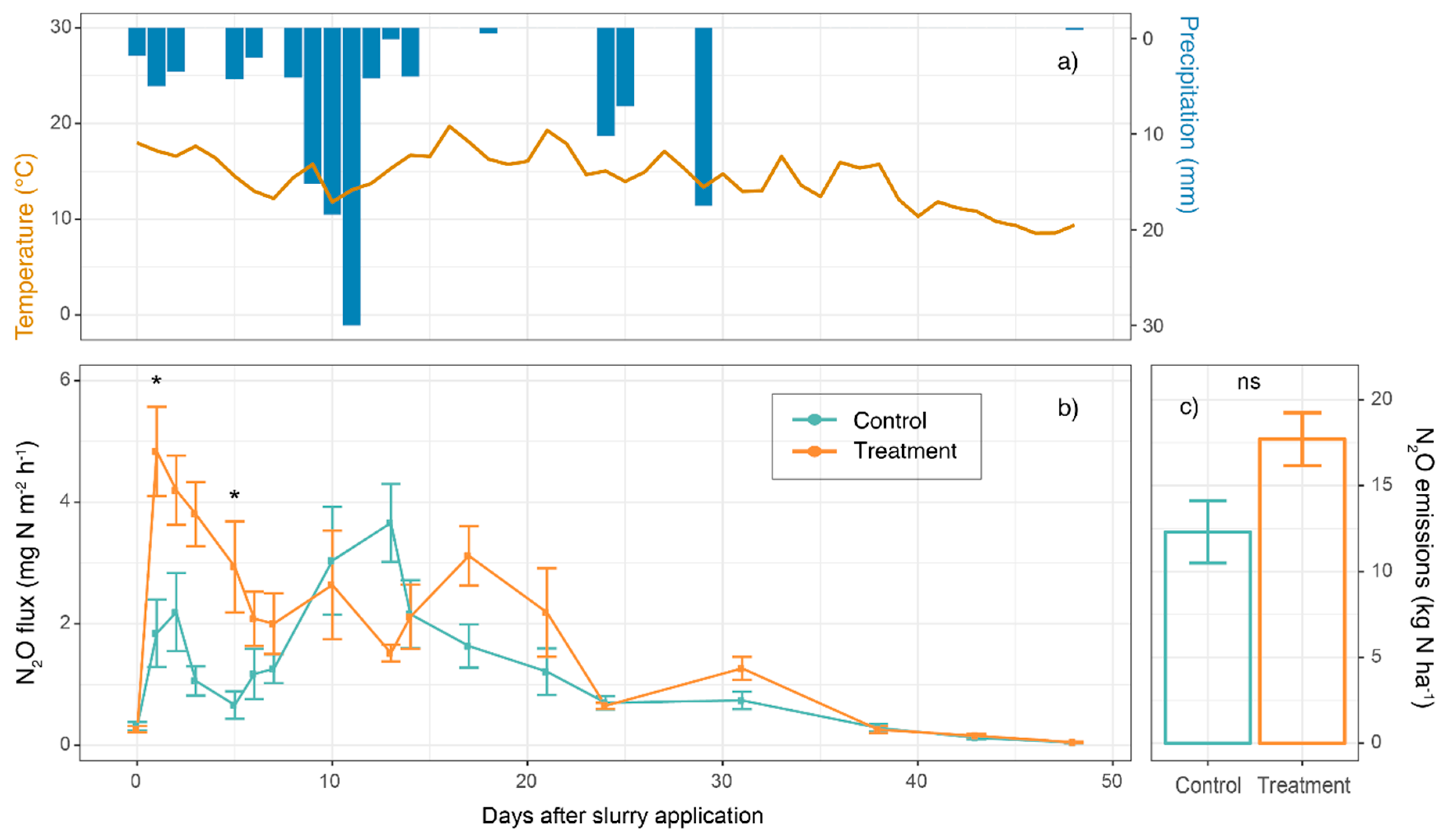
3.1. Nitrous Oxide Flux
3.2. Environmental Data
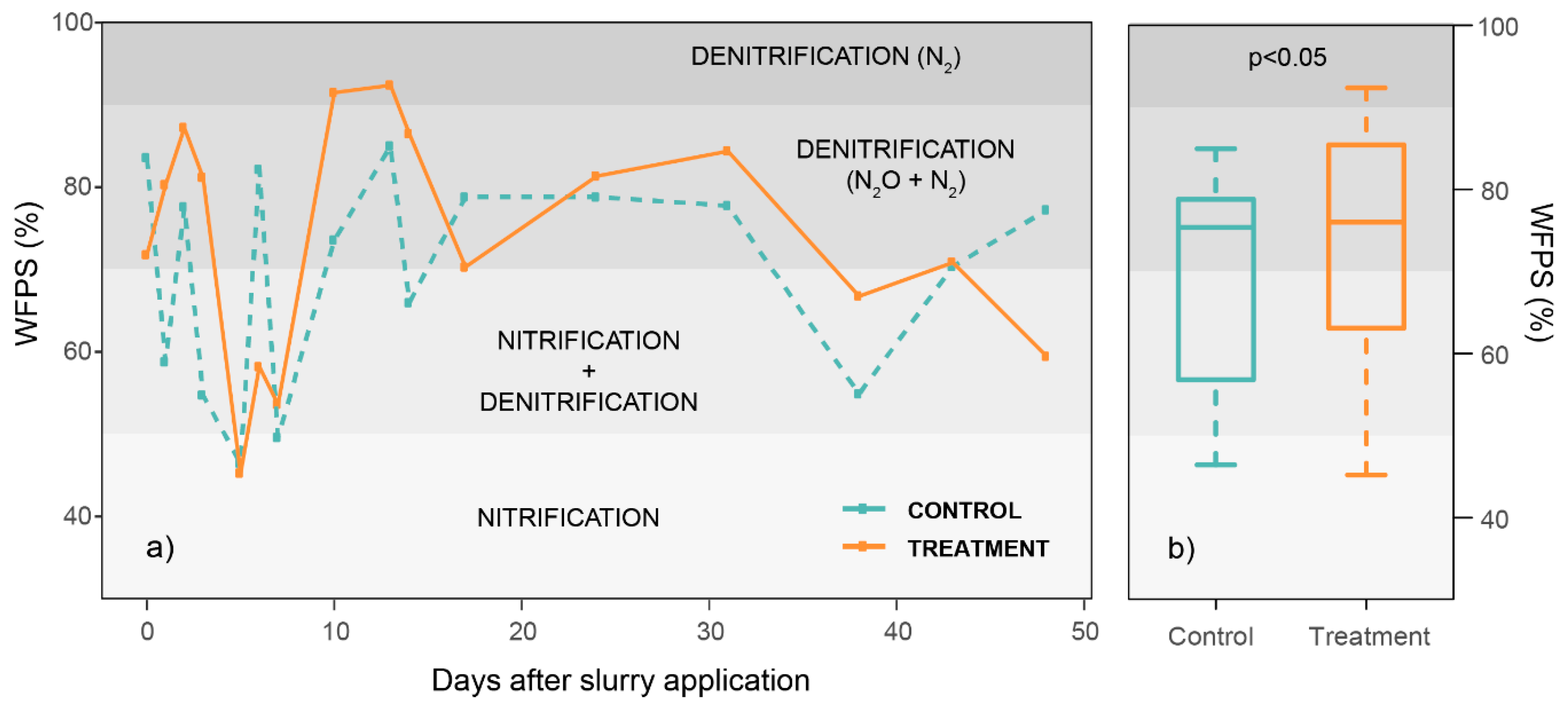
3.3. Soil Parameters
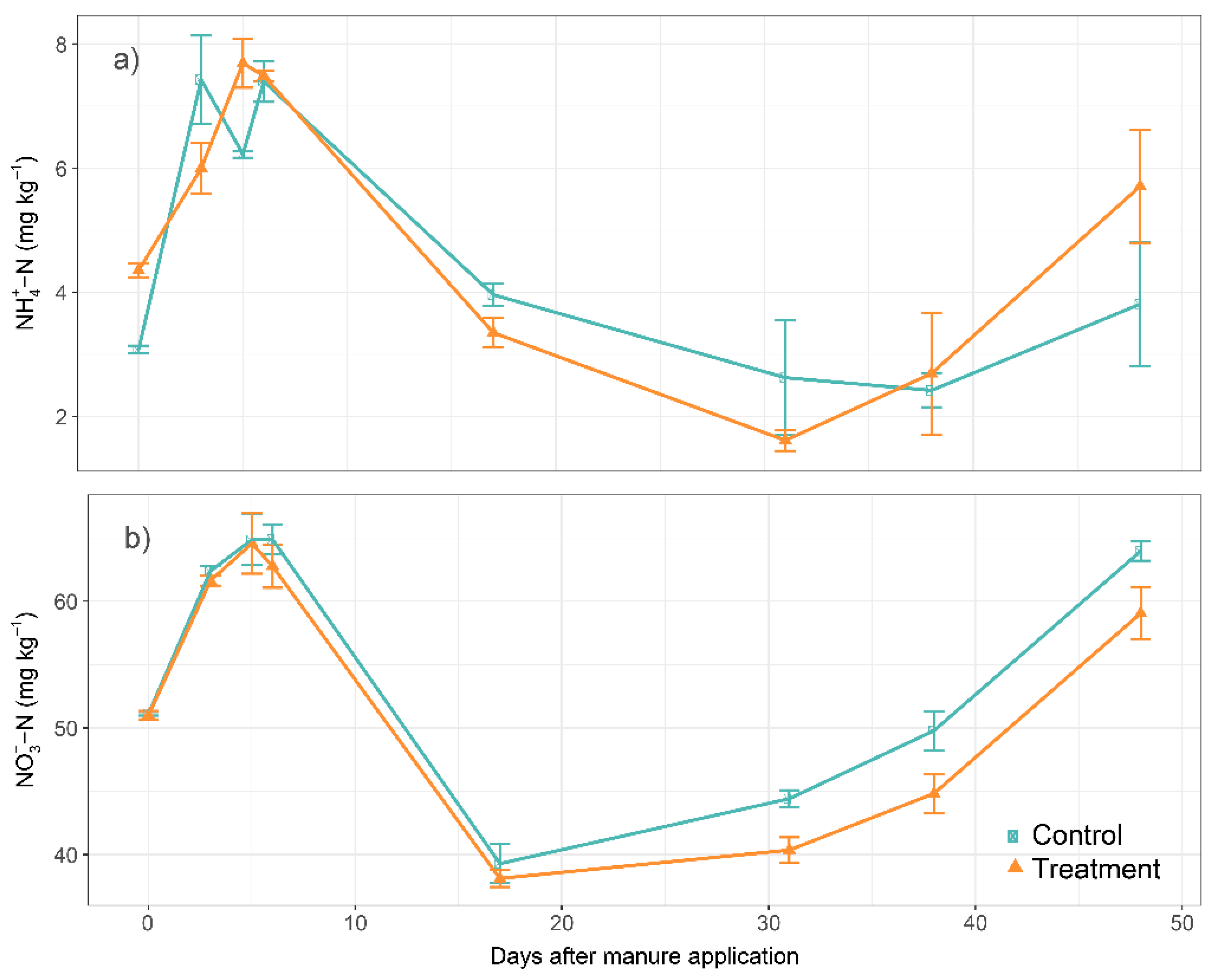
3.4. Soil Chemical Properties
3.5. Leaching
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC. Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas EMISSION pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2018; p. 630.
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team: Geneva, Switzerland, 2014; p. 151. ISBN 978-92-9169-143-2.
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Ussiri, D.; Lal, R. Soil Emission of Nitrous Oxide and Its Mitigation; Springer Netherlands: Dordrecht, The Netherlands, 2013; Volume XVIII, p. 378. [Google Scholar]
- Park, S.; Croteau, P.; Boering, K.A.; Etheridge, D.M.; Ferretti, D.; Fraser, P.J.; Kim, K.R.; Krummel, P.B.; Langenfelds, R.L.; van Ommen, T.D.; et al. Trends and seasonal cycles in the isotopic composition of nitrous oxide since 1940. Nat. Geosci. 2012, 5, 261–265. [Google Scholar] [CrossRef]
- Firestone, M.K.; Davidson, E.A. Microbiological Basis of NO and N2O Production and Consumption in Soils. In Exchange of Trace Gases between Terrestrial Ecosystems and the Atmosphere; Andreae, M.O., Schimel, D.S., Eds.; John Willey and Sons: New York, NY, USA, 1989; pp. 7–21. [Google Scholar]
- Parton, W.J.; Mosier, A.R.; Ojima, D.S.; Valentine, D.W.; Schimel, D.S.; Weier, K.; Kulmala, A.E. Generalized model for N2and N2O production from nitrification and denitrification. Glob. Biogeochem. Cycles 1996, 10, 401–412. [Google Scholar] [CrossRef]
- Bremner, J.M. Sources of nitrous oxide in soils. Nutr. Cycl. Agroecosyst. 1997, 49, 7–16. [Google Scholar] [CrossRef]
- Wrage, N.; van Groenigen, J.W.; Oenema, O.; Baggs, E.M. A novel dual-isotope labelling method for distinguishing between soil sources of N2O. Rapid Commun. Mass Spectrom. 2005, 19, 3298–3306. [Google Scholar] [CrossRef]
- Smith, K.A.; Thomson, P.E.; Clayton, H.; McTaggart, I.P.; Conen, F. Effects of temperature, water content and nitrogen fertilisation on emissions of nitrous oxide by soils. Atmos. Environ. 1998, 32, 3301–3309. [Google Scholar] [CrossRef]
- Braker, G.; Ralf, C. Diversity, Structure, and Size of N2O-Producing Microbial Communities in Soils—What Matters for Their Functioning? In Advances in Applied Microbiology; Academic Press: Cambridge, MA, USA, 2011; Volume 75, pp. 33–70. [Google Scholar]
- Duan, Y.F.; Kong, X.W.; Schramm, A.; Labouriau, R.; Eriksen, J.; Petersen, S.O. Microbial N Transformations and N2O Emission after Simulated Grassland Cultivation: Effects of the Nitrification Inhibitor 3,4-Dimethylpyrazole Phosphate (DMPP). Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef]
- Leininger, S.; Urich, T.; Schloter, M.; Schwark, L.; Qi, J.; Nicol, G.W.; Prosser, J.I.; Schuster, S.C.; Schleper, C. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 2006, 442, 806–809. [Google Scholar] [CrossRef]
- Taylor, A.E.; Zeglin, L.H.; Wanzek, T.A.; Myrold, D.D.; Bottomley, P.J. Dynamics of ammonia-oxidizing archaea and bacteria populations and contributions to soil nitrification potentials. ISME J. 2012, 6, 2024–2032. [Google Scholar] [CrossRef]
- Jia, Z.; Conrad, R. Bacteria rather than Archaea dominate microbial ammonia oxidation in an agricultural soil. Environ. Microbiol. 2009, 11, 1658–1671. [Google Scholar] [CrossRef]
- Morimoto, S.; Hayatsu, M.; Takada Hoshino, Y.; Nagaoka, K.; Yamazaki, M.; Karasawa, T.; Takenaka, M.; Akiyama, H. Quantitative Analyses of Ammonia-oxidizing Archaea (AOA) and Ammonia-oxidizing Bacteria (AOB) in Fields with Different Soil Types. Microbes Environ. 2011, 26, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Di, H.J.; Cameron, K.C.; Shen, J.P.; Winefield, C.S.; O’Callaghan, M.; Bowatte, S.; He, J.Z. Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils. Nat. Geosci. 2009, 2, 621–624. [Google Scholar] [CrossRef]
- Di, H.J.; Cameron, K.C.; Shen, J.P.; Winefield, C.S.; O’Callaghan, M.; Bowatte, S.; He, J.Z. Ammonia-oxidizing bacteria and archaea grow under contrasting soil nitrogen conditions. FEMS Microbiol. Ecol. 2010, 72, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Di, H.J.; Cameron, K.C.; Sherlock, R.R.; Shen, J.-P.; He, J.-Z.; Winefield, C.S. Nitrous oxide emissions from grazed grassland as affected by a nitrification inhibitor, dicyandiamide, and relationships with ammonia-oxidizing bacteria and archaea. J. Soils Sediments 2010, 10, 943–954. [Google Scholar] [CrossRef]
- Di, H.J.; Cameron, K.C.; Podolyan, A.; Robinson, A. Effect of soil moisture status and a nitrification inhibitor, dicyandiamide, on ammonia oxidizer and denitrifier growth and nitrous oxide emissions in a grassland soil. Soil Biol. Biochem. 2014, 73, 59–68. [Google Scholar] [CrossRef]
- Briones, A.M.; Okabe, S.; Umemiya, Y.; Ramsing, N.B.; Reichardt, W.; Okuyama, H. Influence of different cultivars on populations of ammonia-oxidizing bacteria in the root environment of rice. Appl. Environ. Microbiol. 2002, 68, 3067–3075. [Google Scholar] [CrossRef]
- Villar, J.M.; Guillaumes, E. Use of nitrification inhibitor DMPP to improve nitrogen recovery in irrigated wheat on a calcareous soil. Span. J. Agric. Res. 2010, 8. [Google Scholar] [CrossRef]
- Gerardi, M.H. Nitrifying Bacteria. In Nitrification and Denitrification in the Activated Sludge Process; John Wiley & Sons, Inc.: New York, NY, USA, 2002; pp. 43–54. [Google Scholar] [CrossRef]
- Cabello, P.; Roldán, M.D.; Castillo, F.; Moreno-Vivián, C. Nitrogen Cycle. In Encyclopedia of Microbiology; Academic Press: Cambridge, MA, USA, 2009; pp. 299–321. [Google Scholar] [CrossRef]
- Wrage, N.; Velthof, G.L.; van Beusichem, M.L.; Oenema, O. Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol. Biochem. 2001, 33, 1723–1732. [Google Scholar] [CrossRef]
- Dobbie, K.E.; Smith, K.A. Nitrous oxide emission factors for agricultural soils in Great Britain: The impact of soil water-filled pore space and other controlling variables. Glob. Chang. Biol. 2003, 9, 204–218. [Google Scholar] [CrossRef]
- Vinzent, B.; Fuß, R.; Maidl, F.-X.; Hülsbergen, K.-J. N2O emissions and nitrogen dynamics of winter rapeseed fertilized with different N forms and a nitrification inhibitor. Agric. Ecosyst. Environ. 2018, 259, 86–97. [Google Scholar] [CrossRef]
- Halling-Sørensen, B. Process Chemistry and Biochemistry of Nitrification. In Studies in Environmental Science; Halling-Sørensen, B., Jorgensen, S.E., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1993; Volume 54, pp. 55–118. [Google Scholar]
- Hansen, R.; Mander, Ü.; Soosaar, K.; Maddison, M.; Lõhmus, K.; Kupper, P.; Kanal, A.; Sõber, J. Greenhouse gas fluxes in an open air humidity manipulation experiment. Landsc. Ecol. 2012, 28, 637–649. [Google Scholar] [CrossRef]
- Saggar, S.; Jha, N.; Deslippe, J.; Bolan, N.S.; Luo, J.; Giltrap, D.L.; Kim, D.G.; Zaman, M.; Tillman, R.W. Denitrification and N2O:N2 production in temperate grasslands: Processes, measurements, modelling and mitigating negative impacts. Sci. Total Environ. 2013, 465, 173–195. [Google Scholar] [CrossRef]
- Borchard, N.; Schirrmann, M.; Cayuela, M.L.; Kammann, C.; Wrage, M.N.; Estavillo, J.M.; Fuertes, M.T.; Sigua, G.; Spokas, K.; Ippolito, J.A.; et al. Biochar, soil and land-use interactions that reduce nitrate leaching and N2O emissions: A meta-analysis. Sci. Total Environ. 2019, 651, 2354–2364. [Google Scholar] [CrossRef] [PubMed]
- Cowan, N.J.; Norman, P.; Famulari, D.; Levy, P.E.; Reay, D.S.; Skiba, U.M. Spatial variability and hotspots of soil N2O fluxes from intensively grazed grassland. Biogeosciences 2015, 12, 1585–1596. [Google Scholar] [CrossRef]
- Lohila, A.; Aurela, M.; Hatakka, J.; Pihlatie, M.; Minkkinen, K.; Penttilä, T.; Laurila, T. Responses of N2O fluxes to temperature, water table and N deposition in a northern boreal fen. Eur. J. Soil Sci. 2010, 61, 651–661. [Google Scholar] [CrossRef]
- Focht, D.D. Methods for Analysis of Denitrification in Soils. In Soil–Plant–Nitrogen Relationships; Nielsen, D.R., MacDonald, J.G., Eds.; Academic Press: Cambridge, MA, USA, 1978; pp. 433–490. [Google Scholar] [CrossRef]
- Davidson, E.A. Fluxes of nitrous oxide and nitric oxide from terrestrial ecosystems. In Microbial Production and Consumption of Greenhouse Gases: Methane, Nitrogen Oxides, and Halomethanes; Rogers, J.E., Whitman, W.B., Eds.; American Society for Microbiology: Washington, DC, USA, 1991; pp. 219–235. [Google Scholar]
- Bouwman, A.F. Direct emission of nitrous oxide from agricultural soils. Nutr. Cycl. Agroecosyst. 1996, 46, 53–70. [Google Scholar] [CrossRef]
- Ruser, R.; Flessa, H.; Russow, R.; Schmidt, G.; Buegger, F.; Munch, J.C. Emission of N2O, N2 and CO2 from soil fertilized with nitrate: Effect of compaction, soil moisture and rewetting. Soil Biol. Biochem. 2006, 38, 263–274. [Google Scholar] [CrossRef]
- Allison, F.E. Nitrogen and Soil Fertility. In Yearbook of Agriculture 1957; United States Department of Agriculture: Washington, WA, USA, 1957; p. 784. [Google Scholar]
- Bouchet, A.-S.; Laperche, A.; Bissuel-Belaygue, C.; Snowdon, R.; Nesi, N.; Stahl, A. Nitrogen use efficiency in rapeseed. A review. Agron. Sustain. Dev. 2016, 36. [Google Scholar] [CrossRef]
- Yu, L.; Kang, R.; Mulder, J.; Zhu, J.; Dörsch, P. Distinct fates of atmogenic NH4+ and NO3− in subtropical, N-saturated forest soils. Biogeochemistry 2017, 133, 279–294. [Google Scholar] [CrossRef]
- Ruser, R.; Schulz, R. The effect of nitrification inhibitors on the nitrous oxide (N2O) release from agricultural soils-a review. J. Plant Nutr. Soil Sci. 2015, 178, 171–188. [Google Scholar] [CrossRef]
- Di, H.J.; Cameron, K.C. Inhibition of nitrification to mitigate nitrate leaching and nitrous oxide emissions in grazed grassland: A review. J. Soils Sediments 2016, 16, 1401–1420. [Google Scholar] [CrossRef]
- Akiyama, H.; Yan, X.; Yagi, K. Evaluation of effectiveness of enhanced-efficiency fertilizers as mitigation options for N2O and NO emissions from agricultural soils: Meta-analysis. Glob. Chang. Biol. 2009, 16, 1837–1846. [Google Scholar] [CrossRef]
- Gilsanz, C.; Báez, D.; Misselbrook, T.H.; Dhanoa, M.S.; Cárdenas, L.M. Development of emission factors and efficiency of two nitrification inhibitors, DCD and DMPP. Agric. Ecosyst. Environ. 2016, 216, 1–8. [Google Scholar] [CrossRef]
- Yang, M.; Fang, Y.; Sun, D.; Shi, Y. Efficiency of two nitrification inhibitors (dicyandiamide and 3, 4-dimethypyrazole phosphate) on soil nitrogen transformations and plant productivity: A meta-analysis. Sci. Rep. 2016, 6, 22075. [Google Scholar] [CrossRef]
- Kong, X.; Eriksen, J.; Petersen, S.O. Evaluation of the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) for mitigating soil N2O emissions after grassland cultivation. Agric. Ecosyst. Environ. 2018, 259, 174–183. [Google Scholar] [CrossRef]
- Zerulla, W.; Barth, T.; Dressel, J.; Erhardt, K.; Horchler von Locquenghien, K.; Pasda, G.; Rädle, M.; Wissemeier, A. 3,4-Dimethylpyrazole phosphate (DMPP)—A new nitrification inhibitor for agriculture and horticulture. Biol. Fertil. Soils 2001, 34, 79–84. [Google Scholar] [CrossRef]
- Guillaumes, E.; Villar, J.M. Effects of DMPP [3,4-dimethylpyrozole phosphate] on the growth and chemical composition of ryegrass (Lolium perenne L.) raised on calcareous soil. Span. J. Agric. Res. 2004, 2. [Google Scholar] [CrossRef]
- Trenkel, M.E. Slow-and Controlled-Release and Stabilized Fertilizers: An Option for Enhancing Nutrient Use Efficiency in Agriculture; International Fertilizer Industry Association (IFA): Paris, France, 2010. [Google Scholar]
- Byrne, M.P.; Tobin, J.T.; Forrestal, P.J.; Danaher, M.; Nkwonta, C.G.; Richards, K.; Cummins, E.; Hogan, S.A.; O’Callaghan, T.F. Urease and Nitrification Inhibitors—As Mitigation Tools for Greenhouse Gas Emissions in Sustainable Dairy Systems: A Review. Sustainability 2020, 12, 6018. [Google Scholar] [CrossRef]
- Barrena, I.; Menéndez, S.; Correa-Galeote, D.; Vega-Mas, I.; Bedmar, E.J.; González-Murua, C.; Estavillo, J.M. Soil water content modulates the effect of the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) on nitrifying and denitrifying bacteria. Geoderma 2017, 303, 1–8. [Google Scholar] [CrossRef]
- Godfrey, L.V.; Glass, J.B. The geochemical record of the ancient nitrogen cycle, nitrogen isotopes, and metal cofactors. Methods Enzym. 2011, 486, 483–506. [Google Scholar] [CrossRef]
- Velthof, G.L.; Mosquera, J.; Mosquera, J.; Veld, J.W.H.H.i.t.; Hummelink, E.W.J. Effect of Manure Application Technique on Nitrous Oxide Emission from Agricultural Soils; Alterra Wageningen UR: Wageningen, The Netherlands, 2010; p. 74. [Google Scholar]
- Zhu, Y.; Merbold, L.; Leitner, S.; Pelster, D.E.; Okoma, S.A.; Ngetich, F.; Onyango, A.A.; Pellikka, P.; Butterbach-Bahl, K. The effects of climate on decomposition of cattle, sheep and goat manure in Kenyan tropical pastures. Plant Soil 2020, 451, 325–343. [Google Scholar] [CrossRef]
- Mosier, A.R. Soil processes and global change. Biol. Fertil. Soils 1998, 27, 221–229. [Google Scholar] [CrossRef]
- Lessard, R.; Rochette, P.; Gregorich, E.G.; Pattey, E.; Desjardins, R.L. Nitrous Oxide Fluxes from Manure-Amended Soil under Maize. J. Environ. Qual. 1996, 25. [Google Scholar] [CrossRef]
- Flach, B.; Lieberz, S.; Lappin, J.; Bolla, S. EU Biofuels Annual 2018; USDA Foreign Agricultural Service: Washington, DC, USA, 2018. [Google Scholar]
- Pehnelt, G.; Vietze, C. Uncertainties about the GHG Emissions Saving of Rapeseed Biodiesel; Friedrich-Schiller University of Jena: Jena, Germany, 2012. [Google Scholar]
- Astover, A.; Shanskiy, M.; Lauringson, E. Development and Application of the Methodology for the Calculation of Average Greenhouse Gas Emissions from the Cultivation of Rapeseed, Wheat, Rye, Barley and Triticale in Estonia; Ministry of the Environment of the Republic of Estonia: Tartu, Estonia, 2015.
- BioGrace. BioGrace Greenhouse Gas Calculation Tool Version 4d. Available online: http://www.biograce.net/ (accessed on 26 February 2020).
- Vinzent, B.; Fuß, R.; Maidl, F.-X.; Hülsbergen, K.-J. Efficacy of agronomic strategies for mitigation of after-harvest N2O emissions of winter oilseed rape. Eur. J. Agron. 2017, 89, 88–96. [Google Scholar] [CrossRef]
- European Commission. Communication from the Comission: The European Green Deal; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- The European Parliament and of the Council of the European Union. Directive (EU) 2018/2001 of the European Parliament and of the Council of 11 December 2018 on the Promotion of the Use of Energy from Renewable Sources; Council of the European Union: Strasbourg, France, 2018.
- Oo, A.Z.; Gonai, T.; Sudo, S.; Thuzar Win, K.; Shibata, A. Surface application of fertilizers and residue biochar on N2O emission from Japanese pear orchard soil. Plantsoil Environ. 2018, 64, 597–604. [Google Scholar] [CrossRef]
- Carter, M.R.; Gregorich, E.G. Soil Sampling and Methods of Analysis; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]
- Egnér, H.; Riehm, H.; Domingo, W.R. Untersuchungen uber die chemische Bodenanalyse als Grundlage fur die Beurteilung des Nährstoffzustandes der Böden. II. Chemische Extraktionsmethoden zur Phosphor- und Kaliumbestimmung. K. Lantbr. Ann. 1960, 26, 199–215. [Google Scholar]
- Association of official analytical chemists (AOAC). Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Schulte, E.E.; Hopkins, B.G. Estimation of Soil Organic Matter by Weight Loss-On-Ignition. In Soil Organic Matter: Analysis and Interpretation; Magdoff, F.R., Tabatabai, M.A., Jr., Edward, A.H., Eds.; Soil Science Society of America, Inc.: Madison, WI, USA, 1996; Volume 46. [Google Scholar] [CrossRef]
- Ruzicka, J.; Hansen, E.H. Flow Injection Analysis, 2nd ed.; Wiley: New York, NY, USA, 1988. [Google Scholar]
- Hutchinson, G.L.; Livingston, G.P. Use of chamber systems to measure trace gas fluxes. In Agricultural Ecosystem Effects on Trace Gases and Global Climate Change; Harper, L.A., Mosier, A.R., Duxbury, J.M., Rolston, D.E., Eds.; American Society of Agronomy: Madison, WI, USA, 1993; pp. 63–78. [Google Scholar] [CrossRef]
- Mander, U.; Maddison, M.; Soosaar, K.; Teemusk, A.; Kanal, A.; Uri, V.; Truu, J. The impact of a pulsing groundwater table on greenhouse gas emissions in riparian grey alder stands. Environ. Sci. Pollut. Res. Int. 2015, 22, 2360–2371. [Google Scholar] [CrossRef]
- Loftfield, N.; Flessa, H.; Augustin, J.; Beese, F. Automated Gas Chromatographic System for Rapid Analysis of the Atmospheric Trace Gases Methane, Carbon Dioxide, and Nitrous Oxide. J. Environ. Qual. 1997, 26. [Google Scholar] [CrossRef]
- Livingston, G.P.; Hutchinson, G.L. Enclosure-based measurement of trace gas exchange: Applications and sources of error. In Biogenic Trace Gases: Measuring Emissions from Soil and Water; Matson, P.A., Harriss, R.C., Eds.; Blackwell Publishing: Oxford, UK, 1995; pp. 14–51. [Google Scholar]
- Volpi, I.; Ragaglini, G.; Nassi o Di Nasso, N.; Bonari, E.; Bosco, S. Soil N2O emissions in Mediterranean arable crops as affected by reduced tillage and N rate. Nutr. Cycl. Agroecosyst. 2019, 116, 117–133. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Tukey, J.W. Exploratory Data Analysis; Addison-Wesley Publishing Company: Reading, MA, USA, 1977. [Google Scholar]
- Müller, C.; Stevens, R.J.; Laughlin, R.J.; Azam, F.; Ottow, J.C.G. The nitrification inhibitor DMPP had no effect on denitrifying enzyme activity. Soil Biol. Biochem. 2002, 34, 1825–1827. [Google Scholar] [CrossRef]
- Flessa, H.; Dörsch, P.; Beese, F. Seasonal variation of N2O and CH4 fluxes in differently managed arable soils in southern Germany. J. Geophys. Res. 1995, 100. [Google Scholar] [CrossRef]
- Dobbie, K.E.; McTaggart, I.P.; Smith, K.A. Nitrous oxide emissions from intensive agricultural systems: Variations between crops and seasons, key driving variables, and mean emission factors. J. Geophys. Res. Atmos. 1999, 104, 26891–26899. [Google Scholar] [CrossRef]
- Schaufler, G.; Kitzler, B.; Schindlbacher, A.; Skiba, U.; Sutton, M.A.; Zechmeister-Boltenstern, S. Greenhouse gas emissions from European soils under different land use: Effects of soil moisture and temperature. Eur. J. Soil Sci. 2010, 61, 683–696. [Google Scholar] [CrossRef]
- Wang, F.; Li, J.; Wang, X.; Zhang, W.; Zou, B.; Neher, D.A.; Li, Z. Nitrogen and phosphorus addition impact soil N(2)O emission in a secondary tropical forest of South China. Sci. Rep. 2014, 4, 5615. [Google Scholar] [CrossRef]
- Dhadli, H.S.; Brar, B.S.; Kingra, P.K. Temporal Variations in N2O Emissions in Maize and Wheat Crop Seasons: Impact of N-Fertilization, Crop Growth, and Weather Variables. J. Crop Improv. 2016, 30, 17–31. [Google Scholar] [CrossRef]
- Ryden, J.C. Denitrification loss from a grassland soil in the field receiving different rates of nitrogen as ammonium nitrate. J. Soil Sci. 1983, 34, 355–365. [Google Scholar] [CrossRef]
- Mori, T.; Ohta, S.; Ishizuka, S.; Konda, R.; Wicaksono, A.; Heriyanto, J.; Hardjono, A. Effects of phosphorus addition on N2O and NO emissions from soils of anAcacia mangiumplantation. Soil Sci. Plant Nutr. 2010, 56, 782–788. [Google Scholar] [CrossRef]
- Keil, D.; Niklaus, P.A.; von Riedmatten, L.R.; Boeddinghaus, R.S.; Dormann, C.F.; Scherer-Lorenzen, M.; Kandeler, E.; Marhan, S. Effects of warming and drought on potential N2O emissions and denitrifying bacteria abundance in grasslands with different land-use. FEMS Microbiol. Ecol. 2015, 91. [Google Scholar] [CrossRef]
- Stevens, R.J.; Laughlin, R.J. Cattle Slurry Applied Before Fertilizer Nitrate Lowers Nitrous Oxide and Dinitrogen Emissions. Soil Sci. Soc. Am. J. 2002, 66, 647–652. [Google Scholar] [CrossRef]
- Loro, P.J.; Bergstrom, D.W.; Beauchamp, E.G. Intensity and Duration of Denitrification following Application of Manure and Fertilizer to Soil. J. Environ. Qual. 1997, 26, 706–713. [Google Scholar] [CrossRef]
- Simek, M.; Cooper, J.E. The influence of soil pH on denitrification: Progress towards the understanding of this interaction over the last 50 years. Eur. J. Soil Sci. 2002, 53, 345–354. [Google Scholar] [CrossRef]
- Hénault, C.; Bourennane, H.; Ayzac, A.; Ratié, C.; Saby, N.P.A.; Cohan, J.P.; Eglin, T.; Gall, C.L. Management of soil pH promotes nitrous oxide reduction and thus mitigates soil emissions of this greenhouse gas. Sci. Rep. 2019, 9, 20182. [Google Scholar] [CrossRef] [PubMed]
- McMillan, A.M.S.; Pal, P.; Phillips, R.L.; Palmada, T.; Berben, P.H.; Jha, N.; Saggar, S.; Luo, J. Can pH amendments in grazed pastures help reduce N2O emissions from denitrification?—The effects of liming and urine addition on the completion of denitrification in fluvial and volcanic soils. Soil Biol. Biochem. 2016, 93, 90–104. [Google Scholar] [CrossRef]
- Chen, Z.; Ding, W.; Luo, Y.; Yu, H.; Xu, Y.; Müller, C.; Xu, X.; Zhu, T. Nitrous oxide emissions from cultivated black soil: A case study in Northeast China and global estimates using empirical model. Glob. Biogeochem. Cycles 2014, 28, 1311–1326. [Google Scholar] [CrossRef]
- Thornton, F.C.; Shurpali, N.J.; Bock, B.R.; Reddy, K.C. N2O and no emissions from poultry litter and urea applications to Bermuda grass. Atmos. Environ. 1998, 32, 1623–1630. [Google Scholar] [CrossRef]
- Cantarel, A.A.M.; Bloor, J.M.G.; Deltroy, N.; Soussana, J.-F. Effects of Climate Change Drivers on Nitrous Oxide Fluxes in an Upland Temperate Grassland. Ecosystems 2010, 14, 223–233. [Google Scholar] [CrossRef]
- Calderón, F.J.; McCarty, G.W.; Reeves, J.B. Analysis of manure and soil nitrogen mineralization during incubation. Biol. Fertil. Soils 2005, 41, 328–336. [Google Scholar] [CrossRef]
- Clemens, J.; Huschka, A. The effect of biological oxygen demand of cattle slurry and soil moisture on nitrous oxide emissions. Nutr. Cycl. Agroecosyst. 2001, 59, 193–198. [Google Scholar] [CrossRef]
- Guo, X.; Drury, C.F.; Yang, X.; Daniel Reynolds, W.; Fan, R. The Extent of Soil Drying and Rewetting Affects Nitrous Oxide Emissions, Denitrification, and Nitrogen Mineralization. Soil Sci. Soc. Am. J. 2014, 78, 194–204. [Google Scholar] [CrossRef]
- Smith, M.S.; Parsons, L.L. Persistence of Denitrifying Enzyme Activity in Dried Soils. Appl. Environ. Microbiol. 1985, 49, 316–320. [Google Scholar] [CrossRef]
- Smith, M.S.; Firestone, M.K.; Tiedje, J.M. The Acetylene Inhibition Method for Short-term Measurement of Soil Denitrification and its Evaluation Using Nitrogen-131. Soil Sci. Soc. Am. J. 1978, 42. [Google Scholar] [CrossRef]
- Firestone, M.K.; Tiedje, J.M. Temporal change in nitrous oxide and dinitrogen from denitrification following onset of anaerobiosis. Appl. Environ. Microbiol. 1979, 38, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Paul, J.W.; Beauchamp, E.G. Denitrification and fermentation in plant-residue-amended soil. Biol. Fertil. Soils 1989, 7. [Google Scholar] [CrossRef]
- Tenuta, M.; Bergstrom, D.W.; Beauchamp, E.G. Denitrifying enzyme activity and carbon availability for denitrification following manure application. Commun. Soil Sci. Plant Anal. 2008, 31, 861–876. [Google Scholar] [CrossRef]
- Petersen, S.O. Nitrous Oxide Emissions from Manure and Inorganic Fertilizers Applied to Spring Barley. J. Environ. Qual. 1999, 28. [Google Scholar] [CrossRef]
- Beck, H.; Christensen, S. The effect of grass maturing and root decay on N2O production in soil. Plant Soil 1987, 103, 269–273. [Google Scholar] [CrossRef]
- Oenema, O.; Velthof, G.L.; Yamulki, S.; Jarvis, S.C. Nitrous oxide emissions from grazed grassland. Soil Use Manag. 1997, 13, 288–295. [Google Scholar] [CrossRef]
- Beltran-Rendon, D.; Rico-Fragozo, K.; Farfan-Caceres, L.; Restrepo-Diaz, H.; Hoyos-Carvajal, L. The effect of nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) on nitrifying organism populations under in vitro conditions. Agric. Sci. 2011, 2, 198–200. [Google Scholar] [CrossRef]
- De Antoni, M.M.; Scheer, C.; Grace, P.R.; Rowlings, D.W.; Bell, M.; McGree, J. Influence of different nitrogen rates and DMPP nitrification inhibitor on annual N2O emissions from a subtropical wheat–maize cropping system. Agric. Ecosyst. Environ. 2014, 186, 33–43. [Google Scholar] [CrossRef]
- Kou, Y.P.; Wei, K.; Chen, G.X.; Wang, Z.Y.; Xu, H. Effects of 3,4-dimethylpyrazole phosphate and dicyandiamide on nitrous oxide emission in a greenhouse vegetable soil. Plant Soil Environ. 2016, 61, 29–35. [Google Scholar] [CrossRef]
- Wrage-Mönnig, N.; Horn, M.A.; Well, R.; Müller, C.; Velthof, G.; Oenema, O. The role of nitrifier denitrification in the production of nitrous oxide revisited. Soil Biol. Biochem. 2018, 123, A3–A16. [Google Scholar] [CrossRef]
| Parameter | Control Plot | Treatment Plot |
|---|---|---|
| BD (g cm−3) | 1.107 | 1.124 |
| pHKCl | 4.75 | 4.76 |
| DM (%) | 68.36 | 69.04 |
| NO3−-N (mg kg−1) | 51.11 | 50.99 |
| NH4+-N (mg kg−1) | 3.08 | 4.35 |
| P (mg kg−1) | 40.09 | 61.52 |
| K (mg kg−1) | 105 | 147 |
| OM (mg kg−1) | 12.04 | 10.91 |
| N (%) | 0.66 | 0.60 |
| C (%) | 6.82 | 6.09 |
| S (%) | 0.11 | 0.08 |
| DN (mg kg−1) | 110 | 120 |
| DOC (mg kg−1) | 370 | 387 |
| DM (%) | pH KCl | NH4-N (mg kg−1) | NO3-N (mg kg−1) | P (%) | K (%) | Mg (%) | Ca (%) |
| 7.88 | 8.02 | 6132 | 0.00 | 0.96 | 2.37 | 0.59 | 1.42 |
| OM (%) | C (%) | N (%) | S (%) | DN (%) | DIC (%) | DC (%) | DOC (%) |
| 70.97 | 42.02 | 2.65 | 0.75 | 1.67 | 1.30 | 4.70 | 3.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escuer-Gatius, J.; Shanskiy, M.; Mander, Ü.; Kauer, K.; Astover, A.; Vahter, H.; Soosaar, K. Intensive Rain Hampers the Effectiveness of Nitrification Inhibition in Controlling N2O Emissions from Dairy Slurry-Fertilized Soils. Agriculture 2020, 10, 497. https://doi.org/10.3390/agriculture10110497
Escuer-Gatius J, Shanskiy M, Mander Ü, Kauer K, Astover A, Vahter H, Soosaar K. Intensive Rain Hampers the Effectiveness of Nitrification Inhibition in Controlling N2O Emissions from Dairy Slurry-Fertilized Soils. Agriculture. 2020; 10(11):497. https://doi.org/10.3390/agriculture10110497
Chicago/Turabian StyleEscuer-Gatius, Jordi, Merrit Shanskiy, Ülo Mander, Karin Kauer, Alar Astover, Hanna Vahter, and Kaido Soosaar. 2020. "Intensive Rain Hampers the Effectiveness of Nitrification Inhibition in Controlling N2O Emissions from Dairy Slurry-Fertilized Soils" Agriculture 10, no. 11: 497. https://doi.org/10.3390/agriculture10110497
APA StyleEscuer-Gatius, J., Shanskiy, M., Mander, Ü., Kauer, K., Astover, A., Vahter, H., & Soosaar, K. (2020). Intensive Rain Hampers the Effectiveness of Nitrification Inhibition in Controlling N2O Emissions from Dairy Slurry-Fertilized Soils. Agriculture, 10(11), 497. https://doi.org/10.3390/agriculture10110497






