Effect of 3-Phenyllactic Acid and 3-Phenyllactic Acid-Producing Lactic Acid Bacteria on the Characteristics of Alfalfa Silage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lactic Acid Bacteria Screening
2.2. LAB Identification
2.3. Determination of PLA
2.4. Additive Preparation
2.5. Silage Making
2.6. Analytical Methods
2.7. Statistical Analysis
3. Results
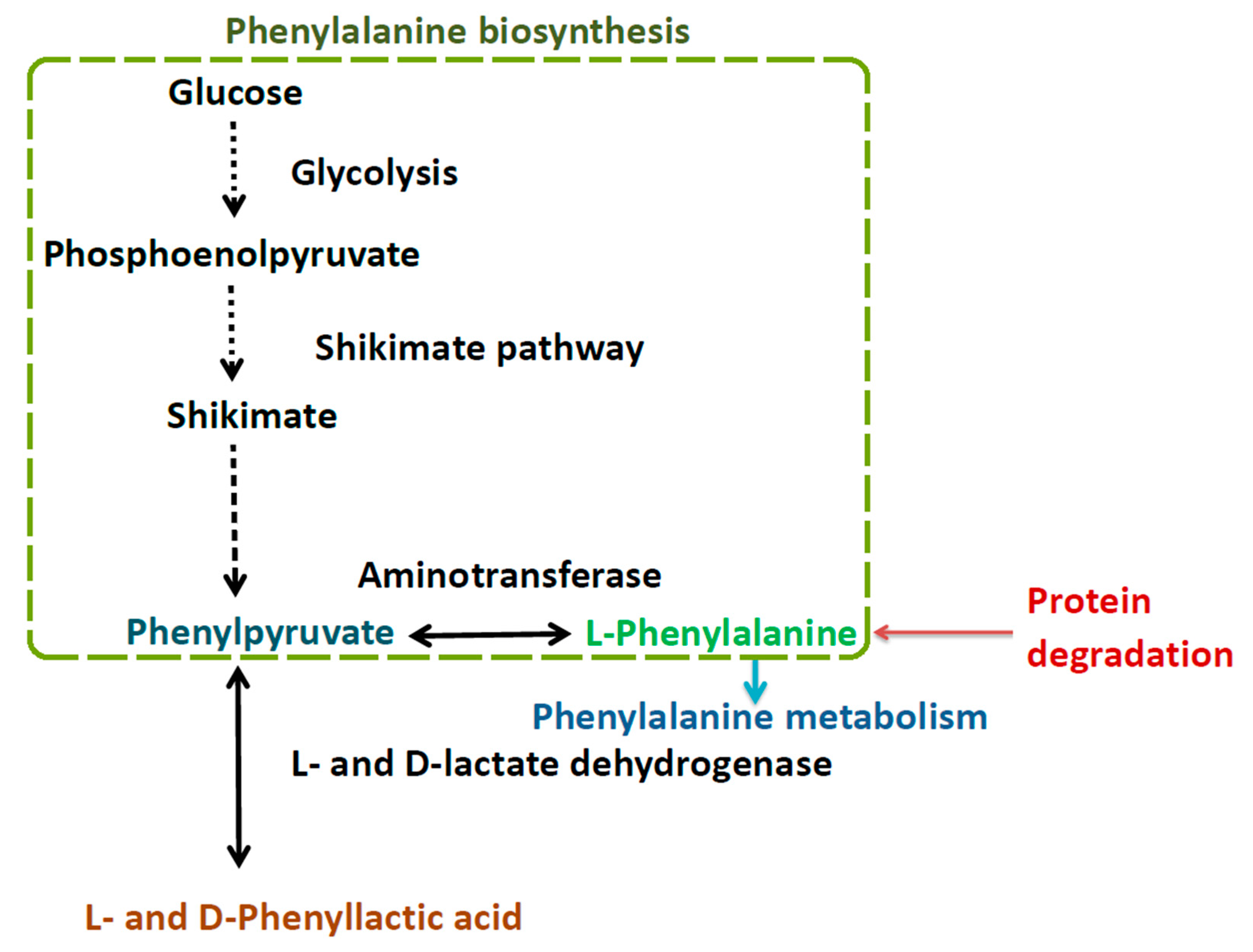
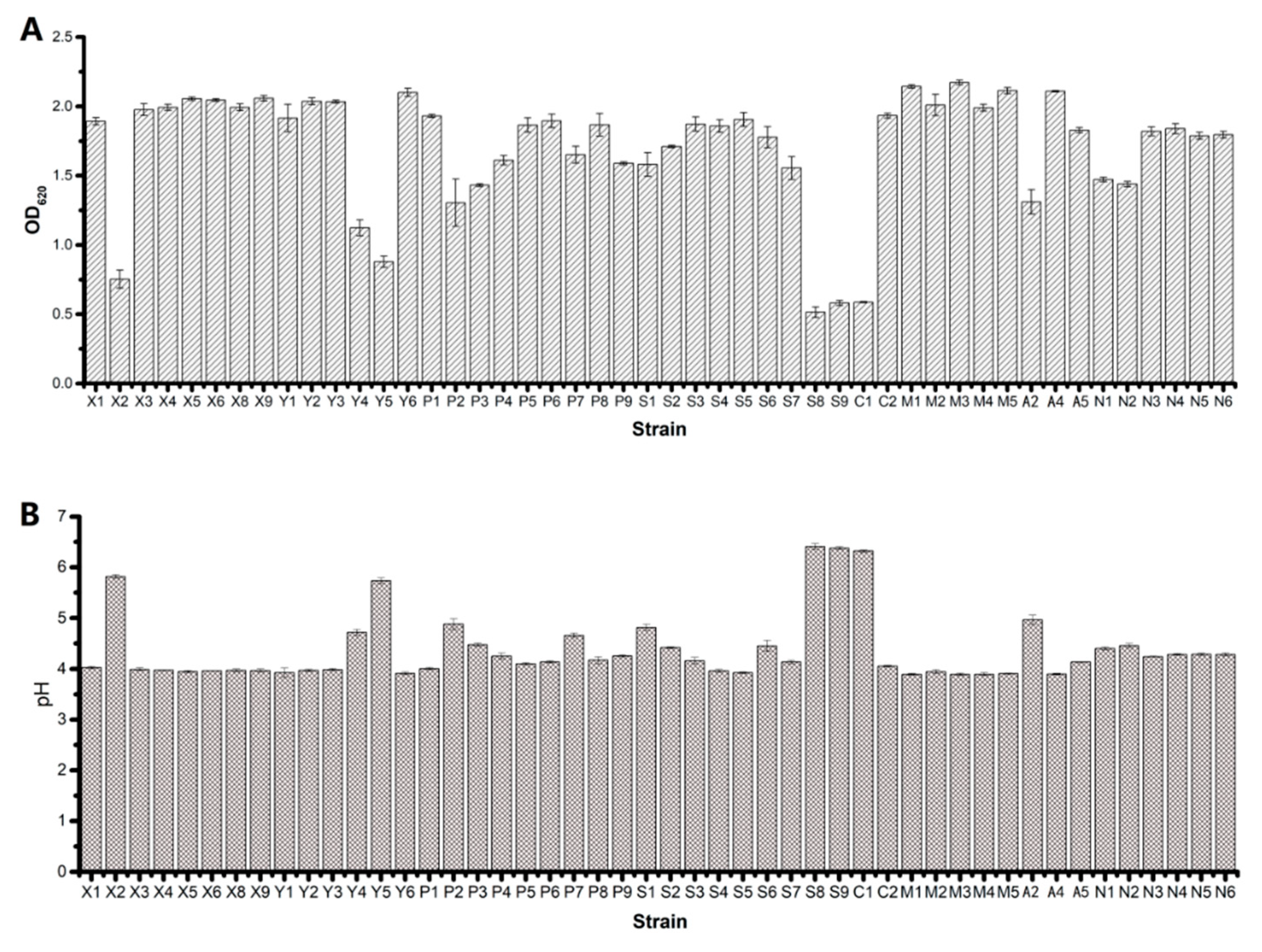
3.1. Strain Screening and Characteristics of the Strains
3.2. Chemical Composition of Fresh Alfalfa
3.3. The Fermentation Characteristics of Alfalfa Silage
3.4. The Nutrient Characteristics of Alfalfa Silage
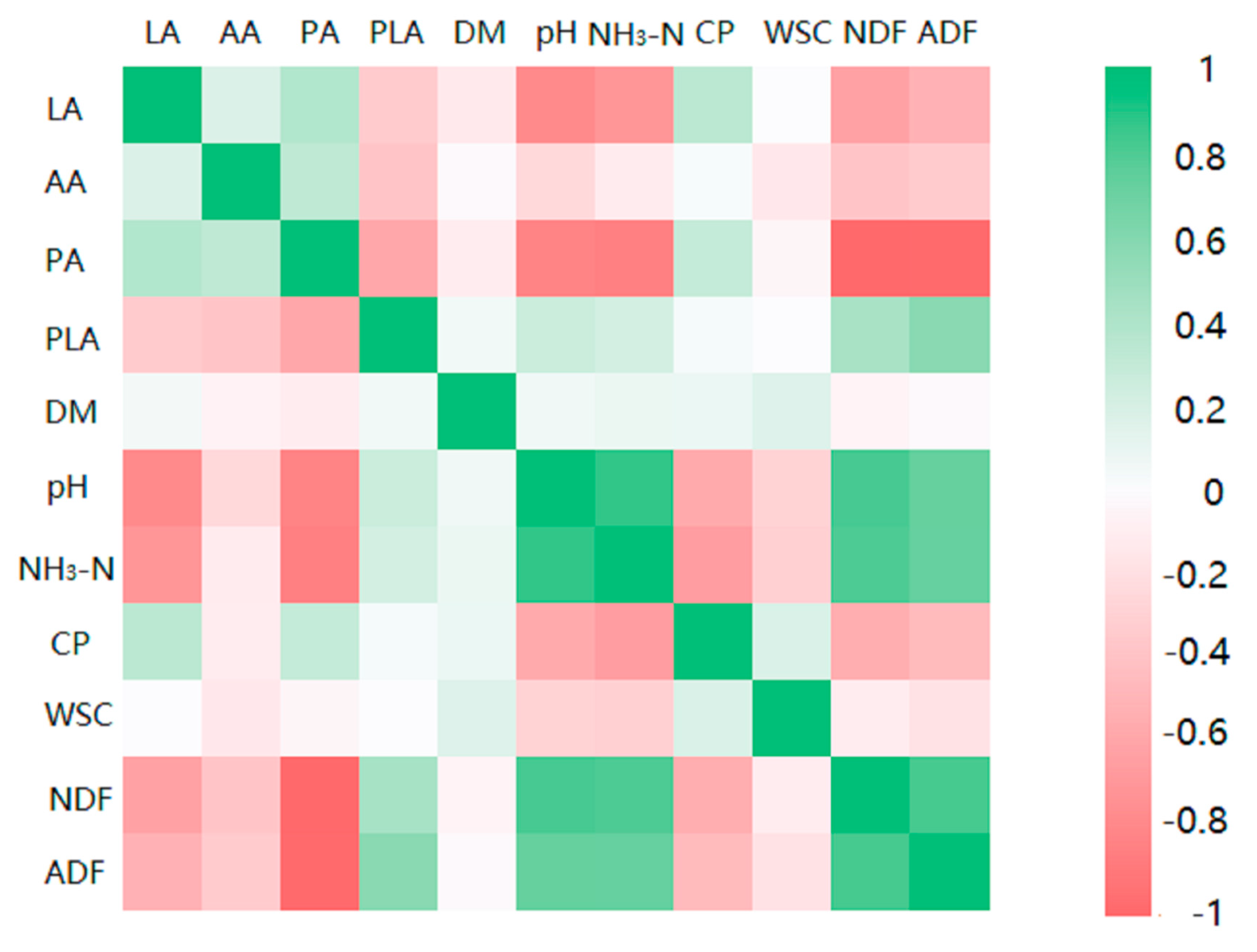
3.5. Correlation Analysis between Silage Fermentation and Treatments
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tabacco, E.; Borreani, G.; Crovetto, G.M.; Galassi, G.; Colombo, D.; Cavallarin, L. Effect of chestnut tannin on fermentation quality, proteolysis, and protein rumen degradability of alfalfa silage. J. Dairy Sci. 2006, 89, 4736–4746. [Google Scholar] [CrossRef] [Green Version]
- Owens, V.N.; Albrecht, K.A.; Muck, R.E. Protein degradation and fermentation characteristics of unwilted red clover and alfalfa silage harvested at various times during the day. Grass Forage Sci. 2002, 57, 329–341. [Google Scholar] [CrossRef] [Green Version]
- Nagel, S.A.; Broderick, G.A. Effect of formic-acid or formaldehyde treatment of alfalfa silage on nutrient utilization by dairy-cows. J. Dairy Sci. 1992, 75, 140–154. [Google Scholar] [CrossRef]
- Su, R.; Ni, K.; Wang, T.; Yang, X.; Zhang, J.; Liu, Y.; Shi, W.; Yan, L.; Jie, C.; Zhong, J. Effects of ferulic acid esterase-producing Lactobacillus fermentum and cellulase additives on the fermentation quality and microbial community of alfalfa silage. PeerJ 2019, 7, e7712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Wang, J.Q.; Zhou, H.; Feng, T. Effects of addition of previously fermented juice prepared from alfalfa on fermentation quality and protein degradation of alfalfa silage. Anim. Feed Sci. Technol. 2009, 151, 280–290. [Google Scholar] [CrossRef]
- Szumacher-Strabel, M.; Stochmal, A.; Cieslak, A.; Kozlowska, M.; Kuznicki, D.; Kowalczyk, M.; Oleszek, W. Structural and quantitative changes of saponins in fresh alfalfa compared to alfalfa silage. J. Sci. Food Agric. 2019, 99, 2243–2250. [Google Scholar] [CrossRef]
- Jia, T.; Sun, Z.; Gao, R.; Yu, Z. Lactic acid bacterial inoculant effects on the vitamin content of alfalfa and Chinese leymus silage. Asian-Australas. J. Anim. Sci. 2019, 32, 1873–1881. [Google Scholar] [CrossRef]
- Strom, K.; Sjogren, J.; Broberg, A.; Schnurer, J. Lactobacillus plantarum MiLAB 393 produces the antifungal cyclic dipeptides cyclo(L-Phe-L-Pro) and cyclo(L-Phe-trans-4-OH-L-Pro) and 3-phenyllactic acid. Appl. Environ. Microb. 2002, 68, 4322–4327. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Ning, Y.; Liu, D.; Yan, A.; Wang, Z.; Wang, S.; Miao, M.; Zhu, H.; Jia, Y. Metabolic mechanism of phenyllactic acid naturally occurring in Chinese pickles. Food Chem. 2015, 186, 265–270. [Google Scholar] [CrossRef]
- Dieuleveux, V.; Lemarinier, S.; Gueguen, M. Antimicrobial spectrum and target site of D-3-phenyllactic acid. Int. J. Food Microbiol. 1998, 40, 177–183. [Google Scholar] [CrossRef]
- Prema, P.; Smila, D.; Palavesam, A.; Immanuel, G. Production and characterization of an antifungal compound (3-Phenyllactic Acid) produced by Lactobacillus plantarum strain. Food Bioprocess Technol. 2010, 3, 379–386. [Google Scholar] [CrossRef]
- Mu, W.M.; Yu, S.H.; Zhu, L.J.; Zhang, T.; Jiang, B. Recent research on 3-phenyllactic acid, a broad-spectrum antimicrobial compound. Appl. Microbiol. Biot. 2012, 95, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Boebel, K.P.; Baker, D.H. Comparative utilization of the isomers of phenylalanine and phenyllactic acid by chicks and rats. J. Nutr. 1982, 112, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.P.; Yoo, J.S.; Lee, J.H.; Zhou, T.X.; Jang, H.D.; Kim, H.J.; Kim, I.H. Effects of phenyllactic acid on production performance, egg quality parameters, and blood characteristics in laying hens. J. Appl. Poultry Res. 2009, 18, 203–209. [Google Scholar] [CrossRef]
- Wang, J.P.; Yoo, J.S.; Lee, J.H.; Jang, H.D.; Kim, H.J.; Shin, S.O.; Seong, S.I.; Kim, I.H. Effects of phenyllactic acid on growth performance, nutrient digestibility, microbial shedding, and blood profile in pigs. J. Anim. Sci. 2009, 87, 3235–3243. [Google Scholar] [CrossRef] [Green Version]
- Assareh, R.; Zahiri, H.S.; Noghabi, K.A.; Aminzadeh, S.; Khaniki, G.B. Characterization of the newly isolated Geobacillus sp T1, the efficient cellulase-producer on untreated barley and wheat straws. Bioresour. Technol. 2012, 120, 99–105. [Google Scholar] [CrossRef]
- Donnell, M.M.O.; Forde, B.M.; Neville, B.; Ross, P.R.; Toole, P.W.O. Carbohydrate catabolic flexibility in the mammalian intestinal commensal Lactobacillus ruminis revealed by fermentation studies aligned to genome annotations. Microb. Cell Fact. 2011, 10. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.G.; Xie, Y.X.; Yu, Z.; Meng, G.; Wu, Z. Effect of Lactobacillus plantarum expressing multifunctional glycoside hydrolases on the characteristics of alfalfa silage. Appl. Microbiol. Biot. 2019, 103, 7983–7995. [Google Scholar] [CrossRef]
- Lavermicocca, P.; Valerio, F.; Visconti, A. Antifungal activity of phenyllactic acid against molds isolated from bakery products. Appl. Environ. Microb. 2003, 69, 634–640. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.M.; Ding, W.R.; Ke, W.C.; Li, F.H.; Zhang, P.; Guo, X.S. Modulation of metabolome and bacterial community in whole crop corn silage by inoculating homofermentative lactobacillus plantarum and heterofermentative Lactobacillus buchneri. Front. Microbiol. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Yang, J.; Pang, H.; Kitahara, M. Lactococcus fujiensis sp. nov., a lactic acid bacterium isolated from vegetable matter. Int. J. Syst. Evol. Microbiol. 2011, 61, 1590–1594. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.H.; Yang, F.Y.; Zhang, J.G.; Shao, T. Characteristics of Lactobacillus parafarraginis ZH1 and its role in improving the aerobic stability of silages. J. Appl. Microbiol. 2014, 117, 405–416. [Google Scholar] [CrossRef]
- Liu, Q.; Lindow, S.E.; Zhang, J. Lactobacillus parafarraginis ZH1 producing anti-yeast substances to improve the aerobic stability of silage. Anim. Sci. J. 2018, 89, 1302–1309. [Google Scholar] [CrossRef]
- Jin, L.; Duniere, L.; Lynch, J.P.; Zaheer, R.; Turkington, K.; Blackshaw, R.E.; Lupwayi, N.Z.; O’Donovan, J.T.; Harker, K.N.; McAllister, T.; et al. Impact of ferulic acid esterase-producing lactobacilli and fibrolytic enzymes on ensiling and digestion kinetics of mixed small-grain silage. Grass Forage Sci. 2017, 72, 80–92. [Google Scholar] [CrossRef]
- Li, J.; Yuan, X.; Desta, S.T.; Dong, Z.; Mugabe, W.; Shao, T. Characterization of Enterococcus faecalis JF85 and Enterococcus faecium Y83 isolated from Tibetan yak (Bos grunniens) for ensiling Pennisetum sinese. Bioresour. Technol. 2018, 257, 76–83. [Google Scholar] [CrossRef]
- Valan Arasu, M.; Jung, M.W.; Ilavenil, S.; Jane, M.; Kim, D.H.; Lee, K.D.; Park, H.S.; Hur, T.Y.; Choi, G.J.; Lim, Y.C.; et al. Isolation and characterization of antifungal compound from Lactobacillus plantarum KCC-10 from forage silage with potential beneficial properties. J. Appl. Microbiol. 2013, 115, 1172–1185. [Google Scholar] [CrossRef]
- Broberg, A.; Jacobsson, K.; Strom, K.; Schnurer, J. Metabolite profiles of lactic acid bacteria in grass silage. Appl. Environ. Microb. 2007, 73, 5547–5552. [Google Scholar] [CrossRef] [Green Version]
- Dickert, S.; Pierik, A.J.; Buckel, W. Molecular characterization of phenyllactate dehydratase and its initiator from Clostridium sporogenes. Mol. Microbiol. 2002, 44, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Valerio, F.; Lavermicocca, P.; Pascale, M.; Visconti, A. Production of phenyllactic acid by lactic acid bacteria: An approach to the selection of strains contributing to food quality and preservation. FEMS Microbiol. Lett. 2004, 233, 289–295. [Google Scholar] [CrossRef]
| Strain | Lactic Acid (mg/mL2) | Acetic Acid (mg/mL) | Propionic Acid (mg/mL) | Butyric Acid (mg/mL) | Phenyllactic Acid (mg/mL) |
|---|---|---|---|---|---|
| X5 | 10.82 ± 0.08de | 2.87 ± 0.02bc | 2.19 ± 0.00a | ND | 0.054 ± 0.001cd |
| X6 | 10.65 ± 0.06ef | 2.87 ± 0.00bc | 2.17 ± 0.01ab | ND | 0.053 ± 0.001cd |
| X9 | 10.50 ± 0.24f | 2.86 ± 0.02cd | 2.15 ± 0.04bc | ND | 0.051 ± 0.000d |
| Y2 | 10.50 ± 0.18f | 2.87 ± 0.01bc | 2.18 ± 0.02ab | ND | 0.058 ± 0.001b |
| Y3 | 10.45 ± 0.14f | 2.89 ± 0.01ab | 2.12 ± 0.01cd | ND | 0.052 ± 0.001cd |
| Y6 | 10.97 ± 0.22bcde | 2.89 ± 0.01ab | 2.17 ± 0.01ab | ND | 0.052 ± 0.001cd |
| M1 | 11.28 ± 0.08ab | 2.80 ± 0.01f | 1.97 ± 0.02f | ND | 0.066 ± 0.000a |
| M2 | 10.90 ± 0.37cde | 2.86 ± 0.02cd | 2.02 ± 0.03e | ND | 0.052 ± 0.001cd |
| M3 | 11.33 ± 0.12a | 2.84 ± 0.01e | 2.13 ± 0.01cd | ND | 0.055 ± 0.000c |
| M5 | 11.02 ± 0.06abcd | 2.84 ± 0.01 de | 2.13 ± 0.01cd | ND | 0.048 ± 0.001d |
| A4 | 11.22 ± 0.07abc | 2.91 ± 0.01a | 2.09 ± 0.01 | ND | 0.050 ± 0.001d |
| Carbohydrate | L. Plantarum M1 | L. Plantarum M3 |
|---|---|---|
| Arabinose | + | + |
| Cellobiose | + | + |
| Fructose | + | + |
| Galactose | + | + |
| Glucose | + | + |
| Lactose | + | + |
| Maltose | + | + |
| Mannose | + | + |
| Raffinose | + | + |
| Rhamnose | − | − |
| Sucrose | + | + |
| Xylose | + | + |
| Strains 1 | Initial Content of PLA | pH | Lactic Acid (% DM 2) | Acetic Acid (% DM) | Propionic Acid (% DM) | Butyric Acid (% DM) | Phenyllactic Acid (‰ DM) |
|---|---|---|---|---|---|---|---|
| CK | 0% | 4.65 ± 0.07a | 4.17 ± 0.02d | 2.75 ± 0.19d | 0.64 ± 0.03c | ND 3 | 0.04 ± 0.00b |
| 0.1% | 4.54 ± 0.04b | 4.36 ± 0.09cd | 3.04 ± 0.04bc | 0.64 ± 0.06c | ND | 0.08 ± 0.00a | |
| 0.2% | 4.43 ± 0.14c | 5.16 ± 0.38ab | 2.94 ± 0.20bcd | 0.55 ± 0.02c | ND | 0.05 ± 0.00a | |
| 0.3% | 4.00 ± 0.03g | 5.85 ± 016a | 2.84 ± 0.06cd | 0.83 ± 0.06b | ND | 0.07 ± 0.01a | |
| M1 | 0% | 4.13 ± 0.03e | 5.14 ± 0.05ab | 3.33 ± 0.06a | 0.91 ± 0.12b | ND | 0.03 ± 0.00b |
| 0.1% | 4.06 ± 0.01efg | 4.95 ± 0.63bc | 2.98 ± 0.21bcd | 0.89 ± 0.07b | ND | 0.04 ± 0.01a | |
| 0.2% | 4.03 ± 0.02fg | 4.96 ± 0.18bc | 2.77 ± 0.08d | 0.93 ± 0.01b | ND | 0.05 ± 0.00a | |
| 0.3% | 3.99 ± 0.02g | 5.86 ± 0.05a | 3.17 ± 0.15ab | 1.12 ± 0.06a | ND | 0.05 ± 0.00a | |
| M3 | 0% | 4.29 ± 0.01d | 4.14 ± 0.17d | 3.16 ± 0.09ab | 1.08 ± 0.10a | ND | 0.03 ± 0.01b |
| 0.1% | 4.10 ± 0.02ef | 5.88 ± 0.06a | 3.06 ± 0.03bc | 1.05 ± 0.00a | ND | 0.02 ± 0.00b | |
| 0.2% | 3.99 ± 0.04g | 5.68 ± 0.15ab | 2.85 ± 0.03 | 1.10 ± 0.02a | ND | 0.03 ± 0.01b | |
| 0.3% | 4.06 ± 0.02efg | 5.71 ± 0.10ab | 3.12 ± 0.10ab | 1.10 ± 0.02a | ND | 0.04 ± 0.00b | |
| Significant | Strains | * | ** | ** | ** | ** | |
| CPLA 4 | ** | ** | ** | ** | ** | ||
| I | ** | ** | ** | * | ** |
| Strains 1 | Initial Content of PLA | Items 2 | |||||
|---|---|---|---|---|---|---|---|
| DM (% FM 3) | CP (%DM) | WSC (%DM) | NDF (%DM) | ADF (%DM) | Ammonia Nitrogen (% TN 4) | ||
| CK | 0‰ | 25.64 ± 0.94b | 20.04 ± 0.27d | 1.95 ± 0.41b | 44.65 ± 0.34a | 34.41 ± 0.29ab | 11.28 ± 0.75a |
| 1.0‰ | 24.26 ± 0.17c | 20.37 ± 0.18bcd | 2.19 ± 0.44ab | 44.62 ± 0.48a | 34.64 ± 0.37ab | 11.01 ± 0.39a | |
| 2.0‰ | 23.76 ± 0.50c | 20.58 ± 0.38bcd | 2.06 ± 0.13ab | 44.20 ± 0.51a | 33.32 ± 0.81cd | 10.39 ± 0.96ab | |
| 3.0‰ | 26.24 ± 0.12b | 20.49 ± 0.14bcd | 2.11 ± 0.09b | 43.11 ± 0.12b | 33.92 ± 0.45bc | 7.58 ± 0.10e | |
| M1 | 0‰ | 24.16 ± 0.29c | 20.38 ± 0.17bcd | 2.37 ± 0.27ab | 42.57 ± 0.19b | 32.52 ± 0.54ef | 8.94 ± 0.67cd |
| 1.0‰ | 26.46 ± 0.44b | 20.77 ± 0.41bc | 2.28 ± 0.16ab | 42.36 ± 0.48b | 32.11 ± 0.11de | 7.75 ± 0.64de | |
| 2.0‰ | 26.53 ± 0.16b | 20.88 ± 0.26b | 2.72 ± 0.51a | 42.53 ± 0.13b | 31.96 ± 0.24fg | 7.16 ± 1.29e | |
| 3.0‰ | 26.13 ± 0.64b | 21.62 ± 0.32a | 2.15 ± 0.14ab | 41.05 ± 0.31c | 31.08 ± 0.25h | 6.82 ± 0.64e | |
| M3 | 0‰ | 28.38 ± 0.36a | 20.19 ± 0.14cd | 1.88 ± 0.28b | 42.61 ± 0.49b | 31.69 ± 0.38gh | 9.33 ± 0.37ab |
| 1.0‰ | 27.81 ± 0.20a | 20.43 ± 0.57bcd | 2.21 ± 0.22ab | 40.09 ± 0.79c | 31.00 ± 0.28h | 8.05 ± 0.20de | |
| 2.0‰ | 28.01 ± 0.63a | 20.53 ± 0.26bcd | 1.95 ± 0.42b | 41.51 ± 0.37c | 31.54 ± 0.35gh | 7.47 ± 0.47e | |
| 3.0‰ | 27.51 ± 0.72a | 20.59 ± 0.37bcd | 2.16 ± 0.24ab | 40.97 ± 007c | 32.10 ± 0.25fg | 7.11 ± 0.15e | |
| Significant | Strains | NS | ** | * | ** | ** | ** |
| CPLA 5 | NS | ** | NS | ** | ** | ** | |
| I 6 | NS | NS | NS | NS | ** | * | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Xu, S.; Yun, Y.; Jia, T.; Yu, Z. Effect of 3-Phenyllactic Acid and 3-Phenyllactic Acid-Producing Lactic Acid Bacteria on the Characteristics of Alfalfa Silage. Agriculture 2020, 10, 10. https://doi.org/10.3390/agriculture10010010
Wu Z, Xu S, Yun Y, Jia T, Yu Z. Effect of 3-Phenyllactic Acid and 3-Phenyllactic Acid-Producing Lactic Acid Bacteria on the Characteristics of Alfalfa Silage. Agriculture. 2020; 10(1):10. https://doi.org/10.3390/agriculture10010010
Chicago/Turabian StyleWu, Zhe, Shengyang Xu, Ying Yun, Tingting Jia, and Zhu Yu. 2020. "Effect of 3-Phenyllactic Acid and 3-Phenyllactic Acid-Producing Lactic Acid Bacteria on the Characteristics of Alfalfa Silage" Agriculture 10, no. 1: 10. https://doi.org/10.3390/agriculture10010010
APA StyleWu, Z., Xu, S., Yun, Y., Jia, T., & Yu, Z. (2020). Effect of 3-Phenyllactic Acid and 3-Phenyllactic Acid-Producing Lactic Acid Bacteria on the Characteristics of Alfalfa Silage. Agriculture, 10(1), 10. https://doi.org/10.3390/agriculture10010010





