Growth Differentiation Factor 15 in Severe Aortic Valve Stenosis: Relationship with Left Ventricular Remodeling and Frailty
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Frailty Assessment
2.3. Echocardiography
2.4. GDF15 and Other Plasma Biomarkers
2.5. Statistical Analyses
3. Results
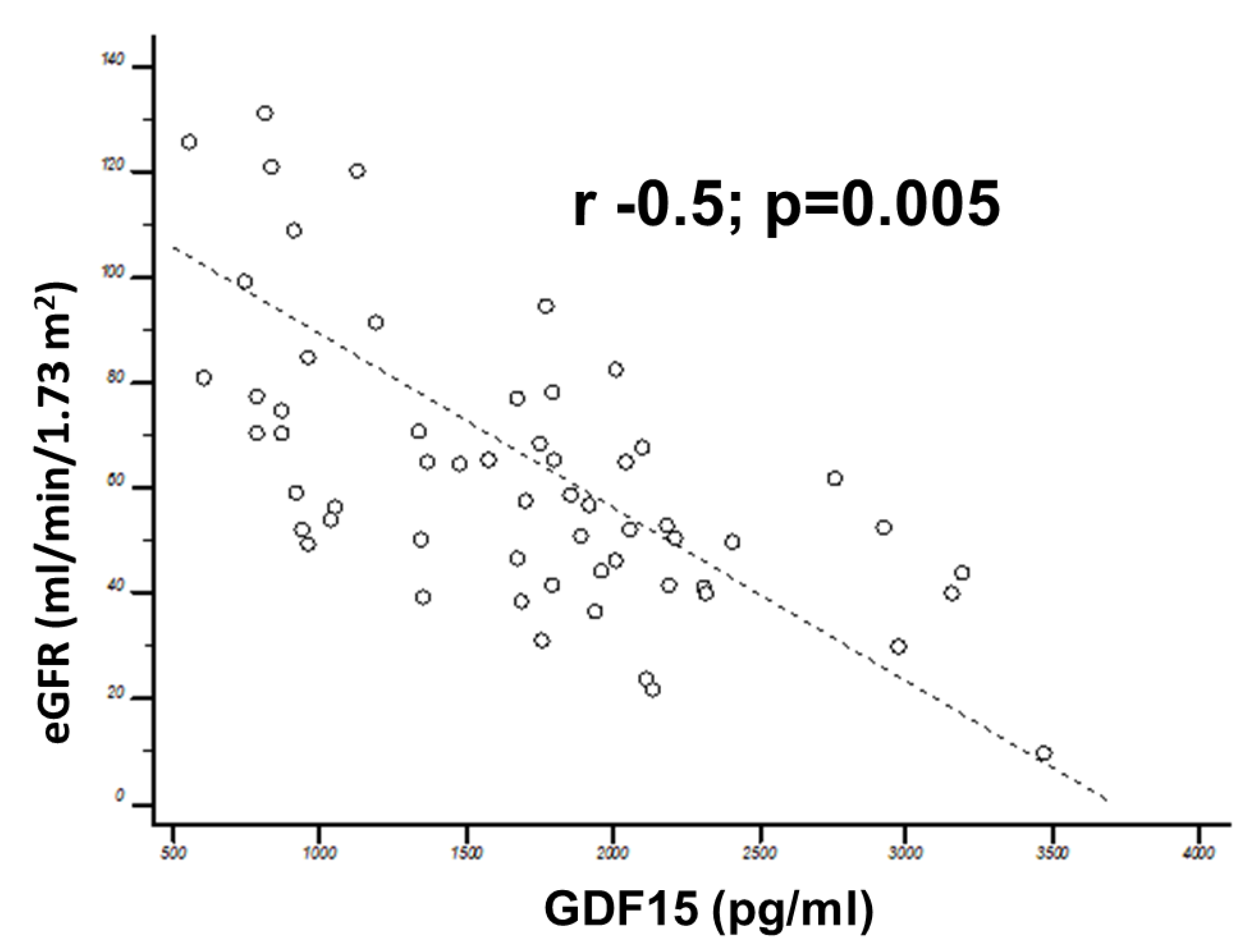
3.1. Correlation of GDF15 with Echocardiographic Data
3.2. Correlations with Frailty
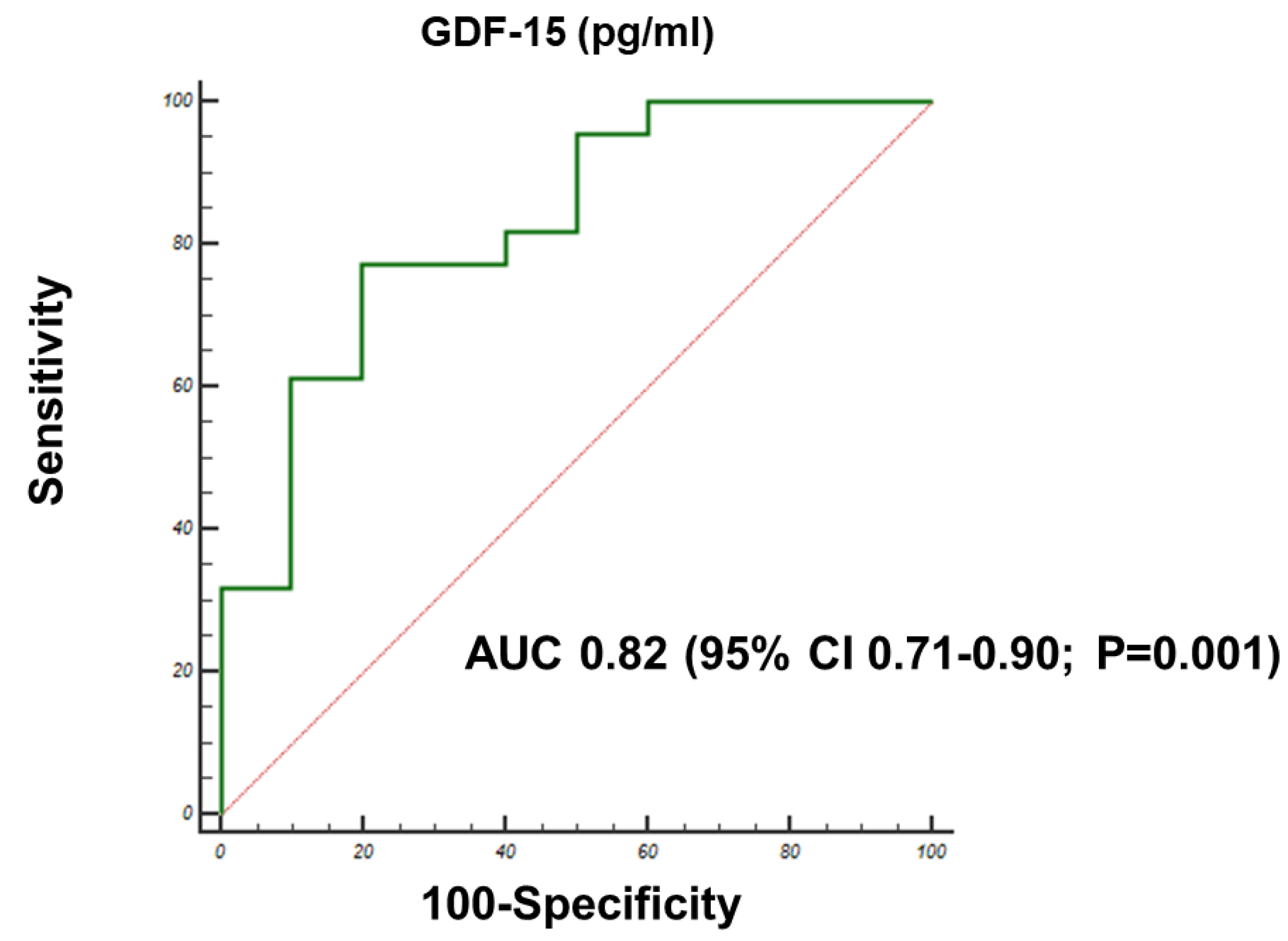
3.3. Correlations with Follow-Up Outcomes
4. Discussion
- Higher GDF15 levels in AVS patients compared to age- and sex-matched controls;
- An association between age, NYHA functional class, renal function and GDF15 levels;
- A differential distribution of GDF15 levels according to parameters of systolic function (GLS) and structure (LAVi; LV mass);
- A differential distribution of GDF15 according to the frailty score (Katz index), with higher GDF15 levels in more frail patients, independent of other clinical, structural and bio-humoral parameters.
4.1. GDF15 in Aortic Valve Disease
4.2. GDF15 and Frailty Before Aortic Valve Replacement
5. Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Rodriguez Munoz, D.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Fleisher, L.A.; Jneid, H.; Mack, M.J.; McLeod, C.J.; O’Gara, P.T.; et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2017, 70, 252–289. [Google Scholar] [CrossRef] [PubMed]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Kappetein, A.P.; Head, S.J.; Genereux, P.; Piazza, N.; van Mieghem, N.M.; Blackstone, E.H.; Brott, T.G.; Cohen, D.J.; Cutlip, D.E.; van Es, G.A.; et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 consensus document. J. Am. Coll. Cardiol. 2012, 60, 1438–1454. [Google Scholar] [CrossRef] [Green Version]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [Green Version]
- Puls, M.; Sobisiak, B.; Bleckmann, A.; Jacobshagen, C.; Danner, B.C.; Hunlich, M.; Beissbarth, T.; Schondube, F.; Hasenfuss, G.; Seipelt, R.; et al. Impact of frailty on short- and long-term morbidity and mortality after transcatheter aortic valve implantation: Risk assessment by Katz Index of activities of daily living. EuroIntervention 2014, 10, 609–619. [Google Scholar] [CrossRef]
- Afilalo, J.; Lauck, S.; Kim, D.H.; Lefevre, T.; Piazza, N.; Lachapelle, K.; Martucci, G.; Lamy, A.; Labinaz, M.; Peterson, M.D.; et al. Frailty in Older Adults Undergoing Aortic Valve Replacement: The FRAILTY-AVR Study. J. Am. Coll. Cardiol. 2017, 70, 689–700. [Google Scholar] [CrossRef]
- Lindman, B.R.; Breyley, J.G.; Schilling, J.D.; Vatterott, A.M.; Zajarias, A.; Maniar, H.S.; Damiano, R.J., Jr.; Moon, M.R.; Lawton, J.S.; Gage, B.F.; et al. Prognostic utility of novel biomarkers of cardiovascular stress in patients with aortic stenosis undergoing valve replacement. Heart 2015, 101, 1382–1388. [Google Scholar] [CrossRef] [Green Version]
- Sinning, J.M.; Wollert, K.C.; Sedaghat, A.; Widera, C.; Radermacher, M.C.; Descoups, C.; Hammerstingl, C.; Weber, M.; Stundl, A.; Ghanem, A.; et al. Risk scores and biomarkers for the prediction of 1-year outcome after transcatheter aortic valve replacement. Am. Heart J. 2015, 170, 821–829. [Google Scholar] [CrossRef]
- Bootcov, M.R.; Bauskin, A.R.; Valenzuela, S.M.; Moore, A.G.; Bansal, M.; He, X.Y.; Zhang, H.P.; Donnellan, M.; Mahler, S.; Pryor, K.; et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc. Natl. Acad. Sci. USA 1997, 94, 11514–11519. [Google Scholar] [CrossRef] [Green Version]
- Desmedt, S.; Desmedt, V.; De Vos, L.; Delanghe, J.R.; Speeckaert, R.; Speeckaert, M.M. Growth differentiation factor 15: A novel biomarker with high clinical potential. Crit. Rev. Clin. Lab. Sci. 2019, 56, 333–350. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Jena, A.; Srivatsan, V.; Muthukumar, R.; Dhandapani, V.E. GDF 15--A Novel Biomarker in the Offing for Heart Failure. Curr. Cardiol. Rev. 2016, 12, 37–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kempf, T.; Zarbock, A.; Widera, C.; Butz, S.; Stadtmann, A.; Rossaint, J.; Bolomini-Vittori, M.; Korf-Klingebiel, M.; Napp, L.C.; Hansen, B.; et al. GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat. Med. 2011, 17, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Shibasaki, I.; Sawaguchi, T.; Haruyama, A.; Kaneda, H.; Nakajima, T.; Hasegawa, T.; Arikawa, T.; Obi, S.; Sakuma, M.; et al. Growth Differentiation Factor-15 (GDF-15) is a Biomarker of Muscle Wasting and Renal Dysfunction in Preoperative Cardiovascular Surgery Patients. J. Clin. Med. 2019, 8, 1576. [Google Scholar] [CrossRef] [Green Version]
- Barma, M.; Khan, F.; Price, R.J.G.; Donnan, P.T.; Messow, C.M.; Ford, I.; McConnachie, A.; Struthers, A.D.; McMurdo, M.E.T.; Witham, M.D. Association between GDF-15 levels and changes in vascular and physical function in older patients with hypertension. Aging Clin. Exp. Res. 2017, 29, 1055–1059. [Google Scholar] [CrossRef] [Green Version]
- Sanchis, J.; Ruiz, V.; Bonanad, C.; Sastre, C.; Ruescas, A.; Diaz, M.; Rodriguez, E.; Valero, E.; Garcia-Blas, S.; Carratala, A.; et al. Growth differentiation factor 15 and geriatric conditions in acute coronary syndrome. Int. J. Cardiol. 2019, 290, 15–20. [Google Scholar] [CrossRef]
- Fabiani, I.; Scatena, C.; Mazzanti, C.M.; Conte, L.; Pugliese, N.R.; Franceschi, S.; Lessi, F.; Menicagli, M.; De Martino, A.; Pratali, S.; et al. Micro-RNA-21 (biomarker) and global longitudinal strain (functional marker) in detection of myocardial fibrotic burden in severe aortic valve stenosis: A pilot study. J. Transl. Med. 2016, 14, 248. [Google Scholar] [CrossRef] [Green Version]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of Illness in the Aged. The Index of Adl: A Standardized Measure of Biological and Psychosocial Function. JAMA 1963, 185, 914–919. [Google Scholar] [CrossRef]
- Townend, J.N.; Ludman, P.F.; Doshi, S.N. Measuring frailty in patients undergoing TAVI: How and why? Eur. Heart J. 2019, 40, 2240–2242. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Edvardsen, T.; Goldstein, S.; Lancellotti, P.; LeFevre, M.; Miller, F., Jr.; Otto, C.M. Recommendations on the Echocardiographic Assessment of Aortic Valve Stenosis: A Focused Update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2017, 30, 372–392. [Google Scholar] [CrossRef] [PubMed]
- Voigt, J.U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a common standard for 2D speckle tracking echocardiography: Consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Breit, S.N.; Johnen, H.; Cook, A.D.; Tsai, V.W.; Mohammad, M.G.; Kuffner, T.; Zhang, H.P.; Marquis, C.P.; Jiang, L.; Lockwood, G.; et al. The TGF-beta superfamily cytokine, MIC-1/GDF15: A pleotrophic cytokine with roles in inflammation, cancer and metabolism. Growth Factors 2011, 29, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Kempf, T.; von Haehling, S.; Peter, T.; Allhoff, T.; Cicoira, M.; Doehner, W.; Ponikowski, P.; Filippatos, G.S.; Rozentryt, P.; Drexler, H.; et al. Prognostic utility of growth differentiation factor-15 in patients with chronic heart failure. J. Am. Coll. Cardiol. 2007, 50, 1054–1060. [Google Scholar] [CrossRef] [Green Version]
- Anand, I.S.; Kempf, T.; Rector, T.S.; Tapken, H.; Allhoff, T.; Jantzen, F.; Kuskowski, M.; Cohn, J.N.; Drexler, H.; Wollert, K.C. Serial measurement of growth-differentiation factor-15 in heart failure: Relation to disease severity and prognosis in the Valsartan Heart Failure Trial. Circulation 2010, 122, 1387–1395. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.B.; Kobayashi, Y.; Moneghetti, K.J.; Brenner, D.A.; O’Malley, R.; Schnittger, I.; Wu, J.C.; Murtagh, G.; Beshiri, A.; Fischbein, M.; et al. GDF-15 (Growth Differentiation Factor 15) Is Associated With Lack of Ventricular Recovery and Mortality After Transcatheter Aortic Valve Replacement. Circ. Cardiovasc. Interv. 2017, 10. [Google Scholar] [CrossRef]
- Xue, H.; Fu, Z.; Chen, Y.; Xing, Y.; Liu, J.; Zhu, H.; Zhou, X. The association of growth differentiation factor-15 with left ventricular hypertrophy in hypertensive patients. PLoS ONE 2012, 7, e46534. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Cui, Y.; Huang, A.; Li, Q.; Jia, W.; Liu, K.; Qi, X. Additional Diagnostic Value of Growth Differentiation Factor-15 (GDF-15) to N-Terminal B-Type Natriuretic Peptide (NT-proBNP) in Patients with Different Stages of Heart Failure. Med. Sci. Monit. 2018, 24, 4992–4999. [Google Scholar] [CrossRef]
- Fabiani, I.; Conte, L.; Pugliese, N.R.; Calogero, E.; Barletta, V.; Di Stefano, R.; Santoni, T.; Scatena, C.; Bortolotti, U.; Naccarato, A.G.; et al. The integrated value of sST2 and global longitudinal strain in the early stratification of patients with severe aortic valve stenosis: A translational imaging approach. Int. J. Cardiovasc. Imaging 2017, 33, 1915–1920. [Google Scholar] [CrossRef]
- Fabiani, I.; Pugliese, N.R.; Calogero, E.; Conte, L.; Mazzanti, M.C.; Scatena, C.; Scopelliti, C.; Tantillo, E.; Passiatore, M.; Angelillis, M.; et al. MicroRNAs distribution in different phenotypes of Aortic Stenosis. Sci. Rep. 2018, 8, 9953. [Google Scholar] [CrossRef] [Green Version]
- Krau, N.C.; Lunstedt, N.S.; Freitag-Wolf, S.; Brehm, D.; Petzina, R.; Lutter, G.; Bramlage, P.; Dempfle, A.; Frey, N.; Frank, D. Elevated growth differentiation factor 15 levels predict outcome in patients undergoing transcatheter aortic valve implantation. Eur. J. Heart Fail. 2015, 17, 945–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjornstad, J.L.; Neverdal, N.O.; Vengen, O.A.; Knudsen, C.W.; Husebye, T.; Pepper, J.; Lie, M.; Christensen, G.; Tonnessen, T. Alterations in circulating activin A, GDF-15, TGF-beta3 and MMP-2, -3, and -9 during one year of left ventricular reverse remodelling in patients operated for severe aortic stenosis. Eur. J. Heart Fail. 2008, 10, 1201–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Guyton, R.A.; O’Gara, P.T.; Ruiz, C.E.; Skubas, N.J.; Sorajja, P.; et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, e521–e643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morley, J.E.; Vellas, B.; van Kan, G.A.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; et al. Frailty consensus: A call to action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.H.; Buth, K.J.; Martin, B.J.; Yip, A.M.; Hirsch, G.M. Frail patients are at increased risk for mortality and prolonged institutional care after cardiac surgery. Circulation 2010, 121, 973–978. [Google Scholar] [CrossRef]
- Stortecky, S.; Schoenenberger, A.W.; Moser, A.; Kalesan, B.; Juni, P.; Carrel, T.; Bischoff, S.; Schoenenberger, C.M.; Stuck, A.E.; Windecker, S.; et al. Evaluation of multidimensional geriatric assessment as a predictor of mortality and cardiovascular events after transcatheter aortic valve implantation. JACC Cardiovasc. Interv. 2012, 5, 489–496. [Google Scholar] [CrossRef] [Green Version]
- Schoenenberger, A.W.; Stortecky, S.; Neumann, S.; Moser, A.; Juni, P.; Carrel, T.; Huber, C.; Gandon, M.; Bischoff, S.; Schoenenberger, C.M.; et al. Predictors of functional decline in elderly patients undergoing transcatheter aortic valve implantation (TAVI). Eur. Heart J. 2013, 34, 684–692. [Google Scholar] [CrossRef] [Green Version]
- Green, P.; Woglom, A.E.; Genereux, P.; Daneault, B.; Paradis, J.M.; Schnell, S.; Hawkey, M.; Maurer, M.S.; Kirtane, A.J.; Kodali, S.; et al. The impact of frailty status on survival after transcatheter aortic valve replacement in older adults with severe aortic stenosis: A single-center experience. JACC Cardiovasc. Interv. 2012, 5, 974–981. [Google Scholar] [CrossRef] [Green Version]
- Kleczynski, P.; Dziewierz, A.; Bagienski, M.; Rzeszutko, L.; Sorysz, D.; Trebacz, J.; Sobczynski, R.; Tomala, M.; Stapor, M.; Dudek, D. Impact of frailty on mortality after transcatheter aortic valve implantation. Am. Heart J. 2017, 185, 52–58. [Google Scholar] [CrossRef]
- Martin, G.P.; Sperrin, M.; Ludman, P.F.; deBelder, M.A.; Gunning, M.; Townend, J.; Redwood, S.R.; Kadam, U.T.; Buchan, I.; Mamas, M.A. Do frailty measures improve prediction of mortality and morbidity following transcatheter aortic valve implantation? An analysis of the UK TAVI registry. BMJ Open 2018, 8, e022543. [Google Scholar] [CrossRef]
- Rogers, T.; Alraies, M.C.; Moussa Pacha, H.; Bond, E.; Buchanan, K.D.; Steinvil, A.; Gai, J.; Torguson, R.; Ben-Dor, I.; Satler, L.F.; et al. Clinical Frailty as an Outcome Predictor After Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2018, 121, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Diez-Villanueva, P.; Ariza-Sole, A.; Vidan, M.T.; Bonanad, C.; Formiga, F.; Sanchis, J.; Martin-Sanchez, F.J.; Ruiz Ros, V.; Sanmartin Fernandez, M.; Bueno, H.; et al. Recommendations of the Geriatric Cardiology Section of the Spanish Society of Cardiology for the Assessment of Frailty in Elderly Patients With Heart Disease. Rev. Esp. Cardiol. 2019, 72, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Meng, X.; Chen, Y.; Leng, S.X.; Zhang, H. The Biology of Aging and Cancer: Frailty, Inflammation, and Immunity. Cancer J. 2017, 23, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Majak, P.; Braathen, B.; Bjornstad, J.L.; Tonnessen, T. GDF-15 is increased during early reperfusion following aortic valve replacement. Scand. Cardiovasc. J. 2012, 46, 99–106. [Google Scholar] [CrossRef] [PubMed]

| Population (n = 62) | Controls (n = 15) | GDF15 T1-T2 (754–1643 ng/L) (n = 23) | GDF15 T3 (2255–2725 ng/L) (n = 39) | T1-T2 vs. T3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N (Median/Mean) | % (95% CI) | N (Median/Mean) | % (95% CI) | p * | N (Median/Mean) | % (95% CI) | N (Median/Mean) | % (95% CI) | p | |
| Gender (Female) | 34 | 54.8% | 8 | 55% | ns | 12 | 52.1% | 22 | 56.4% | 0.5 |
| Age (years) | 79.3 | 77.3–81.3 | 78.2 | 76.5–80.2 | ns | 76.2 | 73.3–79.2 | 81 | 79.4–82.5 | 0.004 |
| BMI (kg/m2) | 26.6 | 25.6–27.6 | 25.5 | 25.2–27.7 | ns | 26.6 | 25–28.2 | 26.6 | 25.2–27.9 | 0.9 |
| BSA (m2) | 1.8 | 1.81–1.95 | 1.75 | 1.71–1.96 | ns | 1.88 | 1.8–1.96 | 1.81 | 1.75–1.87 | 0.2 |
| Heart Rate (bpm) | 74 | 69.3–78.7 | 71 | 68.1–79.6 | ns | 72.1 | 67.9–76.3 | 77.3 | 73–81.7 | 0.1 |
| Atrial Fibrillation | 6 | 9.6% | 0 | 0% | NA | 1 | 4.3% | 5 | 12.8% | 0.06 |
| SAP (mmHg) | 140.2 | 132.9–147.5 | 140.2 | 132.9–147.5 | ns | 135.6 | 128.9–142.3 | 140.2 | 133.2–147.2 | 0.4 |
| DAP (mmHg) | 71.1 | 67.2–74.9 | 71.8 | 67–75.3 | ns | 71 | 66.9–75.1 | 71.3 | 67.1–75.4 | 0.9 |
| Smokers | 17 | 27.4% | 0 | 0% | NA | 7 | 30.4% | 10 | 25.6% | 0.07 |
| Anemia | 20 | 32.2% | 0 | 0% | NA | 6 | 26% | 14 | 35.8% | 0.08 |
| Diabetes Mellitus | 17 | 27.4% | 0 | 0% | NA | 7 | 30.4% | 10 | 25.6% | 0.07 |
| Hypertension | 57 | 92% | 13.5 | 90% | ns | 21 | 91.3% | 36 | 92.3% | 0.07 |
| Coronary Artery Disease | 19 | 30.6% | 0 | 0% | NA | 7 | 30% | 12 | 30.7% | 0.6 |
| COPD | 15 | 24.1% | 0 | 0% | NA | 4 | 17.3% | 11 | 28.2% | 0.06 |
| NYHA class I/II | 38 | 61.2% | 15 | 100% | <0.0001 | 20 | 86.9% | 18 | 46.1% | 0.03 |
| NYHA class III | 24 | 38.7% | 0 | 0% | NA | 7 | 30% | 17 | 43.6% | 0.04 |
| Katz Frailty Score ≥5 | 52 | 83.8% | NA | NA | NA | 22 | 95.6% | 30 | 77% | 0.05 |
| Katz Frailty Score <5 | 10 | 16.1% | NA | NA | NA | 1 | 4.3% | 9 | 23% | 0.0001 |
| eGFR (mL/min/1.73 m2) | 63.3 | 56.6–70 | 73.3 | 68.8–75.4 | <0.0001 | 69.4 | 62.2–73.6 | 54.2 | 47.6–60.9 | 0.0001 |
| Euroscore II (%) | 3.8 | 2.7–4.9 | NA | NA | NA | 3.4 | 1.2–5.7 | 4 | 2.9–5.2 | 0.6 |
| GDF-15 (pg/mL) | 1772.4 | 1573.5–1971.3 | 774.9 | 599.5–950.2 | <0.0001 | 1253.8 | 1115.5–1392.2 | 2490.4 | 2255–2725.7 | <0.0001 |
| NT-pro-BNP (ng/L) | 918 | 588.2–1433.6 | 33.7 | 16.7–50.6 | 0.0001 | 140 | 78–251.2 | 1048.5 | 510.2–1371.4 | <0.0001 |
| TnT-Hs (ng/L) | 89.5 | 20.4–110.7 | 4.8 | 4–5.6 | 0.04 | 24.8 | 4.8–44.8 | 54.1 | 49.5–98.8 | 0.0001 |
| Albumin (g/dl) | 3.8 | 3–4.4 | 4.2 | 4–4.6 | ns | 3.5 | 2.9–3.8 | 3.6 | 3.1–4.1 | 0.8 |
| Population (n = 62) | Controls (n = 15) | GDF15 T1-T2 (754-1643 ng/L) (n = 23) | GDF15 T3 (2255-2725 ng/L) (n = 39) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N (Median/Mean) | % (95% CI) | N (Median/Mean) | % (95% CI) | p * | N (Median/Mean) | % (95% CI) | N (Median/Mean) | % (95% CI) | p | |
| LV EDVi (mL/m2) | 53 | 48.6–57.4 | 56 | 49.6–59.4 | ns | 50 | 44.3–56.9 | 54.3 | 48.3–60.4 | 0.4 |
| LV ESVi (mL/m2) | 28.1 | 24.3–31.9 | 26.1 | 23.3–33.9 | ns | 26.9 | 22.3–31.6 | 28.8 | 23.2–34.3 | 0.6 |
| EF (%) | 60.7 | 57.5–63.8 | 62.7 | 58.5–65.8 | ns | 62.7 | 59.9–65.4 | 59.9 | 56.6–63.3 | 0.2 |
| GLS (%) | 13.9 | 12.9–15 | 17.2 | 15.9–19 | <0.0001 | 15.9 | 14.7–17.2 | 13.7 | 12.7–15.1 | 0.01 |
| RWT | 0.50 | 0.47–0.53 | 0.38 | 0.35–0.42 | <0.0001 | 0.5 | 0.46–0.53 | 0.51 | 0.48–0.54 | 0.5 |
| LVMi (g/m2) | 130.1 | 119.6–140.5 | 80.2 | 65.4–88.3 | <0.0001 | 111.4 | 101.2–121.6 | 132.5 | 124.3–140.7 | 0.001 |
| LAVi (cm2/m2) | 44.1 | 37–47 | 26 | 22.2–31.1 | <0.0001 | 41.7 | 35.1–46.4 | 47.3 | 41.3–50.2 | 0.04 |
| E/A | 0.8 | 0.6–0.9 | 1 | 0.8–1.2 | ns | 0.7 | 0.6–0.8 | 0.8 | 0.7–0.9 | 0.8 |
| E/e’average | 15.8 | 13.1–17.9 | 11.5 | 9.1–13.2 | 0.04 | 16.3 | 13.4–19.1 | 16.9 | 14.4–19.4 | 0.7 |
| Stroke Volume index (mL/m2) | 35 | 31.4–38.6 | 49 | 45–65 | <0.0001 | 36.7 | 31.9–41.5 | 33.4 | 30.2–36.5 | 0.2 |
| AVAi (mL/m2) | 0.36 | 0.34–0.4 | NA | NA | NA | 0.4 | 0.35–0.43 | 0.38 | 0.33–0.41 | 0.4 |
| Peak Trans-aortic velocity (m/s) | 4.4 | 4.2–4.6 | 1.6 | 1.5–2.1 | <0.0001 | 4.4 | 4.2–4.7 | 4.3 | 4.2–4.5 | 0.4 |
| Maximum pressure gradient (mmHg) | 79.9 | 73.6–86.3 | NA | NA | NA | 82.5 | 73.8–91.1 | 77.5 | 73.1–82.4 | 0.3 |
| Mean pressure gradient (mmHg) | 49.7 | 45.7–53.8 | NA | NA | NA | 50.3 | 44.7–55.9 | 47.7 | 44.6–50.7 | 0.4 |
| ZVa (mmHg/mL/m2) | 5.4 | 4.9–5.8 | NA | NA | NA | 5.2 | 4.5–5.8 | 5.4 | 4.9–5.8 | 0.6 |
| Estimated sPAP | 33.1 | 29.8–32.4 | 28.8 | 27.9–31.3 | 0.03 | 30.5 | 27.9–33 | 33.9 | 30.1–37.7 | 0.2 |
| TAPSE (mm) | 19 | 16.7–24 | 20 | 18.3–25.2 | 0.04 | 18 | 17.5–23.3 | 17 | 16.8–22.8 | 0.6 |
| Independent Variables | OR | 95% CI | p | OR | 95%CI | p |
|---|---|---|---|---|---|---|
| GLS% | 1.062 | 1.002–1.112 | 0.04 | 0.07 | ||
| Age (years) | 1.065 | 1.002–1.114 | 0.05 | 0.08 | ||
| GDF-15 (pg/mL) | 1.2 | 1001–1.324 | 0.02 | 1.06 | 1–1.123 | 0.03 |
| LVMi (g/m2) | 0.9 | 0.8–0.987 | 0.04 | 0.1 | ||
| NYHA class | 0.85 | 0.78–0.943 | 0.03 | 0.2 | ||
| eGFR (mL/min/1.73 m2) | 1.12 | 1.004–1.253 | 0.038 | 0.09 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabiani, I.; Santoni, T.; Angelillis, M.; Petricciuolo, S.; Colli, A.; Pellegrini, G.; Mazzei, D.; Pugliese, N.R.; Petronio, A.S.; De Caterina, R. Growth Differentiation Factor 15 in Severe Aortic Valve Stenosis: Relationship with Left Ventricular Remodeling and Frailty. J. Clin. Med. 2020, 9, 2998. https://doi.org/10.3390/jcm9092998
Fabiani I, Santoni T, Angelillis M, Petricciuolo S, Colli A, Pellegrini G, Mazzei D, Pugliese NR, Petronio AS, De Caterina R. Growth Differentiation Factor 15 in Severe Aortic Valve Stenosis: Relationship with Left Ventricular Remodeling and Frailty. Journal of Clinical Medicine. 2020; 9(9):2998. https://doi.org/10.3390/jcm9092998
Chicago/Turabian StyleFabiani, Iacopo, Tatiana Santoni, Marco Angelillis, Serena Petricciuolo, Andrea Colli, Giovanni Pellegrini, Deborah Mazzei, Nicola Riccardo Pugliese, Anna Sonia Petronio, and Raffaele De Caterina. 2020. "Growth Differentiation Factor 15 in Severe Aortic Valve Stenosis: Relationship with Left Ventricular Remodeling and Frailty" Journal of Clinical Medicine 9, no. 9: 2998. https://doi.org/10.3390/jcm9092998
APA StyleFabiani, I., Santoni, T., Angelillis, M., Petricciuolo, S., Colli, A., Pellegrini, G., Mazzei, D., Pugliese, N. R., Petronio, A. S., & De Caterina, R. (2020). Growth Differentiation Factor 15 in Severe Aortic Valve Stenosis: Relationship with Left Ventricular Remodeling and Frailty. Journal of Clinical Medicine, 9(9), 2998. https://doi.org/10.3390/jcm9092998






