Effects of Tillage and Crop Residue Application on Soybean Nitrogen Fixation in a Tropical Ferralsol
Abstract
: This study was aimed at quantifying soybean (Glycine max) nitrogen fixation under reduced tillage (RT) and conventional tillage (CT) in a tropical Ferralsol of the sub-humid zone of western Kenya, using the isotope 15N dilution method. Crop residue (CR) management was a superimposed treatment in soybean-maize rotation and intercropping systems. This study quantified N in abscised soybean leaves. Soybean-N derived from the atmosphere (%NDfA) ranged between 41–65%; it was higher (P < 0.05) in RT (55.6%) than in CT (46.6%). Total fixed-N under ‘RT + CR’ was more than in the other treatments by at least 55% in intercropping and 34% in rotation system. Nitrogen fixed in soybean aboveground parts was 26–48 kg N ha−1 with intercropping and 53–82 kg N ha−1 with rotation. Seasonal litter fall contained about 15 kg N ha−1, with 54% NDfA. Annual nitrogen balances with soybean and maize grain removed were better in RT (−9 to −32 kg N ha−1) than in CT (−40 to −60 kg N ha−1). Application of P increased nodule weight (P < 0.05) by 3 to 16 times over the control. Soybean residues should be returned to the field after harvest to reduce soil N mining. We conclude that ‘RT + CR’ increases biological nitrogen fixation in soybean, over CT, and that phosphorus application is needed for better soybean nodulation in western Kenya.1. Introduction
Relying on the biological nitrogen fixation (BNF) of grain legumes is a strategy to ease the burden that commercial fertilizers exert on poor farmers. In sub-Saharan Africa (SSA), fertilizer use is estimated at 8 kg ha−1, which is only 10% of the worlds' average [1]. Stoorvogel et al. (1993) pointed out gross annual nitrogen mining in SSA averaging 22 kg N ha−1, but up to 100 kg N ha−1 in some cases [2]. The unique socio-economic conditions and wide-spread rural poverty in sub-Saharan Africa limit access to mineral fertilizers, inducing the search for cheap sources of fertilization, such as nitrogen fixation by grain legumes. Soybean has been produced in Kenya for several years on a small scale, but demand for proteins and biodiesel has renewed interest in its production. Promiscuous soybean varieties (varieties that do not require inoculation with specific Rhizobium) have recently been introduced to western Kenya for soil fertility improvement, through the Tropical Soil Biology and Fertility institute of CIAT (TSBF-CIAT), and their performance is being tested in various agro-ecological zones.
The amount of nitrogen fixed by grain legumes such as soybean is affected by the degree of colonization by soil rhizobia [3], by their interaction with other biological, physical and chemical properties of the soil [4-6] and by weather [7]. These factors, except weather, are themselves influenced by management practices such as tillage and crop residue (CR) application as well as by the spatial-temporal arrangement of crop components. Reduced soil disturbance, for example, improves soil physical parameters such as structure, but also the chemical and biological parameters that affect nitrogen fixation. Soil moisture and temperature, for example, which are influenced by tillage and CR, affect biological nitrogen fixation [8]. Legumes such as soybean that transport N fixed from roots to shoots in the form of ureides (allantoin and allantoic acid) are particularly susceptible to water stress [9], and conservation practices involving reduced tillage and surface CR application are expected to positively influence nitrogen fixation. Also, CR management affects decomposition of the residue and rates of N-release to the soil pool, thus affecting nitrogen fixation [5,10]. Small-scale crop production in eastern Africa takes place mostly without mulching, but about 2 t ha−1 of low-quality CR can be applied or just remain from the previous cereal crop. It is not known to which extent such low-quality CR mulch, in quantities available and affordable by small-scale farmers, affects proportion and total amount of nitrogen fixed by soybean in eastern Africa. The effect of CR on nitrogen fixation in grain legumes has been investigated on sandy soils [6], but no work on tropical African Ferralsols could be found.
Within smallholder production systems, soybean is produced in rotation or intercropping with cereals. Quantifying the contribution of soybean to the nitrogen balance in these systems under different tillage and residue management scenarios is important for optimization of BNF. Appreciable amounts of soybean leaves can be shed during the soybean crop cycle thus contributing to the N-balance in these systems. Peoples and Craswell (1992) noted that frequently, only standing shoot data and no leaf-fall data are available, and therefore net N-balances are underestimated in many studies [10].
This study aimed to quantify nitrogen fixation of soybean under reduced and conventional tillage with different CR management in a sub-humid zone of western Kenya. We hypothesized that practicing reduced tillage combined with CR application leads to a greater proportion of nitrogen derived from the atmosphere (%NDfA) and to greater total nitrogen fixed, compared to reduced tillage without CR application, and also compared to conventional tillage systems.
2. Materials and Methods
2.1. Site and Soil Type
The study was conducted in a soybean-maize rotation and intercropping system on a tropical Ferralsol during the long rains in 2007 in Nyabeda, western Kenya. The soils are characterized by 64% clay, 15% sand, pH water 5.1, 1.35% total C, 0.15% total N and 2.99 mg available P kg−1 soil. The site, at 0°07′N 34°24′E and 1420 m.a.s.l receives an annual rainfall of 1200–1600 mm which is distributed in two distinct seasons: long rains season from March to August and short rains season from September to February. The experiment was established as a long-term conservation tillage trial in March 2003 and was set up with a factorial design involving tillage (reduced tillage [RT] and conventional tillage [CT]), CR application (maize stover applied at 0 and 2 t ha−1) and cropping systems (legume-cereal intercropping and rotation). Under RT, tillage was restricted to surface-scratching down to 3 cm depth to remove weeds, while CT involved tillage with hand hoes to about 10–15 cm depth as done by small-scale farmers. The legume crop was soybean (Glycine max (L.) Merr) (TGX 1448-2E, locally known as SB20), except in the first season when common bean (Phaseolus vulgaris) was used. Maize (Zea mays L.) was the cereal crop. Legume residues were left on the respective plots after harvest. Nitrogen in form of urea was applied at 0 and 60 kg N ha−1 (split applied with one third at planting and two thirds when crop was at knee height), and P was applied at 0 and 60 kg P ha−1 as triple super phosphate (TSP). All the treatments received a basal application of 60 kg K ha−1 as muriate of potash.
Maize was planted at 0.25 m (between plants) by 0.75 m (between rows) with two seeds per planting hole and thinned to one plant per hill (53,000 hills ha−1). Soybean was planted at 0.05 m (between plants) by 0.75 m (between rows; 266,000 seeds ha−1) in both rotation and intercropping systems, i.e., the legume in the intercrop was superimposed on maize.
2.2. Soybean Nodulation and Biomass Assessment
Soybean nodulation was investigated at peak biomass in four different cropping seasons between September 2003 and August 2005. One quadrant measuring 0.75 m by 0.75 m was placed on each diagonal of the plot, aboveground shoots cut and roots carefully excavated to 0.2 m depth, and nodules sorted out by hand. After washing to soil-free, nodules were counted, fresh weights determined, and dry weights determined after oven-drying at 60 °C for 48 h.
2.3. Soybean In-Season Leaf Fall
Six plastic nets (traps), each measuring 50 cm by 75 cm, were laid 5 cm above the soil surface at 6 weeks after planting to collect soybean litter-fall. The leaves and flowers falling in the litter traps were collected weekly starting 61 days after planting and twice weekly from 83 days after planting. Weight of the collected leaves and flowers was taken before and after drying at 60 °C for 48 hours. At the end of the season, soil-free leaves and flowers for each treatment were bulked by replicate and analyzed for N content. Soybean leaf and flower debris mixed with soil were also taken, and a subsample ashed at 550 °C for 5 hours to determine the mineral content (i.e., non-organic component) that was used to estimate leaf and flower biomass in the biomass-soil mixture.
2.4. Microplots and 15N Dilution
Microplots measuring 2 × 3 m and 3 × 3 m were established in April 2007 within soybean-maize rotation and intercropping systems, respectively. Urea (−CO(15NH2)2), with atom % 15N of 5.30 +/− 0.05, was applied at 6 g and 9 g in soybean-maize rotation and intercropping systems plots, respectively, split applied ½ at planting and ½ at 50 days after planting. The target application rate was 4.6 kg N ha−1. The fertilizer was applied in solution to ensure uniform distribution in the soil. Although there is a likelihood that 15N was not completely equilibrated in the soil in this study, for instance due to application after crop residue application (see [11,12] for best 15N application practices), it nevertheless provides useful insights into treatment effects. The microplots were planted with nitrogen fixing (F) soybean and non-nitrogen fixing reference (NF) maize plants (maize was used in the absence of a suitable non-fixing soybean cultivar growing in the study area). Limitations of using maize as a reference crop in N-fixation studies, such as seasonal changes in %NdFA and indication of lower %NdFA contribution relative to crops such as rice is discussed by [13]. No inoculation was done, and soybean was expected to self-inoculate with native soil bacteria. Harvesting of the microplots was done at 115 days after planting (at physiological maturity) when the soybean pods turned yellow-to-brown. For aboveground biomass, four maize plants were sampled from each reference plot and their stems, pods and root biomass taken. For soybean, sampling was done for all plants within a distance of 50 cm from the two ends of the microplot (area of 2 m2 for rotation and 4 m2 for intercropping). Fresh weights for all samples were taken in the field. All aboveground biomass was oven-dried at 60 °C for 48 hours before taking dry weights.
Root biomass in microplots was assessed in two quadrats of 0.50 m by 0.75 m instead of the whole microplot to avoid disturbance in the RT plots. The area was dug out to 15 cm depth using a hand-hoe and the roots sorted out by hand. Root fresh weights for all root samples were taken in the laboratory after washing to soil-free with de-ionized water, over a 0.5 mm sieve. The samples were put in the oven immediately after washing and dried at 60 °C for 48 hours.
Laboratory analysis for total N and atom % 15N was done for each of the plant parts (stems and leaves, pods, grains, and roots) at the Institute of Plant Nutrition Laboratory in Bonn, Germany, using an isotope ratio mass spectrometer ANCA-SL (PDZ Europa, Cheshire, UK, 1998). Soils sampled from soybean-maize rotation, soybean-maize intercropping and continuous maize at the beginning of season 9 (in March 2007) were also analyzed for δ15N concentration. Litter N was analyzed by wet oxidation based on Kjeldahl digestion with sulfuric acid [14] in the ICRAF laboratories in Nairobi, Kenya, and its 15N/14N ratio was assumed to be similar to that of stems plus leaves at physiological maturity.
Calculations to quantify N fixation followed standard methods [11,15]. Nitrogen balances were calculated by subtracting total N contained in soybean and maize grain from total N fixed. For rotation, the balances refer to two cropping cycles, while intercropping covers one season.
2.5. Data Analysis
Data were analyzed using SAS version 9.1 statistical software for Windows. Tillage, crop residue and cropping system were fixed effects, while replicates were treated as random effects in the procedure “Mixed”. The statistical analysis for this split-split plot study was done according to the model of [16] for such a design. Pair-wise comparison of treatment means was done using the Tukey-Kramer method. Before analysis, nodule counts were log transformed and then back-transformed after the analysis.
3. Results
3.1. Nodulation
There were no large differences in nodulation between intercrop and the rotation systems (Table 1). Nodulation was always better in CT than in RT. For instance, nodulation was always lower (P < 0.05) under ‘RT − CR’ than in CT treatments (e.g., nodule weight was 38% to 68% lower than any of the other treatments). Application of CR resulted in >33% increases in nodule mass in RT, but was variable in CT (i.e., a positive effect in intercrop but no change in rotation).
The effects of fertilizer N and P on nodulation in a rotation system were analyzed (Figure 1). Application of P alone (−N +P) resulted in higher nodule weight (P < 0.05); it was 3 to 16 times higher than the control (−N −P), and 1.5 to 4 times higher than when N and P were given together (+N +P). Lower nodulation with application of inorganic N fertilizer was expected due to lower plant demand for fixed-N. Since application of P without N was the most promising strategy, soybean nitrogen fixation was investigated further only under this system, using the 15N dilution method.
3.2. Soybean Below- and Above-Ground Biomass
Tillage and CR had no significant effect on products harvested at physiological maturity, except for litter-fall in the rotation system; here the litter-fall was greater (by 29 to 43%; P < 0.05) in ‘RT − CR’ than in the other treatments (Table 2). In the intercropping system, soybean grain yield in ‘RT − CR’ was about 50% of that with CR application (though not significant). The litter-fall at physiological maturity (Table 2) on average constituted about 64% of the total litter-fall observed up to harvest, both for intercropping and rotation (Figure 2). The first leaf abscission occurred about 60 days after planting, followed by a 3-week lag phase after which leaf-fall increased rapidly; this can be related to the physiological stage of the soybean crop.
3.3. Nitrogen Derived from the Atmosphere (NDfA)
Distribution of atom% 15N excess varied significantly between plant parts, with the highest concentration being found in roots, followed by stems and leaves, and similar values in husks and grain (Table 3). Such distribution was expected due to fractionation of 15N and 14N isotopes during transfer and reallocation of N-containing compounds to different plant parts in response to demand (see [17,18]). The relative difference in atom % 15N excess, between the fixing and non-fixing (reference) plants, is used for determining the amount of fixed N. The atom % 15N excess values in the reference plant were only 2% higher than in the fixing plant in the roots, but more than double for grain, husks, and stems and leaves. Thus, differences in root atom % 15N excess levels between fixing and non-fixing crops were small, suggesting that little of the fixed N was held in the roots. The roots were thus excluded from further consideration in the assessment of BNF. Total nitrogen concentration was highest in grain and lowest in roots for both fixing and non-fixing plants. Soybean grain %N was greatly affected by cropping system (5.9% in intercrop and 6.1% in rotation; P < 0.01).
In general, the proportion of soybean N derived from biological fixation ranged from 42 to 57% in the intercropping and 47 to 65% in the rotation system (Table 4). Regardless of the cropping system, practicing RT increased (P < 0.05) total (and also grain) %NDfA (a difference of 9% NDfA) relative to that in CT, and in contrast to nodulation observed at least 2 years earlier. Increased %NDfA in RT resulted in decreased nitrogen derived from the soil (NDfS) of 42% in contrast to 51% in CT (P < 0.05; data not shown).
There was no significant effect of CR on %NDfA. However, in the rotation system, CR application resulted in a large increase (P < 0.05), from 47 to 65% total NDfA in RT, and a slight (insignificant) decrease in CT. The slightly decreased %NDfA in both cropping systems (a difference of 4% and 10% NDfA in intercropping and rotation systems, respectively) following application of CR in CT is attributable to possibly increased available N following mineralization of the incorporated residue.
3.4. Nitrogen Fixed
The amount of fixed N in soybean aboveground plant parts was 26–48 kg N ha−1 in the intercropping and 53–82 kg N ha−1 in the rotation system (Table 5). The lower N fixation in intercropping is attributed to the observed lower plant harvest biomass due to competition effects of the superimposed maize crop. Under both rotation and intercropping, practicing ‘RT + CR’ led to the highest grain and biomass N as well as total fixed N when compared to CT and ‘RT − CR’ treatments. Total fixed N in ‘RT + CR’ was, for instance, higher than in the other treatments by at least 55% in the soybean-maize intercropping and 34% in the rotation system. The lowest total amount of fixed N was observed in the CT system, for both intercropping and rotation systems.
Unlike with %NDfA, application of CR did not significantly increase the total amount of fixed N under RT although this was appreciably raised by 34% and 64% in rotation and intercropping systems, respectively, and slightly suppressed under CT (by 9% and 14% in rotation and intercropping, respectively).
By the time of physiological maturity, the amount of nitrogen biologically fixed in shed leaves ranged from 3.7 to 5.8 kg N ha−1, representing about 54% of the total N contained in leaf-fall by the time of physiological maturity.
3.5. Nitrogen Balance
Overall, the net nitrogen balances varied from −19 to +8 kg N ha−1 when only harvest soybean grain was removed, and from −51 to −9 kg N ha−1 when also maize grain was removed (Table 6). With only soybean grain removed, positive N balances were observed in the two RT treatments (‘RT + CR’ and ‘RT − CR’) in intercropping and in ‘RT + CR’ treatment in the rotation system. Although the balances were all in the negative range when both soybean and maize grain were removed, the balances in the rotation system, for example, were better in ‘RT + CR’ than in the other treatments (P < 0.05). Also, balances with soybean and maize grain removed were better in RT (−10 to −33 kg N ha−1) than in CT (−42 to −66 kg N ha−1), assuming two seasons for the intercrop. The differences in the balances are a result of both the %NDfA and the amount of harvested products.
4. Discussion
4.1. Factors Affecting Nitrogen Fixation
The %NDfA values observed in this study are in agreement with previous results of 54 % NDfA for P fertilized soybean in the high rainfall zone of western Kenya [19]. The greater %NDfA observed in RT when CR was also applied, and the resultant higher amount of fixed N compared to disturbed soils (such as in CT) has been attributed to the greater colonization by arbuscular mycorrhizae that increases P supply to the nodules [4]. We also observed higher fungal presence under RT compared to conventionally tilled plots (data not shown), similar to other studies [20,21]. Legumes are generally dependent on mycorrhizae for efficient uptake of P, and diversity of mycorrhizae is related positively to efficient use of soil P [22]. In the same experimental field used for this study, [23] observed low available P values of 10 mg P kg−1 soil in ‘RT − CR’, compared to over 22 mg P kg−1 soil in the ‘RT + CR’ and the two CT treatments and attributed to increased P losses in surface runoff due to crusting in absence of tillage and crop residue application (the critical P value for soybean growth and nodulation has not been determined but is shown to be 13 ppm elsewhere [24]). Besides mycorrhizae, it was previously shown that also Bradyrhizobia diversity is higher under RT, and N fixation rates of the isolates obtained from RT were observed to be higher than those from CT [25]. This could also apply to our RT treatments, and both diversity of rhizobia and fixation efficiency were likely greater with than without surface CR, leading to the greater nitrogen fixation in ‘RT + CR’ relative to other treatments as observed. However, during the first seasons, microbial communities were probably not yet well established, and available soil P differences between the tillage systems were likely more pronounced (crusting was a greater problem) than during season 9 when the 15N dilution method was employed. This explains the inconsistency between nodulation (higher in CT) and %NDfA (higher in RT) during the two periods.
In addition to microbial contributions and soil P, reduced nodulation (and darker-green soybean leaves and visually observed tendency to delay maturation) following chemical fertilizer N application is due to the suppressive effect of the increased available N. Similar findings have also been reported by other researchers [26,27]. The reduced %NDfA and fixed N in CT as opposed to RT (e.g., in the rotation system) can also be attributed to the suppressive effect of a possibly larger amount of available soil N than in RT systems [10,27,28]. Such greater N in CT than in RT can be the result of the usually higher mineralization of nutrients in organic matter following perturbations by tillage [27]. With CT, the stronger depressive effect of incorporated CR on %NDfA in rotation as opposed to intercropping may be due to the fact that the mineralized N was fully at the disposal of the soybean alone, whereas this was shared among the jointly planted crops (soybean and maize) in the intercropping system. A similar effect was not observed with nodulation (nodulation was higher in CT than in RT), which was perhaps mainly influenced by differences in soil P as already discussed. Unlike with incorporated residue, the positive effect with surface residue (RT) on N fixation might be attributed to the modified micro-climate under residues, reduced plant water stress during the dry periods due to soil moisture conservation (leading to increased plant demand for fixed N [7]), and reduced soil temperature [29]; these factors were likely minimized or absent when surface CR was not applied or when much of it was incorporated into the soil (e.g., in CT).
Lower soybean %NDfA in the intercropping system is in agreement with previous reports such as the 42 and 23% NDfA found under monocrop and intercrop, respectively, as reported by [27]. Fujita et al. (1992) identified shading and light availability as important factors reducing BNF in mixed legume-cereal systems [30]. Perhaps the lack of CR effect on %NDfA observed under the intercropping system was due to soybean-maize interaction effects such as shading and nutrient and water competition. Data for multiple seasons provide further insights. For instance, rainfall would be a plausible explanation for the seasonal differences in nodulation; the amount of seasonal rainfall was low in the short rains (SR) 2003 season, and in long rains (LR) 2004 and SR 2004 seasons, rainfall amount was poorly distributed compared to LR 2005 season.
4.2. Nitrogen Fixed and N Balances
In general, fixed N in the intercropping system was just about 50% of the fixed N in the rotation system. However, assuming two cropping seasons in a year, and assuming that a similar amount of N is fixed during the second intercrop season during the cereal phase of the rotation system, similar quantities of N could be fixed annually in both intercropping and rotation. The amount of fixed N in the rotation system was within the range of 51 to 78 kg N ha−1 observed by [31] in various farms in Nigeria. Other researchers have reported different amounts of fixed N. For instance, Peoples et al. (1995) reported N fixed by soybean of between 44 to 250 kg N ha−1 per season [32]. Our results imply that in both cropping systems, practicing RT while adding CR in quantities accessible to farmers results in elevated (not significant) amounts of biologically fixed N, which is in line with our hypothesis. The reported quantity of fixed N in the current study could be an underestimation, since an appreciable amount of fixed N in soybean, reaching up to 30% of the N being fixed at pod-filling [33], is said to be released by the root system into the soil nutrient medium [30].
Nitrogen balances of crop fields that include grain legumes vary widely and are affected by site conditions, grain harvest and N input [27]. Although in the present study 42% to 65% of soybean N was obtained from BNF, this could not turn then balance into a positive one. Similar soybean N balances have been reported for Argentina [34] and Switzerland [35]. In fact, in their review of BNF studies that had been conducted between 1966 and 2006, [8] observed that the amount of fixed N in soybean (a grain legume as opposed to non-grain legumes or legumes where the grain is usually not harvested) was, in most cases, insufficient to replace all the N removed in harvested seed. Some positive N balances after soybean seed harvest were found in our study mainly in the ‘RT + CR’ treatments, suggesting that adoption of ‘RT + CR’ could save some cash the farmers would otherwise spend on fertilizers. For instance, for two cropping seasons (annually), an additional 10 to 30 kg N ha−1 is required in RT, which is about half or less of the 40 to 60 kg N ha−1 required in CT to compensate for harvested N. The farmers would also achieve a higher soybean grain yield over other treatments of 50 to 160 kg ha−1 in rotation and 70 to 360 kg ha−1 in intercropping by using ‘RT + CR’. Although soybean can have a positive N balance (after soybean grain removal), the fixed N cannot also compensate for the entire N harvested in the maize grain. Chikowo et al. (2004) studied a similar case in a sandy soil in southern Africa and concluded that a combination of legume rotations and mineral fertilizer application would be the best option [36].
In terms of residue management, small-scale farmers usually transfer above-ground biomass to the homestead, where threshing is done with ease, and the residues are heaped in a corner where they rot. Since, residues contain total N of up to 30 kg N ha−1 as for the rotation system, we recommend that soybean residues after removal of grains be returned to the field plots after harvest, especially in the soybean-maize rotation system, to reduce soil N mining. Overall, gross margin analysis showed legume-based systems to dominate all maize-maize rotation system in the same trial [23].
4.3. Litter-Fall- and Root-N Contribution
Nitrogen contained in litter-fall occurring from planting to physiological maturity in this study was 8.2 to 11.8 kg N ha−1, but as much as 40 kg N ha−1 can be observed in legume systems [10]. Our accounting took into consideration the 65% of litter-fall that had occurred before physiological maturity, constituting a fixed N of 4 to 6 kg N ha−1, representing 54% NDfA. Nitrogen contained in such litter-fall is not usually taken into consideration in estimation of N-fixation nutrient balances, perhaps because of the small amount of the fixed N, and some of it can be lost within the season. Combination of nutrient and water stress likely induced early leaf abscission resulting to the greater litter-fall observed in ‘RT − CR’ relative to other rotation treatments at physiological maturity. However, the greater litter-fall did not yet translate to greater soybean grain yield suggesting the need to investigate effect of abscissed litter on soil moisture in future studies.
In both cropping systems, the recovered roots accounted for about 8% of the total plant biomass, consistent with the observation by [37] that underground grain legume plant parts constitute <10% of total standing biomass. The roots may, however, not contribute substantially to fixed N, as they seem to be enriched in 15N due to isotope 15N/14N fractionation. The fractionation is related to form and concentration of N in the soil, nitrate reduction in roots, ammonia assimilation, and plant-fungal associations ([17,38]. Wanek and Arndt (2002) [39] reported δ15N enrichment of soybean roots at high %NDfA, but such enrichment was reversed to the shoots when soil nitrate was high, induced by the switch from nodule activity to mineral N acquisition. This may explain why roots, although not usually reported in many N fixation studies [27], are generally found to have more 15N or δ15N than shoots [18,40].
In summary, ‘RT + CR’ resulted in the highest %NDfA and total N fixed and better N-balances than ‘RT − CR’ and CT treatments. Thus, in agreement to our hypothesis, practicing RT combined with CR application leads to greater utilization of BNF compared to the other treatments.
5. Conclusions
Small-scale farmers in western Kenya can take greater advantage of biological N fixation by: (i) practicing reduced tillage combined with surface application of crop residue, as this increases biologically fixed N by at least 34% over other treatments tested; (ii) applying chemical fertilizer phosphorus; and (iii) returning soybean residues to the farm after grain removal, as such residues were found to contain total N up to as much as 30 kg N ha−1. By practicing ‘reduced tillage plus crop residue’, farmers can achieve extra soybean grain yields of 50 to 160 kg ha−1 in rotation and 70 to 360 kg ha−1 in intercropping, and better N balances can be realized than when practicing CT. Soybean roots become enriched with 15N due to isotope 15N/14N fractionation and do not contribute substantially to total plant fixed N.
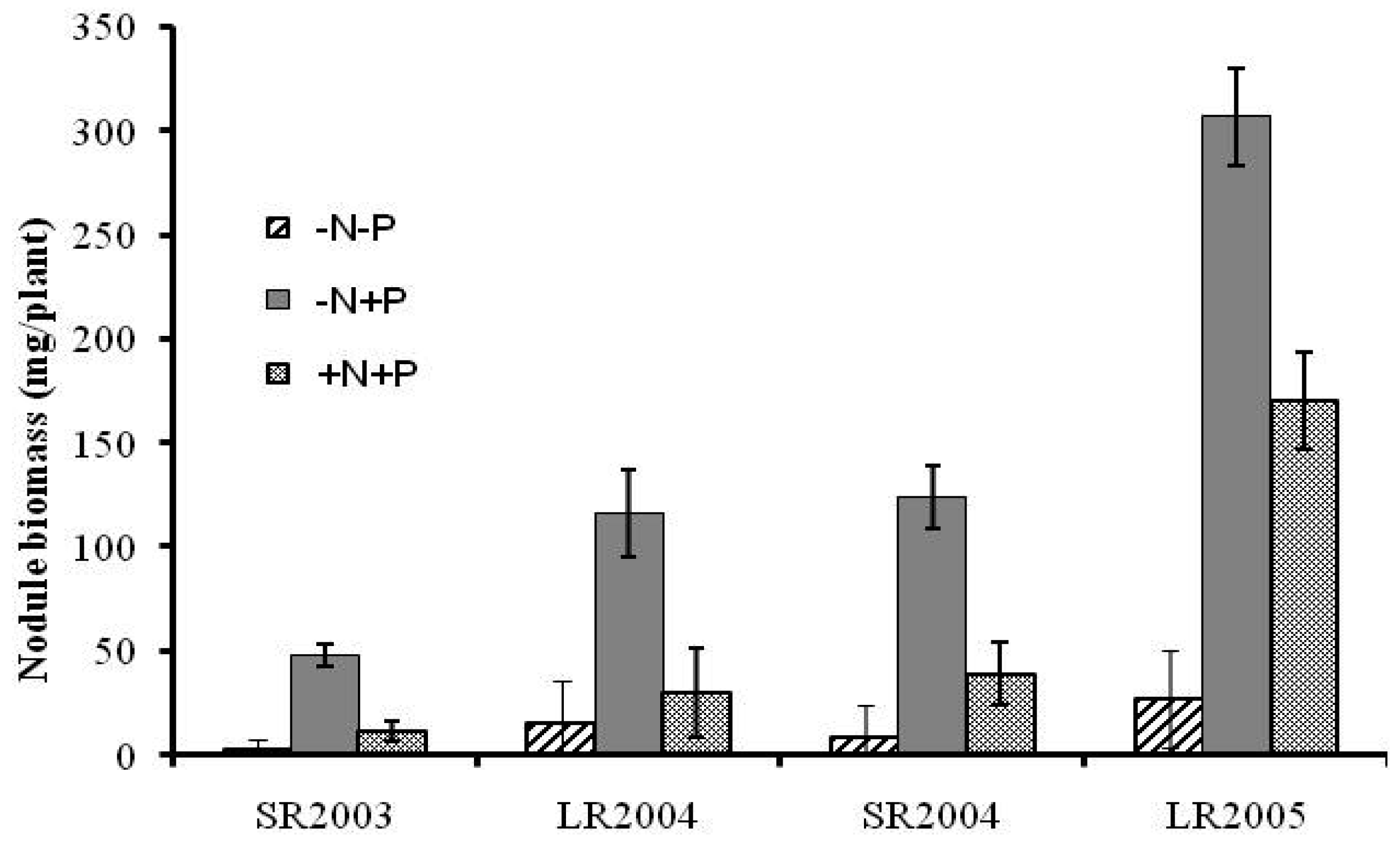
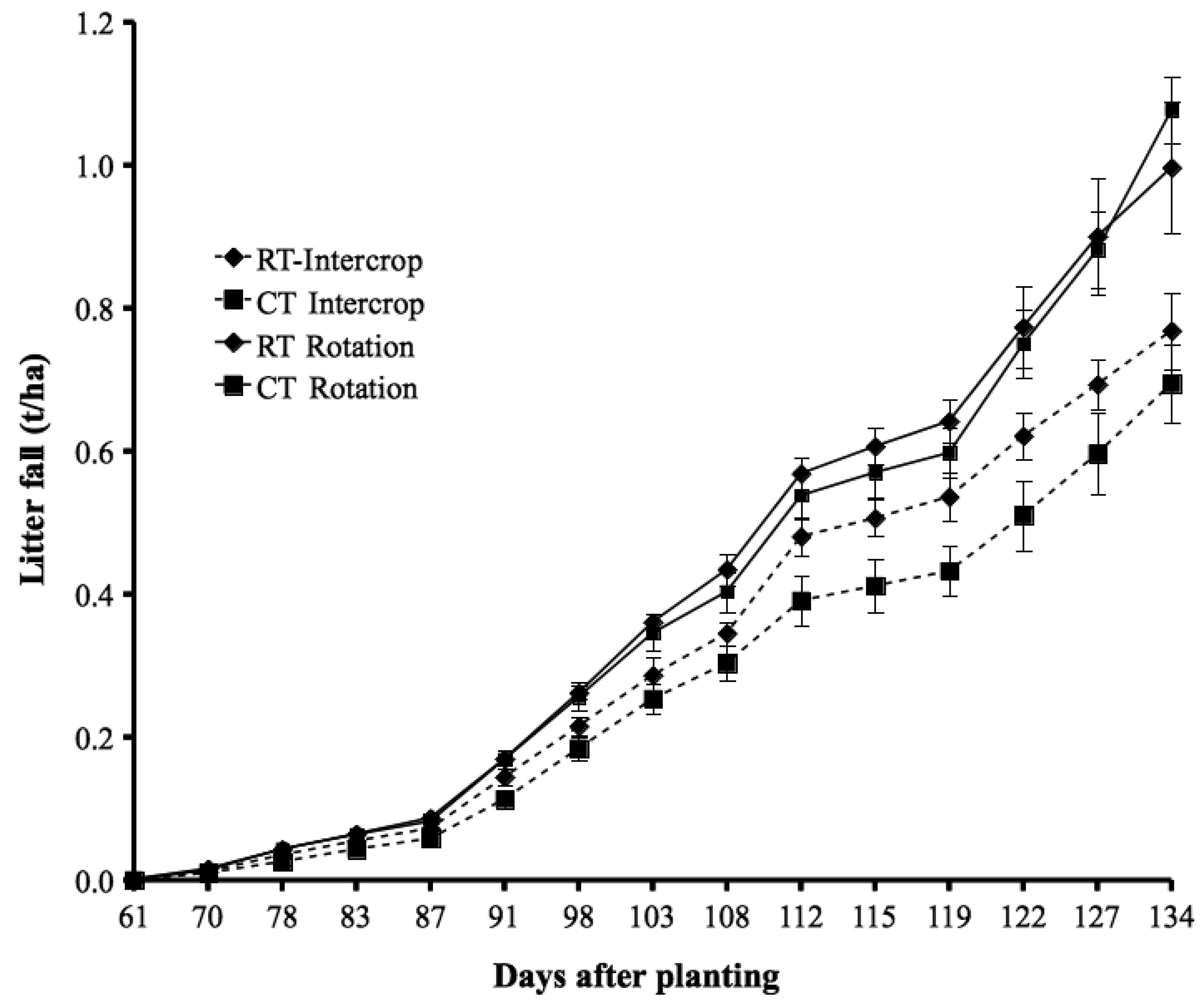
| Treatment | Nodule weight (mg plant−1) | Number of nodules m−2 |
|---|---|---|
| Intercrop | ||
| Conventional tillage −CR | 187 b | 142 a |
| Conventional tillage + CR | 257 a | 198 a |
| Reduced tillage − CR | 93 c | 61 b |
| Reduced tillage + CR | 150 cb | 193 a |
| SED | 33.8 | 27 |
| Rotation | ||
| Conventional tillage − CR | 177 a | 96 a |
| Conventional tillage + CR | 189 a | 122 a |
| Reduced tillage − CR | 92 b | 45 b |
| Reduced tillage + CR | 142 ab | 86 a |
| SED | 26.8 | 19.7 |
CR = crop residue; SED = standard error of differences of means, values in the same column and cropping system followed by the same letter are not significantly different at P = 0.05. All treatments were applied with 60 kg P ha−1 but no chemical N fertilizer.
| Treatment | Grain | Stems + leaves | Husks | Litter-fall | Root |
|---|---|---|---|---|---|
| Intercrop (t ha−1) | |||||
| Conventional tillage − CR | 0.64 a | 1.19 a | 0.52 a | 0.52 a | 0.24 a |
| Conventional tillage + CR | 0.50 a | 1.19 a | 0.51 a | 0.59 a | 0.24 a |
| Reduced tillage − CR | 0.35 a | 0.96 a | 0.44 a | 0.43 a | 0.18 a |
| Reduced tillage + CR | 0.71 a | 1.18 a | 0.49 a | 0.45 a | 0.22 a |
| Average | 0.55 | 1.13 | 0.49 | 0.50 | 0.22 |
| SE | 0.171 | 0.270 | 0.065 | 0.053 | 0.035 |
| Rotation (t ha−1) | |||||
| Conventional tillage −CR | 1.09 a | 1.91 a | 0.53 a | 0.53 b | 0.36 a |
| Conventional tillage +CR | 1.20 a | 2.15 a | 0.48 a | 0.59 b | 0.43 a |
| Reduced tillage −CR | 1.12 a | 2.18 a | 0.50 a | 0.76 a | 0.32 a |
| Reduced tillage +CR | 1.25 a | 2.03 a | 0.52 a | 0.57 b | 0.33 a |
| Average | 1.16 | 2.07 | 0.51 | 0.61 | 0.36 |
| SE | 0.171 | 0.213 | 0.049 | 0.080 | 0.039 |
SE = standard error; CR = crop residue.
| Crop part | Fixing plant | Reference plant | ||
|---|---|---|---|---|
| atom % 15N excess | % N | atom % 15N excess | % N | |
| Stems + leaves | 0.0670 b | 1.33 b (1.19–1.48) | 0.1463 b | 0.51 b (0.45–0.57) |
| Husks | 0.0589 cb | 1.42 b (1.28–1.57) | 0.1237 c | 0.51 b (0.45–0.57) |
| Grain | 0.0547 c | 6.01 a (5.87–6.15) | 0.1156 c | 1.26 a (1.20–1.32) |
| Roots | 0.1700 a | 1.04 c (0.89–1.18) | 0.1738 a | 0.40 c (0.34–0.46) |
| Abscised leaves | - | 1.60 (1.39–1.94) | - | - |
| SE | 0.00612 | 0.072 | 0.00536 | 0.031 |
Numbers in bracket are lower and upper confidence limits, respectively; abscised leaves were not included in analysis of variance for total N, as only few bulked representative samples had been determined; SE = standard error; values in the same column followed by the same letter are not significantly different at P < 0.05.
| Treatment | Grain | Stems + leaves | Husks | Total # |
|---|---|---|---|---|
| Intercrop (%NDfA) | ||||
| Conventional tillage − CR | 44.6 a | 54.6 a | 36.3 a | 46.3 a |
| Conventional tillage + CR | 42.6 a | 43.1 a | 40.9 a | 42.0 a |
| Reduced tillage − CR | 60.4 a | 58.0 a | 53.8 a | 56.5 a |
| Reduced tillage + CR | 57.1 a | 57.2 a | 47.9 a | 54.4 a |
| Average | 51.2 | 53.2 | 44.7 | 49.8 |
| SE | 6.5 | 8.28 | 5.32 | 5.72 |
| Rotation (%NDfA) | ||||
| Conventional tillage −CR | 55.6ab | 59.7 a | 58.8 ab | 57.6 ab |
| Conventional tillage +CR | 41.4 b | 52.3 a | 43.6 c | 47.0 b |
| Reduced tillage −CR | 48.8 b | 45.6 a | 54.3 bc | 46.7 b |
| Reduced tillage +CR | 67.2 a | 65.0 a | 67.6 a | 64.9 a |
| average | 53.2 | 55.6 | 56.1 | 54.1 |
| SE | 4.9 | 6.56 | 7.25 | 4.61 |
| SE § | 5.77 | 7.47 | 6.36 | 3.96 |
#calculated by weighting with respective plant part mass recorded in Table 2; CR = crop residue; SE = standard error; values in the same column and cropping system followed by the same letter are not significantly different at P<0.05;§shows standard errors for comparing across the two cropping systems.
| Treatment | Grain | Stems + leaves | Husks | Litter-fall | Total |
|---|---|---|---|---|---|
| Intercrop (kg N ha−1) | |||||
| Conventional tillage − CR | 14.9 a | 7.4 a | 2.8 a | 5.6 a | 30.7 a |
| Conventional tillage + CR | 12.3 a | 6.8 a | 3.5 a | 3.8 a | 26.3 a |
| Reduced tillage − CR | 12.8 a | 7.9 a | 3.4 a | 4.7 a | 28.9 a |
| Reduced tillage + CR | 28.2 a | 12.2 a | 3.5 a | 3.7 a | 47.5 a |
| Average | 17.0 | 8.6 | 3.3 | 4.5 | 33.4 |
| SE | 7.97 | 3.86 | 0.80 | 1.00 | 11.80 |
| Rotation (kg N ha−1) | |||||
| Conventional tillage − CR | 35.9 a | 14.3 a | 3.7 ab | 4.9 a | 58.7 a |
| Conventional tillage + CR | 30.3 a | 15.7 a | 2.5 b | 4.6 a | 53.2 a |
| Reduced tillage − CR | 37.7 a | 14.8 a | 4.1 ab | 5.1 a | 61.7 a |
| Reduced tillage + CR | 52.0 a | 19.5 a | 5.1 a | 5.8 a | 82.4 a |
| Average | 39.0 | 16.1 | 3.9 | 5.1 | 64.0 |
| SE | 9.18 | 2.62 | 0.72 | 0.63 | 10.51 |
CR = crop residue; SE = standard error; values in the same column and cropping system followed by the same letter are not significantly different at P < 0.05.
| Treatment | Total N fixed | Total N harvested in grain | Net balances | ||
|---|---|---|---|---|---|
| Soybean | Maize | Soybean grain removed | Soybean + maize grain removed | ||
| Intercrop (kg N ha−1) | |||||
| Conventional tillage − CR | 30.7 a | 38.3 a | 23.1 a | −10.0 b | −33.1 b |
| Conventional tillage + CR | 26.3 a | 28.3 a | 18.0 a | −3.3 ab | −21.3 ab |
| Reduced tillage − CR | 28.9 a | 21.2 a | 17.4 a | 7.9 a | −9.4 a |
| Reduced tillage + CR | 47.5 a | 43.5 a | 18.2 a | 3.4 ab | −14.9 ab |
| Average | 33.4 | 32.8 | 19.2 | −0.5 | −19.7 |
| SE | 11.80 | 10.87 | 4.02 | 4.3 | 6.1 |
| Rotation (kg N ha−1) | |||||
| Conventional tillage −CR | 58.7 a | 63.8 a | 47.1 a | −4.3 ab | −51.4 b |
| Conventional tillage +CR | 53.2 a | 72.7 a | 25.3 b | −18.6 c | −43.9 b |
| Reduced tillage −CR | 61.7 a | 72.5 a | 21.4 b | −11.3 bc | −32.7 b |
| Reduced tillage +CR | 82.4 a | 76.3 a | 15.1 b | 4.7 a | −10.4 a |
| Average | 64.0 | 71.3 | 27.2 | −7.4 | −34.6 |
| SE | 10.51 | 10.75 | 4.92 | 3.3 | 6.6 |
CR = crop residue; SE = standard error; values in the same column and cropping system followed by the same letter are not significantly different at P < 0.05.
Acknowledgments
This study was financially supported by both the Germany Federal Ministry for Economic Cooperation and Development (BMZ) through the Deutscher Akademischer Austauschdienst (DAAD) and the African Network for Soil Biology and Fertility (AfNet) of the Tropical Soil Biology and Fertility (TSBF) research area of CIAT. The authors acknowledge the assistance of Livingstone Chibore in sample preparation and of Deborah Rupprecht in laboratory analysis.
References
- Maatman, A.; Wopereis, M.C.S.; Debrah, K.S.; Groot, J.J.R. From thousands to millions: Accelerating agricultural intensification and economic growth in Sub-Saharan Africa. In Advances in Integrated Soil Fertility Management in Sub-Saharan Africa: Challenges and Opportunities; Bationo, A., Waswa, B., Kihara, J., Kimetu, J., Eds.; Springer: Dordrecht, The Netherlands, 2008; p. 1091. [Google Scholar]
- Stoorvogel, J.J.; Smaling, E.M.A.; Janssen, B.H. Calculating soil nutrient balances in Africa at different scales. Nutr. Cycl. Agroecosyst. 1993, 35, 227–235. [Google Scholar]
- Mabood, F.; Zhou, X.; Smith, D. Bradyrhizobium japonicum preincubated with Methyl Jasmonate increases soybean nodulation and nitrogen fixation. Agron. J. 2006, 98, 289–294. [Google Scholar]
- Goss, M.J.; de Varennes, A. Soil disturbance reduces the efficacy of myccorrhizal associations for early soybean growth and N2 fixation. Soil Biol. Biochem. 2002, 34, 1167–1173. [Google Scholar]
- Giller, K.E.; Cadisch, G. Future benefits from biological nitrogen fixation: An ecological approach to agriculture. Plant Soil 1995, 174, 255–277. [Google Scholar]
- Rebafka, F.P.; Mndunguru, B.J.; Marschner, H. Crop residue application increases nitrogen fixation and dry matter production in groundnut (Arachis hypogaea L.) grown on an acid sandy soil in Niger, West Africa. Plant Soil 1993, 150, 213–222. [Google Scholar]
- Streeter, J.G. Effects of drought on nitrogen fixation in soybean root nodules. Plant Cell Environ. 2003, 26, 1199–1204. [Google Scholar]
- Salvagiotti, F.; Cassman, K.G.; Specht, J.E.; Walters, D.T.; Weiss, A.; Dobermann, A. Nitrogen uptake, fixation and response to fertilizer N in soybeans: A review. Field Crops Res. 2008, 108, 1–13. [Google Scholar]
- Sinclair, T.R.; Purcell, L.C.; King, C.A.; Sneller, C.H.; Chen, P.; Vadez, V. Drought tolerance and yield increase of soybean resulting from improved symbiotic N2 fixation. Field Crops Res. 2007, 101, 68–71. [Google Scholar]
- Peoples, M.B.; Craswell, E.T. Biological nitrogen fixation: Investments, expectations and actual contributions to agriculture. Plant Soil 1992, 141, 13–39. [Google Scholar]
- Unkovich, M.; Herridge, D.; Peoples, M.; Cadisch, G.; Boddey, B.; Giller, K.; Alves, B.; Chalk, P. Measuring Plant-Associated Nitrogen Fixation in Agricultural Systems; ACIAR Monogragh Series No. 136; Australian Centre for International Agricultural Research: Canberra, Australia, 2008; pp. 1–258. [Google Scholar]
- Witty, J.F. Estimating N2-fixation in the field using 15N-labelled fertilizer: Some problems and solutions. Soil Biol. Biochem. 1983, 15, 631–639. [Google Scholar]
- Okereke, G.U.; Dauda, G.; Ayama, G. Selection of the most appropriate reference crop for the assessment of nitrogen fixation by soya bean in Anambra state, Nigeria. J. Sci. Food Agric. 2006, 57, 101–109. [Google Scholar]
- Laboratory Methods of Soil and Plant Analysis; International Centre for Research in Agroforestry (ICRAF): Nairobi, Kenya, 1995.
- Use of Isotope and Radiation Methods in Soil and Water Management and Crop Nutrition; Training Course Series No. 14; International Atomic Energy Agency (IAEA): Vienna, Austria, 2001.
- Federer, W.T.; King, F. Variations on Split Plot and Split Block Experiment Designs; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2007; p. 270. [Google Scholar]
- Yoneyama, T.; Ito, O.; Engelaar, W.M.H.G. Uptake, metabolism and distribution of nitrogen in crop plants traced by enriched and natural 15N: Progress over the last 30 years. Phytochem. Rev. 2003, 2, 121–132. [Google Scholar]
- Steele, K.W.; Bonish, P.M.; Daniel, R.M.; O'Hara, G.W. Effect of rhizobial strain and host plant on nitrogen isotopic fractionation in legumes. Plant Physiol. 1983, 72, 1001–1004. [Google Scholar]
- Ojiem, J.O.; Vanlauwe, B.; de Ridder, N.; Giller, K.E. Niche-based assessment of contributions of legumes to the nitrogen economy of western Kenya smallholder farmers. Plant Soil 2007, 292, 119–135. [Google Scholar]
- Beare, M.H.; Pohland, B.R.; Wright, D.H.; Coleman, D.C. Residue placement and fungicide effects on fungal communities in conventional and no-tillage soils. Soil Sci. Soc. Am. J. 1993, 57, 392–399. [Google Scholar]
- Degens, B.P.; Sparling, G.P.; Abbott, L.K. Increasing the length of hyphae in a sandy soil increases the amount of water-stable aggregates. Appl. Soil Ecol. 1996, 3, 149–159. [Google Scholar]
- Vance, C.P. Symbiotic nitrogen fixation and phosphorus acquisition. Plant nutrition in a world of declining renewable resources. Plant Physiol. 2001, 127, 390–397. [Google Scholar]
- Maguta, J.K. Conservation Tillage in Kenya: The Biophysical Processes Affecting Its Effectiveness. Ph.D. Thesis, University of Bonn, Bonn, Germany, 2009. [Google Scholar]
- Waluyo, S.H. Significance of pelleting the seed with phosphate and lime on the cultivation of soybean in acid soils in Sitiung, West Sumatra. J. Tanah Lingk. 2005, 7, 58–65. [Google Scholar]
- Ferreira, M.C.; Andradeb, D.S.; Chueirea, L.M.O.; Takemuraa, S.M.; Hungria, M. Tillage method and crop rotation effects on the population sizes and diversity of bradyrhizobia nodulating soybean. Soil Biol. Biochem. 2000, 32, 627–637. [Google Scholar]
- Ralston, E.J.; Imsande, J. Nodulation of hydroponically grown soybean plants and inhibition of nodule development by nitrate. Exp. Bot. 1983, 34, 1371–1378. [Google Scholar]
- Van Kessel, C.; Hartley, C. Agricultural management of grain legumes: Has it led to an increase in nitrogen fixation? Field Crops Res. 2000, 65, 165–181. [Google Scholar]
- Wheatley, D.M.; Macleod, D.A.; Jessop, R.S. Influence of tillage treatments on N2 fixation of soybean. Soil Biol. Biochem. 1995, 27, 571–574. [Google Scholar]
- Power, J.F.; Wilhelm, W.; Doran, J.W. Crop residue effects on soil environment and dryland maize and soya bean production. Soil Tillage Res. 1986, 8, 101–111. [Google Scholar]
- Fujita, K.; Ofosu-budu, K.G.; Ogata, S. Biological nitrogen fixation in mixed legume-cereal cropping systems. Plant Soil 1992, 141, 155–175. [Google Scholar]
- Osunde, A.O.; Bala, A.; Gwam, M.S.; Tsado, P.A.; Sanginga, N.; Okogun, J.A. Residual benefits of Promiscuous soybean to maize (Zea mays L.) grown on farmer's field around Minna in the Southern Guinea savanna zone of Nigeria. Agric. Ecosyst. Environ. 2003, 100, 209–220. [Google Scholar]
- Peoples, M.B.; Herridge, D.E.; Ladha, J.K. Biological nitrogen fixation: An efficient source of nitrogen for sustainable agricultural production? Plant Soil 1995, 174, 3–28. [Google Scholar]
- Ofosu-budu, K.G.; Fujita, K.; Ogata, S. Excretion of ureide and other nitrogenous compounds by the root system of soybean at different growth stages. Plant Soil 1990, 128, 135–142. [Google Scholar]
- Di Ciocco, C.; Coviella, C.; Penón, E.; Díaz-Zorita, M.; López, S. Biological fixation of nitrogen and N balance in soybean crops in the pampas region. Span. J. Agric. Res. 2008, 6, 114–119. [Google Scholar]
- Oberson, A.; Nanzer, S.; Bosshard, C.; Dubois, D.; Mäder, P.; Frossard, E. Symbiotic N2 fixation by soybean in organic and conventional cropping systems estimated by 15N dilution and 15N natural abundance. Plant Soil 2007, 290, 69–83. [Google Scholar]
- Chikowo, R.; Mapfumo, P.; Nyamugafata, P.; Giller, K.E. Maize productivity and mineral N dynamics following different soil fertility management practices on a depleted sandy soil in Zimbabwe. Agric. Ecosyst. Environ. 2004, 102, 119–131. [Google Scholar]
- Danso, S.K.; Hardarson, G.; Zapata, F. Misconceptions and practical problems in the use of 15N soil enrichment techniques for estimating N2 fixation. Plant Soil 1993, 152, 25–52. [Google Scholar]
- Högberg, P. 15N natural abundance in soil-plant systems. New Phytol. 1997, 137, 179–203. [Google Scholar]
- Wanek, W.; Arndt, S.K. Differences in δ15N signatures between nodulated roots and shoots of soybean is indicative of the contribution of symbiotic N2 fixation to plant N. J. Exp. Bot. 2002, 53, 1109–1118. [Google Scholar]
- Ruschel, A.P.; Salati, E.; Vose, P.B. Nitrogen enrichment of soil and plant by Rhizobium phaseoli-Phaseolus vulgaris symbiosis. Plant Soil 1979, 51, 425–429. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kihara, J.; Martius, C.; Bationo, A.; Vlek, P.L.G. Effects of Tillage and Crop Residue Application on Soybean Nitrogen Fixation in a Tropical Ferralsol. Agriculture 2011, 1, 22-37. https://doi.org/10.3390/agriculture1010022
Kihara J, Martius C, Bationo A, Vlek PLG. Effects of Tillage and Crop Residue Application on Soybean Nitrogen Fixation in a Tropical Ferralsol. Agriculture. 2011; 1(1):22-37. https://doi.org/10.3390/agriculture1010022
Chicago/Turabian StyleKihara, Job, Christopher Martius, Andre Bationo, and Paul L. G. Vlek. 2011. "Effects of Tillage and Crop Residue Application on Soybean Nitrogen Fixation in a Tropical Ferralsol" Agriculture 1, no. 1: 22-37. https://doi.org/10.3390/agriculture1010022
APA StyleKihara, J., Martius, C., Bationo, A., & Vlek, P. L. G. (2011). Effects of Tillage and Crop Residue Application on Soybean Nitrogen Fixation in a Tropical Ferralsol. Agriculture, 1(1), 22-37. https://doi.org/10.3390/agriculture1010022




