Utility of Magnetic Resonance Imaging for Differentiating Necrotizing Fasciitis from Severe Cellulitis: A Magnetic Resonance Indicator for Necrotizing Fasciitis (MRINEC) Algorithm
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Definition of Necrotizing Fasciitis
2.2. Analysis of Magnetic Resonance Imaging
2.3. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Patients
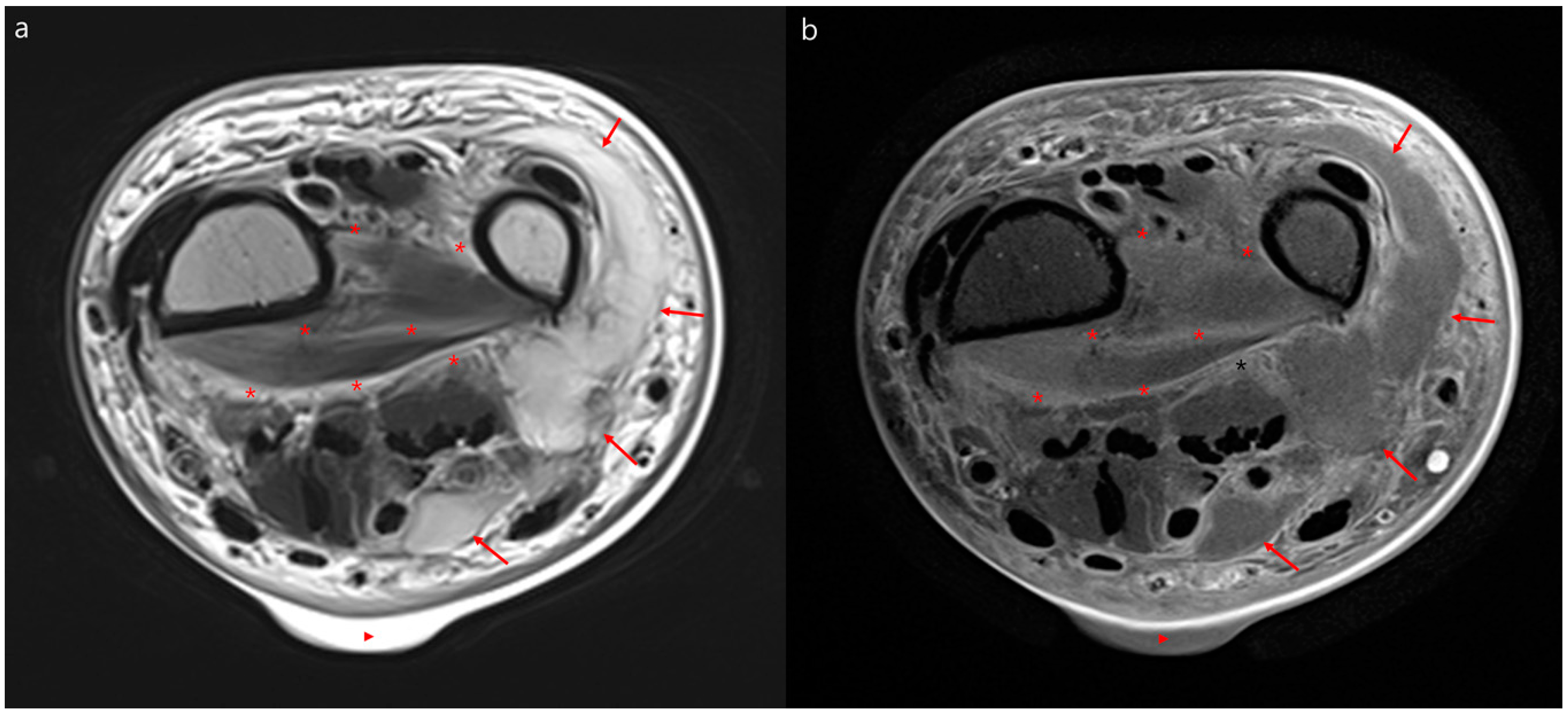
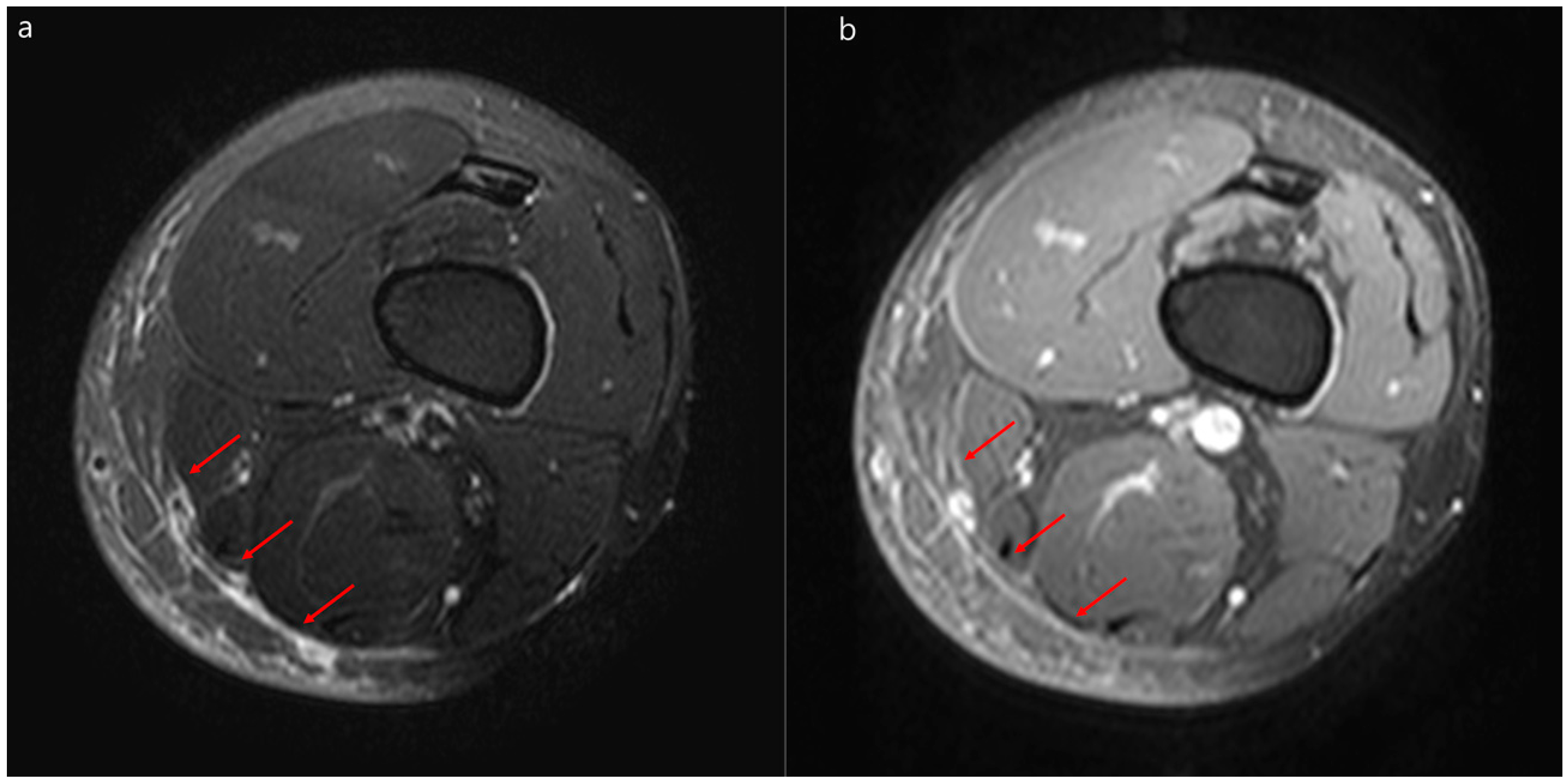
3.2. MRI Findings of Necrotizing Fasciitis and Severe Cellulitis
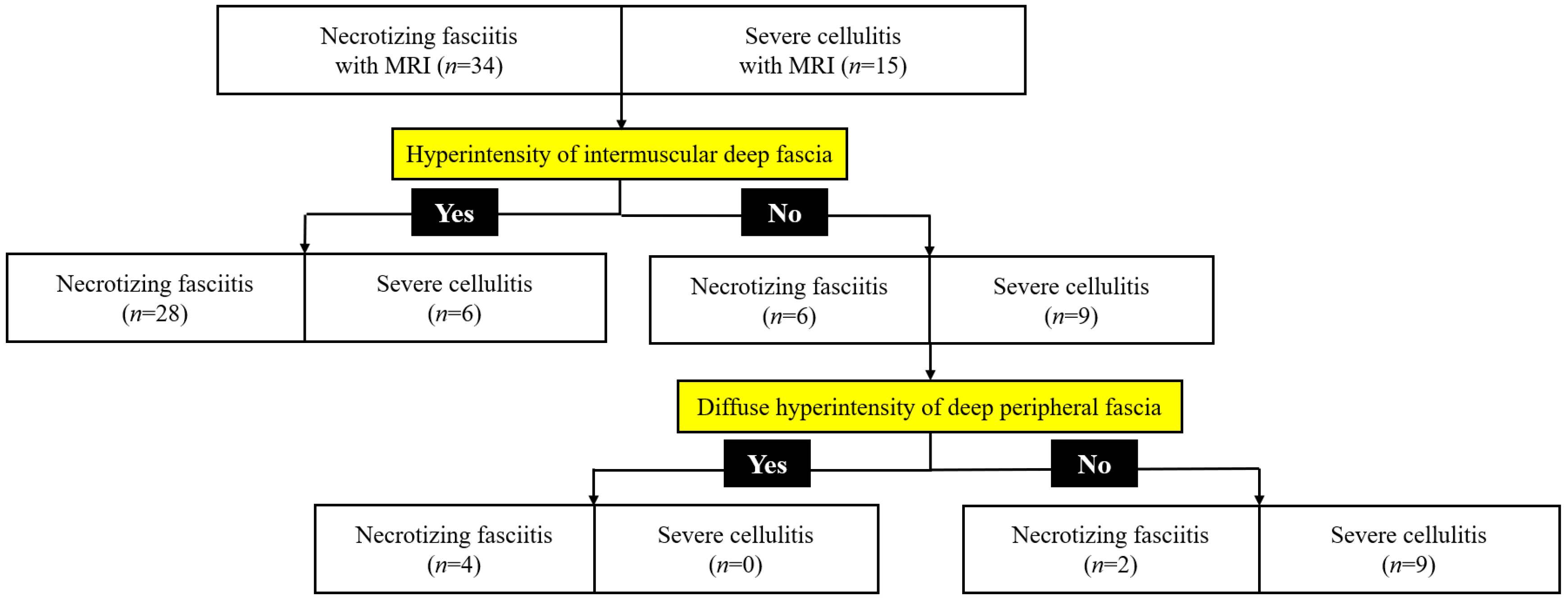
3.3. Diagnostic Performance of the MRINEC Algorithm
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Stevens, D.L.; Bryant, A.E. Necrotizing Soft-Tissue Infections. N. Engl. J. Med. 2018, 378, 971. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Dellinger, E.P.; Goldstein, E.J.; Gorbach, S.L.; Hirschmann, J.V.; Kaplan, S.L.; Montoya, J.G.; Wade, J.C.; et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2014, 59, e10–e52. [Google Scholar] [CrossRef] [Green Version]
- McHenry, C.R.; Piotrowski, J.J.; Petrinic, D.; Malangoni, M.A. Determinants of mortality for necrotizing soft-tissue infections. Ann. Surg. 1995, 221, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Fernando, S.M.; Tran, A.; Cheng, W.; Rochwerg, B.; Kyeremanteng, K.; Seely, A.J.E.; Inaba, K.; Perry, J.J. Necrotizing Soft Tissue Infection: Diagnostic Accuracy of Physical Examination, Imaging, and LRINEC Score: A Systematic Review and Meta-Analysis. Ann. Surg. 2019, 269, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Anaya, D.A.; Dellinger, E.P. Necrotizing soft-tissue infection: Diagnosis and management. Clin. Infect. Dis. 2007, 44, 705–710. [Google Scholar] [PubMed] [Green Version]
- Wong, C.H.; Khin, L.W.; Heng, K.S.; Tan, K.C.; Low, C.O. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: A tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit. Care Med. 2004, 32, 1535–1541. [Google Scholar] [CrossRef]
- Burner, E.; Henderson, S.O.; Burke, G.; Nakashioya, J.; Hoffman, J.R. Inadequate Sensitivity of Laboratory Risk Indicator to Rule Out Necrotizing Fasciitis in the Emergency Department. West. J. Emerg. Med. 2016, 17, 333–336. [Google Scholar] [CrossRef]
- Kim, K.T.; Kim, Y.J.; Won Lee, J.; Kim, Y.J.; Park, S.W.; Lim, M.K.; Suh, C.H. Can necrotizing infectious fasciitis be differentiated from nonnecrotizing infectious fasciitis with MR imaging? Radiology 2011, 259, 816–824. [Google Scholar] [CrossRef]
- Brothers, T.E.; Tagge, D.U.; Stutley, J.E.; Conway, W.F.; Del Schutte, H., Jr.; Byrne, T.K. Magnetic resonance imaging differentiates between necrotizing and non-necrotizing fasciitis of the lower extremity. J. Am. Coll. Surg. 1998, 187, 416–421. [Google Scholar] [CrossRef]
- Ali, S.Z.; Srinivasan, S.; Peh, W.C. MRI in necrotizing fasciitis of the extremities. Br. J. Radiol. 2014, 87, 20130560. [Google Scholar] [CrossRef] [Green Version]
- Schmid, M.R.; Kossmann, T.; Duewell, S. Differentiation of necrotizing fasciitis and cellulitis using MR imaging. AJR Am. J. Roentgenol. 1998, 170, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Malghem, J.; Lecouvet, F.E.; Omoumi, P.; Maldague, B.E.; Vande Berg, B.C. Necrotizing fasciitis: Contribution and limitations of diagnostic imaging. Jt. Bone Spine 2013, 80, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees; Chapman and Hall/CRC: London, UK, 1984. [Google Scholar]
- Yoon, M.A.; Chung, H.W.; Yeo, Y.; Yoo, H.J.; Kang, Y.; Chee, C.G.; Lee, M.H.; Lee, S.H.; Shin, M.J. Distinguishing necrotizing from non-necrotizing fasciitis: A new predictive scoring integrating MRI in the LRINEC score. Eur. Radiol. 2019, 29, 3414–3423. [Google Scholar] [CrossRef] [PubMed]
- Hodgins, N.; Damkat-Thomas, L.; Shamsian, N.; Yew, P.; Lewis, H.; Khan, K. Analysis of the increasing prevalence of necrotising fasciitis referrals to a regional plastic surgery unit: A retrospective case series. J. Plast. Reconstr. Aesthet Surg. 2015, 68, 304–311. [Google Scholar] [CrossRef]
- Wilson, M.P.; Schneir, A.B. A case of necrotizing fasciitis with a LRINEC score of zero: Clinical suspicion should trump scoring systems. J. Emerg. Med. 2013, 44, 928–931. [Google Scholar] [CrossRef]
- Swain, R.A.; Hatcher, J.C.; Azadian, B.S.; Soni, N.; De Souza, B. A five-year review of necrotising fasciitis in a tertiary referral unit. Ann. R. Coll. Surg. Engl. 2013, 95, 57–60. [Google Scholar] [CrossRef] [Green Version]
- Holland, M.J. Application of the Laboratory Risk Indicator in Necrotising Fasciitis (LRINEC) score to patients in a tropical tertiary referral centre. Anaesth. Intensive Care 2009, 37, 588–592. [Google Scholar] [CrossRef] [Green Version]
- van Stigt, S.F.; de Vries, J.; Bijker, J.B.; Mollen, R.M.; Hekma, E.J.; Lemson, S.M.; Tan, E.C. Review of 58 patients with necrotizing fasciitis in the Netherlands. World J. Emerg. Surg. 2016, 11, 21. [Google Scholar] [CrossRef] [Green Version]
- Borschitz, T.; Schlicht, S.; Siegel, E.; Hanke, E.; von Stebut, E. Improvement of a Clinical Score for Necrotizing Fasciitis: ‘Pain Out of Proportion’ and High CRP Levels Aid the Diagnosis. PLoS ONE 2015, 10, e0132775. [Google Scholar] [CrossRef] [Green Version]
- Putnam, L.R.; Richards, M.K.; Sandvall, B.K.; Hopper, R.A.; Waldhausen, J.H.; Harting, M.T. Laboratory evaluation for pediatric patients with suspected necrotizing soft tissue infections: A case-control study. J. Pediatric Surg. 2016, 51, 1022–1025. [Google Scholar] [CrossRef]
- Gelbard, R.B.; Ferrada, P.; Yeh, D.D.; Williams, B.H.; Loor, M.; Yon, J.; Mentzer, C.; Khwaja, K.; Khan, M.A.; Kohli, A.; et al. Optimal timing of initial debridement for necrotizing soft tissue infection: A Practice Management Guideline from the Eastern Association for the Surgery of Trauma. J. Trauma Acute Care Surg. 2018, 85, 208–214. [Google Scholar] [CrossRef] [PubMed]

| Variables | Necrotizing Fasciitis (n = 61) | Severe Cellulitis (n = 28) | p-Value |
|---|---|---|---|
| Male sex | 31 (51) | 17 (61) | 0.39 |
| Age, mean years ± SD | 59 ± 16 | 59 ± 15 | 0.88 |
| Acquisition site of infection | 0.03 | ||
| Community-acquired infection | 41 (67) | 25 (89) | |
| Healthcare-associated infection | 20 (33) | 3 (11) | |
| Infection site | |||
| Leg | 41 (67) | 20 (71) | 0.69 |
| Arm | 13 (21) | 4 (14) | 0.43 |
| Abdomen & pelvis | 4 (7) | 1 (4) | >0.99 |
| Others | 3 (5) | 3 (11) | 0.37 |
| Underlying disease | |||
| Diabetes | 24 (39) | 9 (32) | 0.51 |
| Trauma | 22 (36) | 4 (14) | 0.04 |
| Tinea pedis or onychomycosis | 10 (16) | 9 (32) | 0.09 |
| Malignancy | 11 (18) | 6 (21) | 0.71 |
| Liver cirrhosis | 11 (18) | 1 (4) | 0.09 |
| Peripheral artery disease | 7 (12) | 0 | 0.09 |
| Chronic kidney disease | 6 (10) | 3 (11) | >0.99 |
| Lymphedema | 3 (5) | 1 (4) | >0.99 |
| Symptoms and signs | |||
| Pain and erythema | 61 (100) | 28 (100) | >0.99 |
| Fever | 42 (69) | 14 (50) | 0.09 |
| Discharge | 27 (44) | 4 (14) | 0.006 |
| Bullae | 25 (41) | 0 | <0.001 |
| Necrosis | 17 (28) | 0 | 0.002 |
| Altered mental status | 16 (26) | 1 (4) | 0.01 |
| Shock | 15 (25) | 1 (4) | 0.02 |
| Petechiae/hemorrhage | 11 (22) | 1 (4) | 0.05 |
| Metastatic infections | 6 (10) | 2 (7) | >0.99 |
| Laboratory findings | |||
| White blood cell, mean/mm3 ± SD | 14,000 ± 8500 | 10,000 ± 3800 | 0.05 |
| Hemoglobin, g/dL ± SD | 11.4 ± 2.6 | 13.5 ± 1.9 | 0.001 |
| Platelet, × 103/mm3 ± SD | 225 ± 131 | 217 ± 87 | 0.90 |
| INR ± SD | 1.3 ± 0.3 | 1.1 ± 0.2 | 0.07 |
| Bilirubin, mg/dL ± SD | 1.5 ± 2.9 | 0.9 ± 0.8 | 0.56 |
| Creatinine, mg/dL ± SD | 1.2 ± 1.0 | 1.0 ± 0.5 | 0.77 |
| Na, mEq/L ± SD | 135 ± 6 | 137 ± 4 | 0.02 |
| Glucose, mg/dL ± SD | 171 ± 124 | 153 ± 74 | 0.81 |
| C-reactive protein, mg/dL ± SD | 15.7 ± 11.4 | 9.4 ± 9.2 | 0.02 |
| Procalcitonin, ng/mL ± SD | 4.8 ± 5.3 | 6.7 ± 6.8 | 0.39 |
| Lactate, mmol/L ± SD | 2.5 ± 2.2 | 1.4 ± 0.2 | 0.54 |
| LRINEC score ± SD | 6.0 ± 3.6 | 3.1 ± 3.4 | 0.001 |
| High risk (≥8) | 24 (39) | 2 (7) | 0.002 |
| Moderate risk (6–7) | 9 (15) | 4 (14) | >0.99 |
| Low risk (≤5) | 28 (46) | 22 (79) | 0.004 |
| Microbiology | |||
| Overall culture positivity | 38 (62) | 5 (18) | <0.001 |
| Blood culture positivity | 18 (30) | 2 (7) | 0.02 |
| Wound culture positivity | 27 (44) | 3 (11) | 0.002 |
| Monobacterial infection | 30 (49) | 5 (18) | 0.005 |
| Polybacterial infection | 8 (13) | 0 | 0.05 |
| Staphylococcus aureus | 11 (18) | 1 (4) | 0.09 |
| Group A streptococcus | 5 (8) | 2 (7) | >0.99 |
| Group B and C streptococcus | 4 (7) | 1 (4) | >0.99 |
| Gram negative bacteria | 18 (30) | 1 (4) | 0.006 |
| Anaerobes | 0 | 0 | |
| Surgical debridement | 35 (57) | 0 | <0.001 |
| Amputation | 4 (11) | 0 | 0.30 |
| Interval between admission and surgery, mean days ± SD | 6 ± 8 | NA | NA |
| Second look operation | 18 (51) | 0 | 0.001 |
| Antibiotics | |||
| Total days of antibiotics use ± SD | 35 ± 25 | 20 ± 12 | 0.007 |
| Days of intravenous antibiotics use ± SD | 23 ± 19 | 8 ± 6 | <0.001 |
| Days of oral antibiotics use ± SD | 20 ± 16 | 13 ± 9 | 0.03 |
| Clinical outcomes | |||
| Intensive care unit admission | 16 (26) | 1 (4) | 0.01 |
| Mechanical ventilator | 10 (16) | 0 | 0.03 |
| Relapse within 1 month | 10 (16) | 1 (4) | 0.16 |
| In hospital mortality | 14 (23) | 0 | 0.004 |
| Variables | Necrotizing Fasciitis (n = 34) | Severe Cellulitis (n = 15) | p-Value |
|---|---|---|---|
| Interval from admission to MRI, mean days ± SD | 4 ± 7 | 4 ± 5 | 0.71 |
| T2 hyperintensity of deep peripheral fascia | 31 (91) | 12 (80) | 0.35 |
| Diffuse | 20 (59) | 3 (20) | 0.01 |
| Localized | 11 (32) | 9 (60) | 0.07 |
| Thickness of superficial fascia, mm ± SD | 6.9 ± 4.8 | 5.5 ± 3.0 | 0.52 |
| T2 hyperintensity of intermuscular deep fascia | 28 (82) | 6 (40) | 0.006 |
| Diffuse | 17 (50) | 2 (13) | 0.02 |
| Localized | 11 (32) | 4 (27) | 0.75 |
| Fascial enhancement | 28/31 a (90) | 12/14 b (86) | 0.64 |
| Irregular | 6/31 a (19) | 0 | 0.16 |
| Diffuse | 22/31 a (71) | 12/14 b (86) | 0.46 |
| Myositis | 24 (71) | 6 (40) | 0.04 |
| Abscess | 17 (50) | 1 (7) | 0.004 |
| Intermuscular abscess | 11 (32) | 0 | 0.01 |
| Subcutaneous | 6 (18) | 1 (7) | 0.41 |
| Subcutaneous fat edema | 30 (88) | 14 (93) | >0.99 |
| Univariable Logistic Regression Analysis. | Odds Ratio (95% CI) | p-Value | Multivariable Logistic Regression Analysis | Odds Ratio (95% CI) | p-Value |
|---|---|---|---|---|---|
| Diffuse T2 hyperintensity of deep peripheral fascia | 5.1 (1.3–20.3) | 0.02 | Diffuse T2 hyperintensity of deep peripheral fascia | 4.4 (0.9–22.8) | 0.074 |
| T2 hyperintensity of intermuscular deep fascia | 6.4 (1.7–24.6) | 0.01 | |||
| Diffuse T2 hyperintensity of intermuscular deep fascia | 5.4 (1.2–25.2) | 0.03 | Diffuse T2 hyperintensity of intermuscular deep fascia | 5.5 (0.9–33.8) | 0.065 |
| Abscess | 9.6 (1.5–61.7) | 0.02 | Abscess | 15.1 (1.6–143.5) | 0.02 |
| Intermuscular abscess | 15.2 (0.7–313.7) | 0.078 |
| NF by MRINEC/Patients with NF (n = 34) | SC by MRINEC/Patients with SC (n = 15) | Sensitivity, % (95% CI) | Specificity, % (95% CI) | PLR (95% CI) | NLR (95% CI) | |
|---|---|---|---|---|---|---|
| Total (n = 49) | 32/34 | 9/15 | 94 (80–99) | 60 (32–84) | 2.4 (1.3–4.4) | 0.1 (0.02–0.4) |
| High risk, LRINEC score ≥8 (n = 15) | 15/15 | 0 | 100 (78–100) | NA | 1.0 | NA |
| Intermediate risk, LRINEC score 6–7 (n = 5) | 3/3 | 0/2 | 100 (29–100) | 0 (0–84) | 1.0 | NA |
| Low risk, LRINEC score ≤5 (n = 29) | 14/16 | 9/13 | 88 (62–98) | 69 (39–91) | 2.8 (1.2–6.6) | 0.2 (0.05–0.7) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-C.; Kim, S.; Cho, E.B.; Lee, G.Y.; Choi, S.-H.; Kim, S.O.; Chung, J.-W. Utility of Magnetic Resonance Imaging for Differentiating Necrotizing Fasciitis from Severe Cellulitis: A Magnetic Resonance Indicator for Necrotizing Fasciitis (MRINEC) Algorithm. J. Clin. Med. 2020, 9, 3040. https://doi.org/10.3390/jcm9093040
Kim M-C, Kim S, Cho EB, Lee GY, Choi S-H, Kim SO, Chung J-W. Utility of Magnetic Resonance Imaging for Differentiating Necrotizing Fasciitis from Severe Cellulitis: A Magnetic Resonance Indicator for Necrotizing Fasciitis (MRINEC) Algorithm. Journal of Clinical Medicine. 2020; 9(9):3040. https://doi.org/10.3390/jcm9093040
Chicago/Turabian StyleKim, Min-Chul, Sujin Kim, Eun Been Cho, Guen Young Lee, Seong-Ho Choi, Seon Ok Kim, and Jin-Won Chung. 2020. "Utility of Magnetic Resonance Imaging for Differentiating Necrotizing Fasciitis from Severe Cellulitis: A Magnetic Resonance Indicator for Necrotizing Fasciitis (MRINEC) Algorithm" Journal of Clinical Medicine 9, no. 9: 3040. https://doi.org/10.3390/jcm9093040





