Remedial Application of Urea Eliminates Yield Losses in Wheat Waterlogged during Stem Elongation
Abstract
1. Introduction
2. Material and Methods
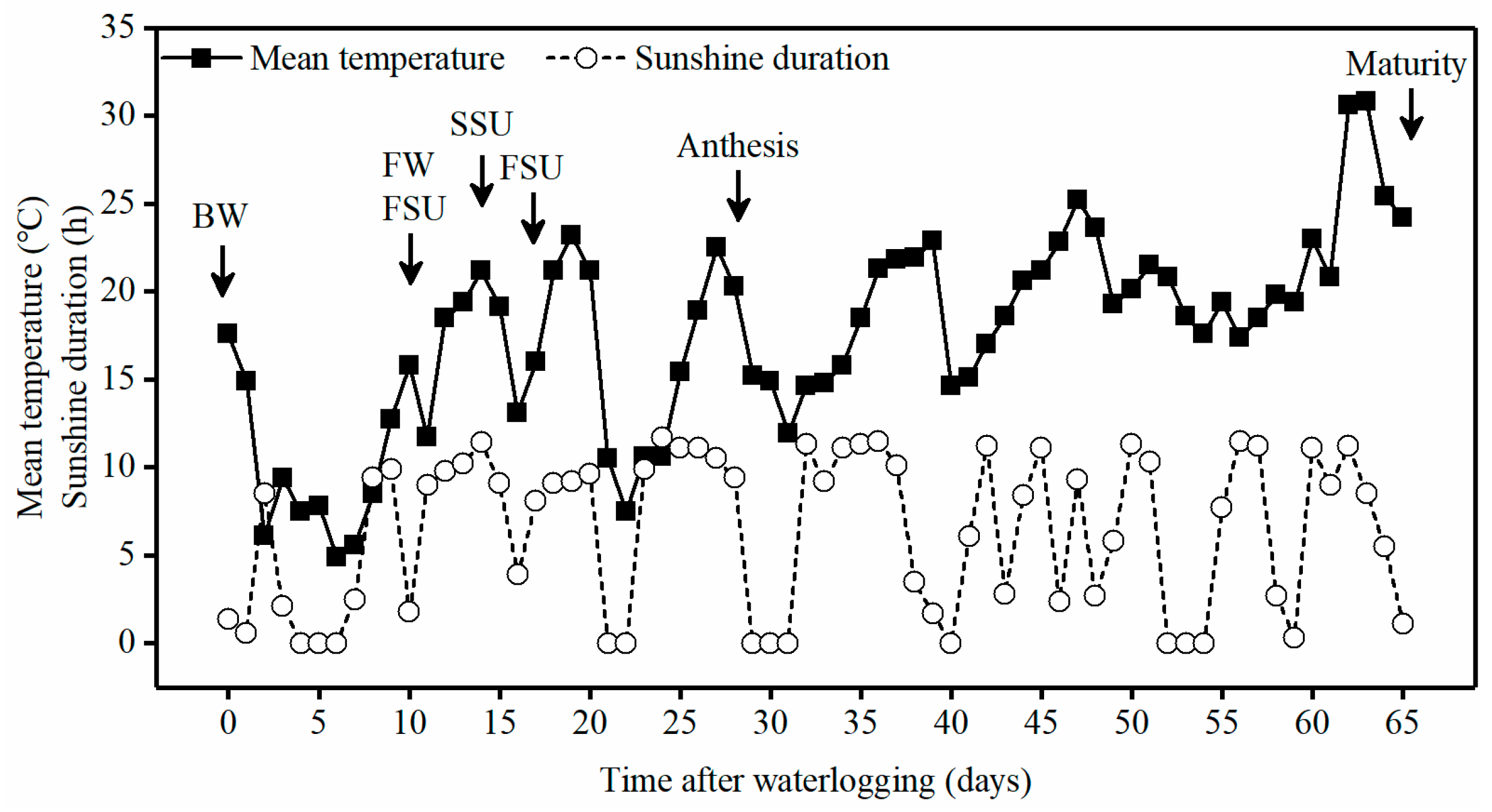
2.1. Growth Conditions
2.2. Experiment Design
2.3. Measurements and Calculations
2.3.1. Chlorophyll Content and Green Leaf Area
2.3.2. Biomass
2.3.3. Grain Yield and Yield Components
2.3.4. N Uptake, N Efficiency, and 15N Recovery
2.4. Statistical Analysis
3. Results
3.1. Grain Yield and Yield Components
3.2. Biomass
3.3. Spike Characters
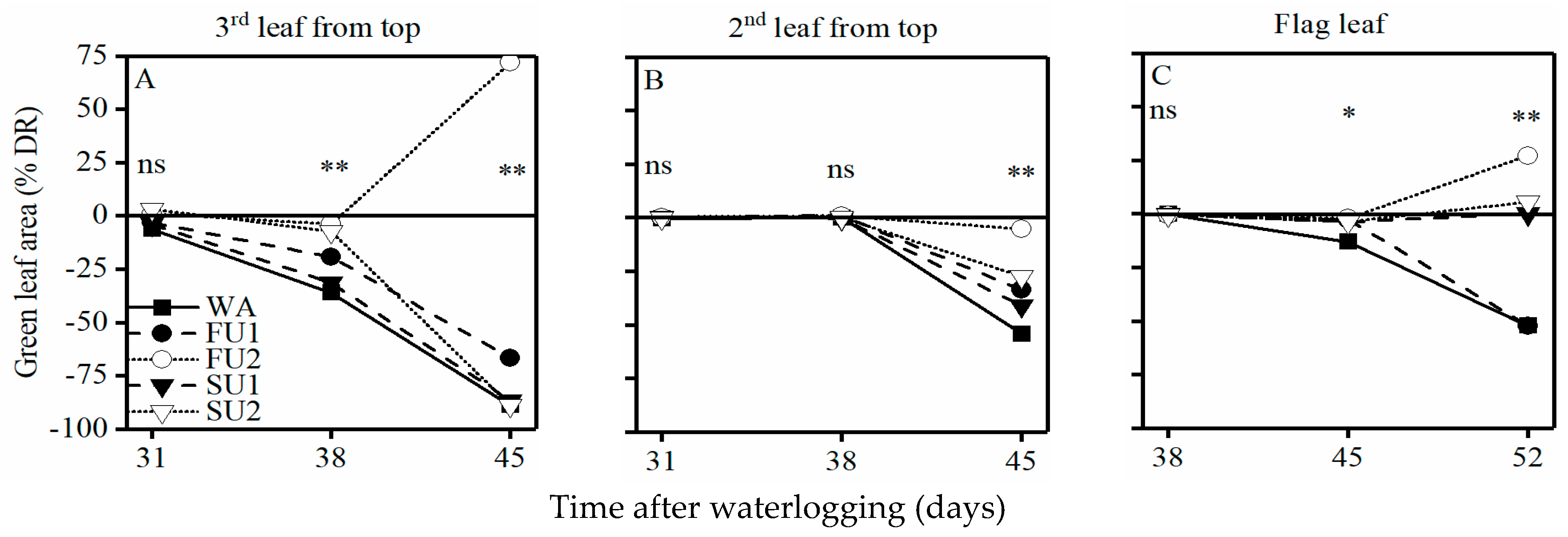
3.4. Green Leaf Area of the Top Three Leaves
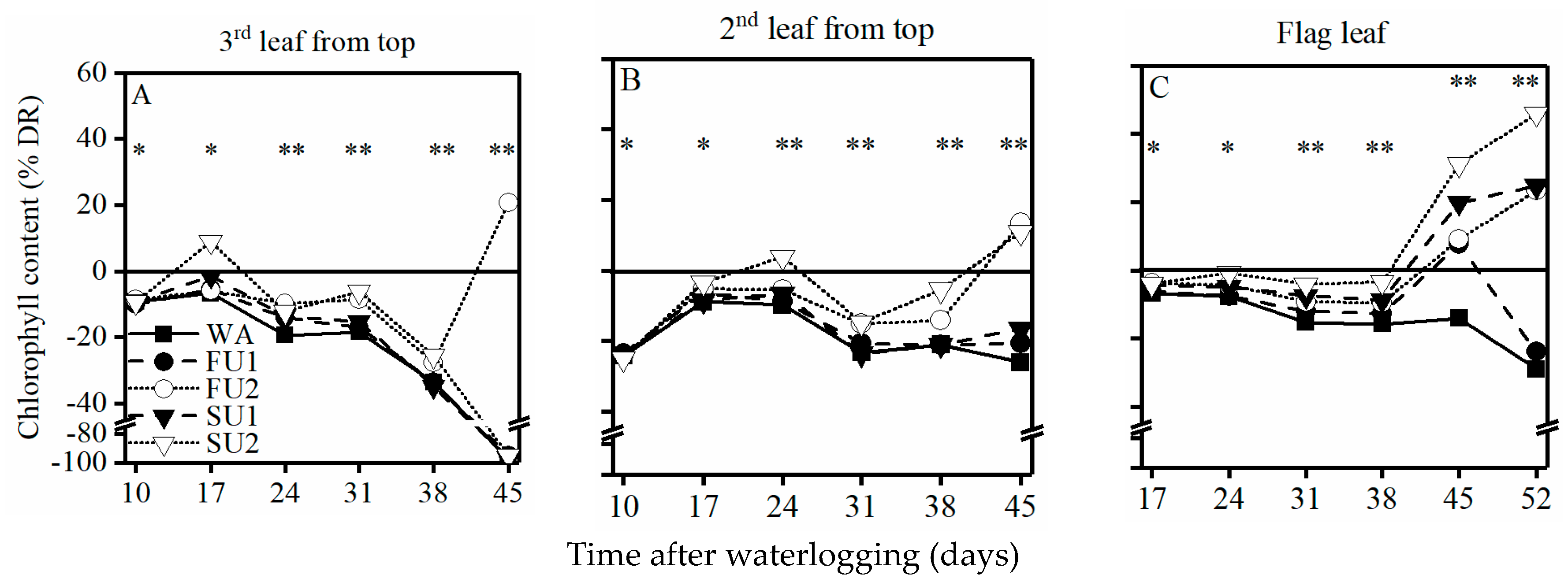
3.5. Chlorophyll Content of the Top Three Leaves
3.6. N Uptake, N Efficiency, 15NREC, and %15NREC
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Manik, S.N.; Pengilley, G.; Dean, G.; Field, B.; Shabala, S.; Zhou, M. Soil and crop management practices to minimize the impact of waterlogging on crop productivity. Front. Plant Sci. 2019, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Huang, M.; Huang, X.; Liu, J.; Wang, X.; Cai, J.; Dai, T.; Cao, W.; Jiang, D. Effect of post-anthesis waterlogging on biosynthesis and granule size distribution of starch in wheat grains. Plant Physiol. Biochem. 2018, 132, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Acuña, T.B.; Dean, G.; Riffkin, P. Constraints to achieving high potential yield of wheat in a temperate, high-rainfall environment in south-eastern Australia. Crop Pasture Sci. 2011, 62, 125–136. [Google Scholar] [CrossRef]
- Ding, J.; Liang, P.; Wu, P.; Zhu, M.; Li, C.; Zhu, X.; Chen, Y.; Guo, W. Effects of waterlogging on grain yield and associated traits of historic wheat cultivars in the middle and lower reaches of the Yangtze River, China. Field Crops Res. 2020, 246, 107695. [Google Scholar] [CrossRef]
- Yaduvanshi, N.P.S.; Setter, T.L.; Sharma, S.K.; Singh, K.N.; Kulshreshtha, N. Influence of waterlogging on yield of wheat (Triticum aestivum), redox potentials, and concentrations of microelements in different soils in India and Australia. Soil Res. 2012, 50, 489–499. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change. Expert Reviewers, Government Reviewers and Other Scientific Advisors of the IPCC WGIII Fifth Assessment Report. In Climate Change 2014: Mitigation of Climate Change: Working Group III Contribution to the IPCC Fifth Assessment Report; Cambridge University Press: Cambridge, UK, 2015; pp. 1371–1414. [Google Scholar] [CrossRef]
- Setter, T.L.; Waters, I. Review of prospects for germplasm improvement for waterlogging tolerance in wheat, barley and oats. Plant Soil 2003, 253, 1–34. [Google Scholar] [CrossRef]
- Hossain, M.A.; Uddin, S.N. Mechanisms of waterlogging tolerance in wheat: Morphological and metabolic adaptations under hypoxia or anoxia. Aust. J. Crop Sci. 2011, 5, 1094. [Google Scholar]
- Liu, C.; Allan, R.P. Observed and simulated precipitation responses in wet and dry regions 1850–2100. Environ. Res. Lett. 2013, 8, 034002. [Google Scholar] [CrossRef]
- Setter, T.L.; Waters, I.; Sharma, S.K.; Singh, K.N.; Kulshreshtha, N.; Yaduvanshi, N.P.S.; Ram, P.C.; Singh, B.N.; Rane, J.; Khabaz-Saberi, G.M.H.; et al. Review of wheat improvement for waterlogging tolerance in Australia and India: The importance of anaerobiosis and element toxicities associated with different soils. Ann. Bot. 2008, 103, 221–235. [Google Scholar] [CrossRef]
- Saqib, M.; Akhtar, J.; Qureshi, R.H.; Nasim, M. Selection and characterization of wheat genotypes for saline soils prone to water-logging. J. Plant Nutr. Soil Sci. 2013, 176, 131–137. [Google Scholar] [CrossRef]
- Shao, G.C.; Lan, J.J.; Yu, S.E.; Liu, N.; Guo, R.Q.; She, D.L. Photosynthesis and growth of winter wheat in response to waterlogging at different growth stages. Photosynthetica 2013, 51, 429–437. [Google Scholar] [CrossRef]
- Wu, X.; Tang, Y.; Li, C.; McHugh, A.D.; Li, Z.; Wu, C. Individual and combined effects of soil waterlogging and compaction on physiological characteristics of wheat in southwestern China. Field Crops Res. 2018, 215, 163–172. [Google Scholar] [CrossRef]
- De San Celedonio, R.P.; Abeledo, L.G.; Brihet, J.M.; Miralles, D.J. Waterlogging affects leaf and tillering dynamics in wheat and barley. J. Agron. Crop Sci. 2016, 202, 409–420. [Google Scholar] [CrossRef]
- De San Celedonio, R.P.; Abeledo, L.G.; Miralles, D.J. Physiological traits associated with reductions in grain number in wheat and barley under waterlogging. Plant Soil 2018, 429, 469–481. [Google Scholar] [CrossRef]
- Pampana, S.; Masoni, A.; Arduini, I. Grain yield of durum wheat as affected by waterlogging at tillering. Cereal Res. Commun. 2016, 44, 706–716. [Google Scholar] [CrossRef]
- Malik, A.I.; Colmer, T.D.; Lambers, H.; Setter, T.L.; Schortemeyer, M. Short-term waterlogging has long-term effects on the growth and physiology of wheat. New Phytol. 2002, 153, 225–236. [Google Scholar] [CrossRef]
- De San Celedonio, R.P.; Abeledo, L.G.; Mantese, A.I.; Miralles, D.J. Differential root and shoot biomass recovery in wheat and barley with transient waterlogging during preflowering. Plant Soil 2017, 417, 481–498. [Google Scholar] [CrossRef]
- Collaku, A.; Harrison, S.A. Heritability of waterlogging tolerance in wheat. Crop Sci. 2005, 45, 722–727. [Google Scholar] [CrossRef]
- Dickin, E.; Bennett, S.; Wright, D. Growth and yield responses of UK wheat cultivars to winter waterlogging. J. Agric. Sci. 2009, 147, 127–140. [Google Scholar] [CrossRef]
- Dickin, E.; Wright, D. The effects of winter waterlogging and summer drought on the growth and yield of winter wheat (Triticum aestivum L.). Eur. J. Agron. 2008, 28, 234–244. [Google Scholar] [CrossRef]
- Araki, H.; Hamada, A.; Hossain, M.A.; Takahashi, T. Waterlogging at jointing and/or after anthesis in wheat induces early leaf senescence and impairs grain filling. Field Crops Res. 2012, 137, 27–36. [Google Scholar] [CrossRef]
- De San Celedonio, R.P.; Abeledo, L.G.; Miralles, D.J. Identifying the critical period for waterlogging on yield and its components in wheat and barley. Plant Soil 2014, 378, 265–277. [Google Scholar] [CrossRef]
- Ding, J.; Huang, Z.; Zhu, M.; Li, C.; Zhu, X.; Guo, W. Does cyclic water stress damage wheat yield more than a single stress? PLoS ONE 2018, 13, e0195535. [Google Scholar] [CrossRef]
- Brisson, N.; Rebiere, B.; Zimmer, D.; Renault, P. Response of the root system of a winter wheat crop to waterlogging. Plant Soil 2002, 243, 43–55. [Google Scholar] [CrossRef]
- Arguello, M.N.; Mason, R.E.; Roberts, T.L.; Subramanian, N.; Acuna, A.; Addison, C.K.; Lozada, D.N.; Miller, R.G.; Gbur, E. Performance of soft red winter wheat subjected to field soil waterlogging: Grain yield and yield components. Field Crops Res. 2016, 194, 57–64. [Google Scholar] [CrossRef]
- Tan, W.; Liu, J.; Dai, T.; Jing, Q.; Cao, W.; Jiang, D. Alterations in photosynthesis and antioxidant enzyme activity in winter wheat subjected to post-anthesis waterlogging. Photosynthetica 2008, 46, 21–27. [Google Scholar] [CrossRef]
- Hossain, M.A.; Araki, H.; Takahashi, T. Poor grain filling induced by waterlogging is similar to that in abnormal early ripening in wheat in Western Japan. Field Crops Res. 2011, 123, 100–108. [Google Scholar] [CrossRef]
- Li, H.; Cai, J.; Liu, F.; Jiang, D.; Dai, T.; Cao, W. Generation and scavenging of reactive oxygen species in wheat flag leaves under combined shading and waterlogging stress. Funct. Plant Biol. 2012, 39, 71–81. [Google Scholar] [CrossRef]
- Herzog, M.; Striker, G.G.; Colmer, T.D.; Pedersen, O. Mechanisms of waterlogging tolerance in wheat–a review of root and shoot physiology. Plant Cell Environ. 2016, 39, 1068–1086. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Osanai, Y.; Anderson, I.C.; Bange, M.P.; Tissue, D.T.; Singh, B.K. Flooding and prolonged drought have differential legacy impacts on soil nitrogen cycling, microbial communities and plant productivity. Plant Soil 2018, 431, 371–387. [Google Scholar] [CrossRef]
- Harris, R.H.; Armstrong, R.D.; Wallace, A.J.; Belyaeva, O.N. Effect of nitrogen fertiliser management on soil mineral nitrogen, nitrous oxide losses, yield and nitrogen uptake of wheat growing in waterlogging-prone soils of south-eastern Australia. Soil Res. 2016, 54, 619–633. [Google Scholar] [CrossRef][Green Version]
- Robertson, D.; Zhang, H.; Palta, J.A.; Colmer, T.; Turner, N.C. Waterlogging affects the growth, development of tillers, and yield of wheat through a severe, but transient, N deficiency. Crop Pasture Sci. 2009, 60, 578–586. [Google Scholar] [CrossRef]
- Jiang, D.; Fan, X.; Dai, T.; Cao, W. Nitrogen fertiliser rate and post-anthesis waterlogging effects on carbohydrate and nitrogen dynamics in wheat. Plant Soil 2008, 304, 301–314. [Google Scholar] [CrossRef]
- Simpson, N.L.; Brennan, R.F.; Anderson, W.K. Grain yield increases in wheat and barley to nitrogen applied after transient waterlogging in the high rainfall cropping zone of western Australia. J. Plant Nutr. 2016, 39, 974–992. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, Z.; Zhang, M.; Shi, Y.; Zhu, Q.; Sun, Y.; Zhou, H.; Li, C.; Yang, Y.; Geng, J. Improving crop yields, nitrogen use efficiencies, and profits by using mixtures of coated controlled-released and uncoated urea in a wheat-maize system. Field Crops Res. 2017, 205, 106–115. [Google Scholar] [CrossRef]
- Swarup, A.; Sharma, D. Influence of top-dressed nitrogen in alleviating adverse effects of flooding on growth and yield of wheat in a sodic soil. Field Crops Res. 1993, 35, 93–100. [Google Scholar] [CrossRef]
- Najeeb, U.; Bange, M.P.; Tan, D.K.; Atwell, B.J. Consequences of waterlogging in cotton and opportunities for mitigation of yield losses. AoB Plants 2015, 7, plv080. [Google Scholar] [CrossRef]
- Pang, J.; Ross, J.; Zhou, M.; Mendham, N.; Shabala, S. Amelioration of detrimental effects of waterlogging by foliar nutrient sprays in barley. Funct. Plant Biol. 2007, 34, 221–227. [Google Scholar] [CrossRef]
- Wu, J.D.; Li, J.C.; Wei, F.Z.; Wang, C.Y.; Zhang, Y.; Sun, G. Effects of nitrogen spraying on the post-anthesis stage of winter wheat under waterlogging stress. Acta Physiol. Plant. 2014, 36, 207–216. [Google Scholar] [CrossRef]
- Cheng, S.; Guo, W.; Wang, L. Wheat in South China; Jiangsu Science and Technology Press: Nanjing, China, 2012. [Google Scholar]
- Chen, Y.; Huang, J.; Song, X.; Gao, P.; Wan, S.; Shi, L.; Wang, X. Spatiotemporal Characteristics of Winter Wheat Waterlogging in the Middle and Lower Reaches of the Yangtze River, China. Adv. Meteorol. 2018, 2018, 3542103. [Google Scholar] [CrossRef]
- Scheiner, D. Determination of ammonia and Kjeldahl nitrogen by indophenol method. Water Res. 1976, 10, 31–36. [Google Scholar] [CrossRef]
- Hauck, R.D.; Bremner, J.M. Use of tracers for soil and fertilizer nitrogen research. Adv. Agron. 1976, 28, 219–266. [Google Scholar] [CrossRef]
- Bronson, K.F.; Fillery, I.R.P. Fate of nitrogen-15-labelled urea applied to wheat on a waterlogged texture-contrast soil. Nutr. Cycl. Agroecosyst. 1998, 51, 175–183. [Google Scholar] [CrossRef]
- Ren, B.; Dong, S.; Zhao, B.; Liu, P.; Zhang, J. Responses of nitrogen metabolism, uptake and translocation of maize to waterlogging at different growth stages. Front. Plant Sci. 2017, 8, 1216. [Google Scholar] [CrossRef]
- Camargo, J.A.; Alonso, Á. Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: A global assessment. Environ. Int. 2006, 32, 831–849. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Tuan, P.A.; Mukherjee, S.; Son, S.; Ayele, B.T. Hormonal regulation in adventitious roots and during their emergence under waterlogged conditions in wheat. J. Exp. Bot. 2018, 69, 4065–4082. [Google Scholar] [CrossRef]
- Liu, R.; Yang, C.; Zhang, G.; Zhang, L.; Yang, F.; Guo, W. Root recovery development and activity of cotton plants after waterlogging. Agron. J. 2015, 107, 2038–2046. [Google Scholar] [CrossRef]
- Fageria, N.K.; Filho, M.B.; Moreira, A.; Guimarães, C.M. Foliar fertilization of crop plants. J. Plant Nutr. 2009, 32, 1044–1064. [Google Scholar] [CrossRef]
- Demotes-Mainard, S.; Jeuffroy, M.H.; Robin, S. Spike dry matter and nitrogen accumulation before anthesis in wheat as affected by nitrogen fertilizer: Relationship to kernels per spike. Field Crops Res. 1999, 64, 249–259. [Google Scholar] [CrossRef]
| Treatments | Spikes per Pot | Kernels per Spike | 1000-Kernel Weight (g) | Grain Yield (g pot−1) | Biomass (g·pot−1) | ||
|---|---|---|---|---|---|---|---|
| Pre-Anthesis | Post-Anthesis | Total | |||||
| DR | 34 a 1 | 42 bc | 46 a | 66 ab | 90 b | 33 ab | 123 bc |
| WA | 36 a | 35 d | 46 a | 59 c | 90 b | 22 d | 112 d |
| FU1 | 36 a | 37 cd | 47 a | 62 bc | 91 b | 31 bc | 121 c |
| FU2 | 37 a | 49 a | 39 b | 73 a | 96 a | 38 a | 133 a |
| SU1 | 38 a | 45 b | 36 c | 62 bc | 97 a | 28 c | 124 bc |
| SU2 | 38 a | 51 a | 35 c | 69 ab | 97 a | 30 bc | 127 b |
| Treatments | Single Spike Yield | Spikelets per Spike | Spikelet Fertility (%) | Kernels per Spikelet |
|---|---|---|---|---|
| DR | 1.9 a 1 | 19 a | 89 c | 2.2 b |
| WA | 1.6 b | 19 a | 75 e | 1.9 c |
| FU1 | 1.7 b | 19 a | 78 d | 2.0 c |
| FU2 | 1.9 a | 19 a | 94 a | 2.7 a |
| SU1 | 1.6 b | 18 a | 88 c | 2.4 ab |
| SU2 | 1.8 ab | 19 a | 91 b | 2.7 a |
| Time after Waterlogging (Days) | Spikelet Fertility | Kernels per Spikelet | Kernels per Spike | Post-Anthesis Biomass |
|---|---|---|---|---|
| 3rd leaf from top | ||||
| 31 | 0.80 1 | 0.79 | 0.80 | 0.73 |
| 38 | 0.70 | 0.55 | 0.57 | 0.86 * |
| 45 | 0.57 | 0.44 | 0.42 | 0.80 |
| 2nd leaf from top | ||||
| 31 | 0.76 | 0.63 | 0.58 | 0.64 |
| 38 | −0.08 | −0.11 | −0.06 | 0.48 |
| 45 | 0.71 | 0.49 | 0.49 | 0.91 * |
| Flag leaf | ||||
| 38 | −0.22 | −0.34 | −0.38 | −0.40 |
| 45 | 0.66 | 0.43 | 0.43 | 0.86 * |
| 52 | 0.98 ** 2 | 0.92 ** | 0.90 * | 0.71 |
| Time after Waterlogging (Days) | Spikelet Fertility | Kernels per Spikelet | Kernels per Spike | Post-Anthesis Biomass |
|---|---|---|---|---|
| 3rd leaf from top | ||||
| 10 | −0.09 1 | −0.26 | −0.26 | |
| 17 | 0.33 | 0.47 | 0.48 | |
| 24 | 0.57 | 0.31 | 0.30 | |
| 31 | 0.69 | 0.54 | 0.54 | 0.64 |
| 38 | 0.25 | 0.03 | 0.02 | 0.32 |
| 45 | 0.57 | 0.34 | 0.32 | 0.80 |
| 2nd leaf from top | ||||
| 10 | 0.13 | −0.13 | −0.16 | |
| 17 | 0.50 | 0.32 | 0.34 | |
| 24 | 0.66 | 0.66 | 0.68 | |
| 31 | 0.44 | 0.22 | 0.22 | 0.57 |
| 38 | 0.56 | 0.44 | 0.45 | 0.48 |
| 45 | 0.89 * | 0.86 * | 0.88 * | 0.78 |
| Flag leaf | ||||
| 17 | 0.64 | 0.41 | 0.39 | |
| 24 | 0.72 | 0.63 | 0.63 | |
| 31 | 0.68 | 0.54 | 0.53 | 0.51 |
| 38 | 0.68 | 0.55 | 0.54 | 0.50 |
| 45 | 0.66 | 0.78 | 0.80 | 0.32 |
| 52 | 0.88 * 2 | 0.97 ** | 0.96 ** | 0.42 |
| Treatments | Total N Uptake (g·pot−1) | N Partial Factor Productivity (g·g−1) | 15NREC (mg·pot−1) | %15NREC |
|---|---|---|---|---|
| DR | 1.6 b 1 | 37 a | ||
| WA | 1.3 d | 32 c | ||
| FU1 | 1.4 c | 33 bc | 23 d | 46 c |
| FU2 | 1.7 b | 38 a | 56 b | 56 b |
| SU1 | 1.4 c | 33 bc | 32 c | 64 a |
| SU2 | 1.8 a | 36 ab | 68 a | 68 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, J.; Liang, P.; Guo, D.; Liu, D.; Yin, M.; Zhu, M.; Li, C.; Zhu, X.; Guo, W. Remedial Application of Urea Eliminates Yield Losses in Wheat Waterlogged during Stem Elongation. Agriculture 2020, 10, 23. https://doi.org/10.3390/agriculture10010023
Ding J, Liang P, Guo D, Liu D, Yin M, Zhu M, Li C, Zhu X, Guo W. Remedial Application of Urea Eliminates Yield Losses in Wheat Waterlogged during Stem Elongation. Agriculture. 2020; 10(1):23. https://doi.org/10.3390/agriculture10010023
Chicago/Turabian StyleDing, Jinfeng, Peng Liang, Desheng Guo, Dejun Liu, Mingxiao Yin, Min Zhu, Chunyan Li, Xinkai Zhu, and Wenshan Guo. 2020. "Remedial Application of Urea Eliminates Yield Losses in Wheat Waterlogged during Stem Elongation" Agriculture 10, no. 1: 23. https://doi.org/10.3390/agriculture10010023
APA StyleDing, J., Liang, P., Guo, D., Liu, D., Yin, M., Zhu, M., Li, C., Zhu, X., & Guo, W. (2020). Remedial Application of Urea Eliminates Yield Losses in Wheat Waterlogged during Stem Elongation. Agriculture, 10(1), 23. https://doi.org/10.3390/agriculture10010023









