Ground Reaction Forces Are Predicted with Functional and Clinical Tests in Healthy Collegiate Students
Abstract
:1. Introduction
2. Experimental Section
2.1. Test 1—Fat Free Mass (FFM) Assessment
2.2. Test 2—Ankle Dorsiflexion Passive Range of Motion (DPROM)
2.3. Test 3—Two-Legged, Overhead Deep Squat (ODS)
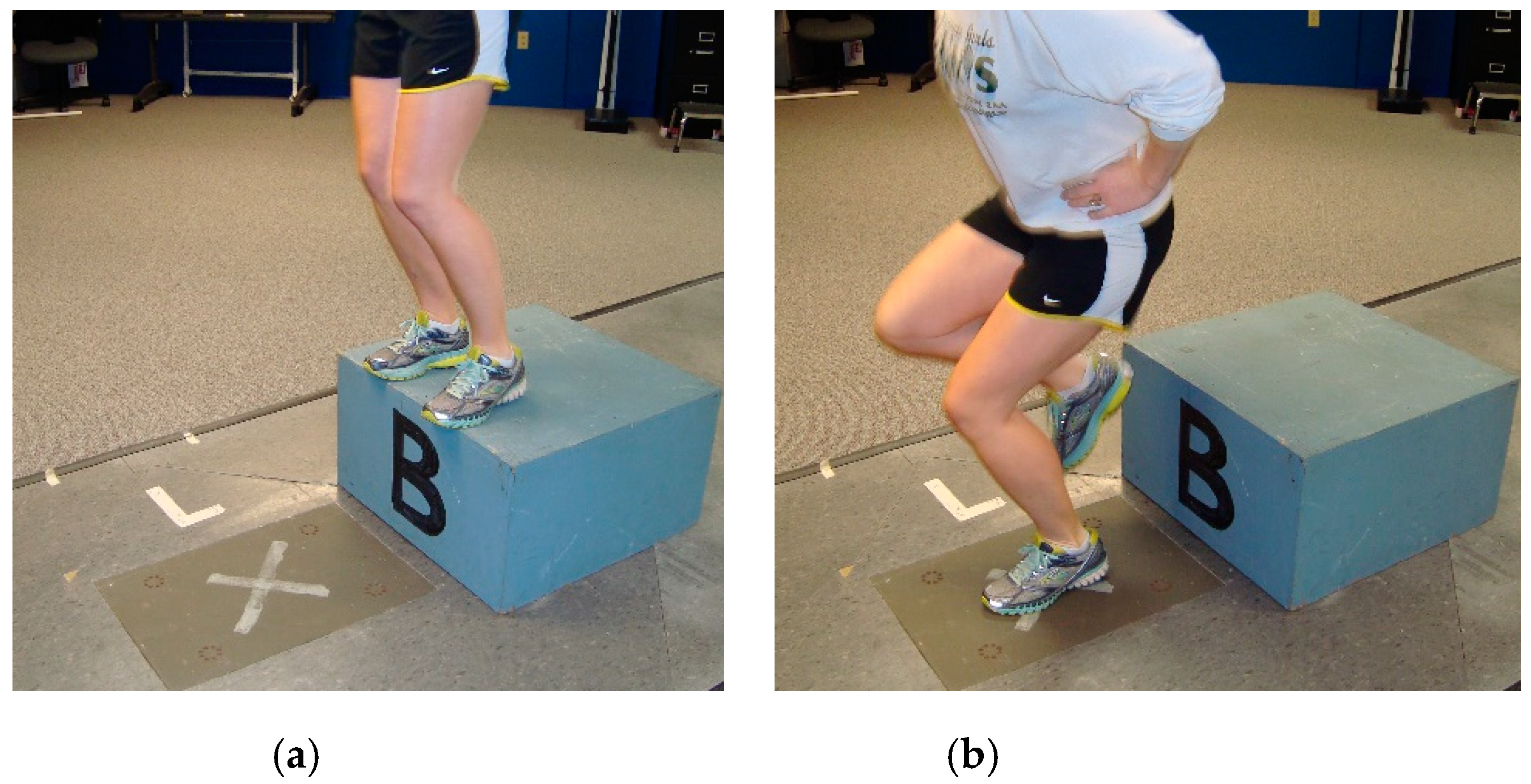
2.4. Test 4—Landing Kinetics
2.5. Test 5—Lower Extremity Power Measured with the Margaria–Kalamen Test (MK)
2.6. Test 6—Test E—Single-Limb Triple Hop (SLTH)
2.7. Test 7—Hamstring to Quadriceps Ratio of Isometric Peak Force Contraction (H:Q)
2.7.1. Knee Flexor Isometric Peak Force Contraction (KF)
2.7.2. Knee Extensor Isometric Peak Force Contraction (KE)
2.8. Test 8—Hip Lateral Rotator Isometric Peak Force Contraction (HipLR)
2.8.1. Statistical Analyses
2.8.2. Power Analysis
3. Results Section
3.1. Demographic Analysis
3.2. Descriptive Analysis
3.3. Correlation and Chi-Squared Analysis
3.4. Regression Analysis with the Examination of Residuals and Outliers
3.5. Post Hoc Power Analysis
4. Discussion
4.1. Examination of the nGRFz Model
4.2. Examination of the nGRFy Model
4.3. Selection of Examined Variables
4.4. Clinical Implications
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Griffin, L.Y.; Albohm, M.J.; Arendt, E.A.; Bahr, R.; Beynnon, B.D.; DeMaio, M.; Dick, R.W.; Engebretsen, L.; Garrett, W.E., Jr. Understanding and preventing noncontact anterior cruciate ligament injuries: A review of the Hunt Valley II meeting, January 2005. Am. J. Sports Med. 2006, 34, 1512–1532. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Bosque, J.; Meehan, J.; Jamali, A.; Marder, R. Increase in outpatient knee arthroscopy in the United States: A comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J. Bone Jt. Surg. 2011, 93, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Mall, N.A.; Chalmers, P.N.; Moric, M.; Tanaka, M.J.; Cole, B.J.; Bach, B.R.; Paletta, G.A. Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am. J. Sports Med. 2014, 42, 2363–2370. [Google Scholar] [CrossRef] [PubMed]
- Mather, R.C.; Koenig, L.; Kocher, M.S.; Dall, T.M.; Gallo, P.; Scott, D.J.; Bach, B.B., Jr.; the MOON Knee Group; Spindler, K.P. Societal and economic impact of anterior cruciate ligament tears. J. Bone Jt. Surg. 2013, 95, 1751. [Google Scholar] [CrossRef] [Green Version]
- Shelbourne, K.D.; Gray, T. Minimum 10-Year results after anterior cruciate ligament reconstruction: How the loss of normal knee motion compounds other factors related to the development of osteoarthritis after surgery. Am. J. Sports Med. 2009, 37, 471–480. [Google Scholar] [CrossRef]
- Hewett, T.E.; Di Stasi, S.L.; Myer, G.D. Current concepts for injury prevention in athletes after anterior cruciate ligament reconstruction. Am. J. Sports Med. 2012, 41, 216–224. [Google Scholar] [CrossRef] [Green Version]
- Kvist, J.; Ek, A.; Sporrstedt, K.; Good, L. Fear of re-injury: A hindrance for returning to sports after anterior cruciate ligament reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2005, 13, 393–397. [Google Scholar] [CrossRef]
- Österberg, A.; Kvist, J.; Dahlgren, M.A. Ways of experiencing participation and factors affecting the activity level after nonreconstructed anterior cruciate ligament injury: A qualitative study. J. Orthop. Sports Phys. Ther. 2013, 43, 172–183. [Google Scholar] [CrossRef] [Green Version]
- van Mechelen, W. Sports injury surveillance systems, “One size fits all?”. Sports Med. 1997, 24, 164–168. [Google Scholar] [CrossRef]
- Renstrom, P.; Ljungqvist, A.; Arendt, E.; Beynnon, B.; Fukubayashi, T.; Garrett, W.; Georgoulis, T.; Hewett, T.E.; Johnson, R.; Krosshaug, T.; et al. Non-contact ACL injuries in female athletes: An International Olympic Committee current concepts statement. Br. J. Sports Med. 2008, 42, 394–412. [Google Scholar] [CrossRef] [Green Version]
- Huston, L.J.; Greenfield, M.L.V.; Wojtys, E.M. Anterior cruciate ligament injuries in the female athlete. Clin. Orthop. Relat. Res. 2000, 372, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Orchard, J.; Seward, H.; McGivern, J.; Hood, S. Intrinsic and extrinsic risk factors for anterior cruciate ligament injury in Australian footballers. Am. J. Sports Med. 2001, 29, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Ireland, M.L. Anterior cruciate ligament injury in female athletes: Epidemiology. J. Athl. Train. 1999, 34, 150–154. [Google Scholar] [PubMed]
- Smith, H.C.; Johnson, R.J.; Shultz, S.J.; Tourville, T.; Holterman, L.A.; Slauterbeck, J.; Vacek, P.M.; Beynnon, B.D. A prospective evaluation of the Landing Error Scoring System (LESS) as a screening tool for anterior cruciate ligament injury risk. Am. J. Sports Med. 2011, 40, 521–526. [Google Scholar] [CrossRef]
- Hewett, T.E.; Myer, G.D.; Ford, K.R.; Heidt, R.S.; Colosimo, A.J.; McLean, S.G.; van den Bogert, A.J.; Paterno, M.V.; Succop, P. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes. Am. J. Sports Med. 2005, 33, 492–501. [Google Scholar] [CrossRef] [Green Version]
- Podraza, J.T.; White, S.C. Effect of knee flexion angle on ground reaction forces, knee moments and muscle co-contraction during an impact-like deceleration landing: Implications for the non-contact mechanism of ACL injury. Knee 2010, 17, 291–295. [Google Scholar] [CrossRef]
- Lin, C.-F.; Liu, H.; Gros, M.T.; Weinhold, P.; Garrett, W.E.; Yu, B. Biomechanical risk factors of non-contact ACL injuries: A stochastic biomechanical modeling study. J. Sport. Health Sci. 2012, 1, 36–42. [Google Scholar] [CrossRef] [Green Version]
- Cacolice, P.A.; Carcia, C.R.; Scibek, J.S.; Phelps, A.L. The Use of Function Tests to Predict Sagittal Plane Knee Kinematics In NCAA-D1 Female Athletes. Int. J. Sports Phys. Ther. 2015, 10, 493–504. [Google Scholar]
- Noyes, F.R.; Barber, S.D.; Mangine, R.E. Abnormal lower limb symmetry determined by function hop tests after anterior cruciate ligament rupture. Am. J. Sports Med. 1991, 19, 513–518. [Google Scholar] [CrossRef]
- Hewett, T.E.; Stroupe, A.L.; Nance, T.A.; Noyes, F.R. Plyometric training in female athletes decreased impact forces and increased hamstring torques. Am. J. Sports Med. 1996, 24, 765–773. [Google Scholar] [CrossRef]
- Hewett, T.E.; Myer, G.D.; Ford, K.R. Decreases in neuromuscular control about the knee with maturation in female athletes. J. Bone Jt. Surg. 2004, 86, 1601–1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heyward, V.H.; Wagner, D.R. Applied Body Composition Assessment, 2nd ed.; Human Kinetics Publishers: Champaign, IL, USA, 2004; ISBN 978-0-7360-4630-5. [Google Scholar]
- Jackson, A.S.; Pollock, M.L.; Ward, A. Generalized equations for predicting body density of women. Med. Sci. Sports Exerc. 1980, 12, 175–182. [Google Scholar] [CrossRef] [Green Version]
- Israel, R.G.; Houmard, J.A.; O’Brien, K.F.; McCammom, M.R.; Zamora, B.S.; Eaton, A.W. Validity of a near-infrared spectrophotometry device for estimating human body composition. Res. Q. 1989, 60, 379–383. [Google Scholar] [CrossRef]
- Watson, C.J.; Propps, M.; Ratner, J.; Zeigler, D.L.; Horton, P.; Smith, S.S. Reliability and responsiveness of the Lower Extremity Functional Scale and the anterior knee pain scale in patients with anterior knee pain. J. Orthop. Sports Phys. Ther. 2005, 35, 136–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeung, T.S.M.; Wessel, J.; Stratford, P.; MacDermid, J. Reliability, validity, and responsiveness of the Lower Extremity Functional Scale for inpatients of an orthopaedic rehabilitation ward. J. Orthop. Sports Phys. Ther. 2009, 39, 468–477. [Google Scholar] [CrossRef] [Green Version]
- Binkley, J.M.; Stratford, P.W.; Lott, S.A.; Riddle, D.L. The Lower Extremity Functional Scale (LEFS): Scale development, measurement properties, and clinical application. Phys. Ther. 1999, 79, 371–383. [Google Scholar] [PubMed]
- Beighton, P.; Horan, F. Orthopaedic aspects of Ehlers-Danlos Syndrome. J. Bone Jt. Surg. 1969, 51-B, 444–453. [Google Scholar] [CrossRef]
- Smits-Engelsman, B.; Klerks, M.; Kirby, A. Beighton score: A valid measure for generalized hypermobility in children. J. Pediatr. 2011, 158, 119–123.e4. [Google Scholar] [CrossRef] [PubMed]
- Knapik, J.J.; Bauman, C.L.; Jones, B.H.; Harris, J.M.; Vaughan, L. Preseason strength and flexibility imbalances associated with athletic injuries in female collegiate athletes. Am. J. Sports Med. 1991, 19, 76–81. [Google Scholar] [CrossRef]
- Padua, D.A.; Carcia, C.R.; Arnold, B.L.; Granata, K.P. Gender differences in leg stiffness and stiffness recruitment strategy during two-legged hopping. J. Motor. Behav. 2005, 37, 111–126. [Google Scholar] [CrossRef] [Green Version]
- Schantz, P.; Randall-Fox, E.; Hutchison, W.; Tydén, A.; Åstrand, P.-O. Muscle fibre type distribution, muscle cross-sectional area and maximal voluntary strength in humans. Acta Physiol. Scand. 1983, 117, 219–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maughan, R.J.; Watson, J.S.; Weir, J. Muscle strength and cross-sectional area in man: A comparison of strength-trained and untrained subjects. Br. J. Sports Med. 1984, 18, 149–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuda, K.; Kikuhara, N.; Takahashi, H.; Yamanaka, K. The relationship between muscle cross-sectional area and strength in various isokinetic movements among soccer players. J. Sports Sci. 2003, 21, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Baechle, T.R.; Earle, R.W. Essentials of Strength Training & Conditioning, 2nd ed.; Human Kinetics Publishers: Champaign, IL, USA, 2000; ISBN 0-7360-0089-5. [Google Scholar]
- Jackson, A.S.; Pollock, M.L.; Gettman, L.R. Intertester reliability of selected skinfold and circumference measurements and percent fat estimates. Res. Q. 1978, 49, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Lohman, T.G.; Pollock, M.L. Which caliper? How much training? JOPER 1981, 52, 27–29. [Google Scholar] [CrossRef]
- Gruber, J.J.; Pollock, M.L.; Graves, J.E.; Colvin, A.B.; Braith, R.W. Comparison of Harpenden and Lange calipers in predicting body composition. Res. Q. Exerc. Sport 1990, 61, 184–190. [Google Scholar] [CrossRef]
- Katch, F.I.; Katch, V.L. Measurement and prediction errors in body composition assessment and the search for the perfect prediction equation. Res. Q. Exerc. Sport 1980, 51, 249–260. [Google Scholar] [CrossRef]
- Portney, L.G.; Watkins, M.P. Foundations of Clinical Research: Applications to Practice, 3rd ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2009; ISBN 978-0-13-171640-7. [Google Scholar]
- Martin, R.L.; Davenport, T.E.; Paulseth, S.; Wukich, D.K.; Godges, J.J. Ankle stability and movement coordination impairments: Ankle ligament sprains: Clinical Practice Guidelines linked to the International Classification of Functioning, Disability and Health from the Orthopaedic section of the American Physical Therapy Association. J. Ortho. Sport Phys. Ther. 2013, 43, A1–A40. [Google Scholar] [CrossRef] [Green Version]
- Fong, C.M.; Blackburn, J.T.; Norcross, M.F.; McGrath, M.; Padua, D.A. Ankle-dorsiflexion range of motion and landing biomechanics. J. Athl. Train. 2011, 46, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Konor, M.M.; Morton, S.; Eckerson, J.M.; Grindstaff, T.L. Reliability of three measures of ankle dorsiflexion range of motion. Int. J. Sports Phys. Ther. 2012, 7, 279–287. [Google Scholar]
- Zhang, S.-N.; Bates, B.T.; Dufek, J.S. Contribution of lower extremity joints to energy dissipation during landings. Med. Sci. Sports Exerc. 2000, 32, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Chappell, J.D.; Yu, B.; Kirkendall, D.T.; Garrett, W.E. A comparison of knee kinetics between male and female recreational athletes in stop-jump tasks. Am. J. Sports Med. 2002, 30, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Hetzler, R.K.; Vogelpohl, R.E.; Stickley, C.D.; Kuramoto, A.N.; DeLaura, M.R.; Kimura, I.F. Development of a modified Margaria-Kalamen anaerobic power test for American football athletes. J. Strength Cond. Res. 2010, 24, 978–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayhew, J.; Piper, F.; Etheridge, G.; Schwegler, T.; Beckenholdt, S.; Thomas, M. The Margaria-Kalamen anaerobic power test: Norms and correlates. J. Hum. Mov. Stud. 1990, 18, 141–150. [Google Scholar]
- Munro, A.; Herrington, L.; Comfort, P. Comparison of landing knee valgus angle between female basketball and football athletes: Possible implications for anterior cruciate ligament and patellofemoral joint injury rates. Phys. Ther. Sport 2012, 13, 259–264. [Google Scholar] [CrossRef]
- Ross, M.D.; Langford, B.; Whelan, P.J. Test-retest reliability of 4 single-leg horizontal hop tests. J. Strength Cond. Res. 2002, 16, 617–622. [Google Scholar]
- Drouin, J.M.; Riemann, B.L. Lower extremity functional-performance testing, Part 1. Int. J. Athl. Ther. Train. 2004, 9, 46–49. [Google Scholar] [CrossRef]
- Bolga, L.A.; Keskula, D.R. Reliability of lower extremity functional performance tests. J. Ortho. Sport Phys. Ther. 1997, 26, 138–142. [Google Scholar] [CrossRef]
- Hislop, H.J.; Montgomery, J. Daniels & Worthinghams’s Muscle Testing: Techniques of Manual Examination, 7th ed.; W.B. Saunders Company: St Louis, MO, USA, 2002; ISBN 0-7216-9299-0. [Google Scholar]
- Kelln, B.M.; McKeon, P.O.; Gontkof, L.M.; Hertel, J. Hand-held dynamometry: Reliability of lower extremity muscle testing in healthy, physically active, young adults. J. Sport Rehabil. 2008, 17, 160–170. [Google Scholar] [CrossRef]
- Bojsen-Møller, J.; Magnusson, S.P.; Rasmussen, L.R.; Kjaer, M.; Aagaard, P. Muscle performance during maximal isometric and dynamic contractions is influenced by the stiffness of the tendinous structures. J. Appl. Physiol. 2005, 99, 986–994. [Google Scholar] [CrossRef] [Green Version]
- Haff, G.; Triplett, N.T.; National Strength & Conditioning Association (U.S.) (Eds.) Essentials of Strength Training and Conditioning, 4th ed.; Human Kinetics: Champaign, IL, USA, 2016; ISBN 978-1-4925-0162-6. [Google Scholar]
- Lawrence, R.K.; Kernozek, T.W.; Miller, E.J.; Torry, M.R.; Reuteman, P. Influences of hip external rotation strength on knee mechanics during single-leg drop landings in females. Clin. Biomech. 2008, 23, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Lynall, R.C.; Mauntel, T.C.; Padua, D.A.; Mihalik, J.P. Acute lower extremity injury rates increase following concussion in college athletes. Med. Sci. Sports Exerc. 2015, 47, 2487–2492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, B.; Lin, C.-F.; Garrett, W.E. Lower extremity biomechanics during the landing of a stop-jump task. Clin. Biomech. 2006, 21, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Decker, M.J.; Torry, M.R.; Wyland, D.J.; Sterett, W.I.; Richard Steadman, J. Gender differences in lower extremity kinematics, kinetics and energy absorption during landing. Clin. Biomech. 2003, 18, 662–669. [Google Scholar] [CrossRef]
- Ford, K.R.; Myer, G.D.; Hewett, T.E. Valgus knee motion during landing in high school female and male basketball players. Med. Sci. Sports Exerc. 2003, 35, 1745–1750. [Google Scholar] [CrossRef] [Green Version]
- Chappell, J.D.; Creighton, R.A.; Giuliani, C.; Yu, B.; Garrett, W.E. Kinematics and electromyography of landing preparation in vertical stop-jump. Am. J. Sports Med. 2007, 35, 235–241. [Google Scholar] [CrossRef]
- Sell, T.C.; Ferris, C.M.; Abt, J.P.; Tsai, Y.-S.; Myers, J.B.; Fu, F.H.; Lephart, S.M. The effect of direction and reaction on the neuromuscular and biomechanical characteristics of the knee during tasks that simulate the noncontact anterior cruciate ligament injury mechanism. Am. J. Sports Med. 2006, 34, 43–54. [Google Scholar] [CrossRef]
- Krosshaug, T.; Nakamae, A.; Boden, B.P.; Engebretsen, L.; Smith, G.; Slauterbeck, J.R.; Hewett, T.E.; Bahr, R. Mechanisms of anterior cruciate ligament injury in basketball: Video analysis of 39 cases. Am. J. Sports Med. 2006, 35, 359–367. [Google Scholar] [CrossRef]
- Kernozek, T.W.; Torry, M.R.; Van Hoof, H.; Cowley, H.; Tanner, S. Gender differences in frontal and sagittal plane biomechanics during drop landings. Med. Sci. Sports Exerc. 2005, 37, 1003–1012. [Google Scholar] [CrossRef]
- Shultz, S.J.; Schmitz, R.J. Effects of transverse and frontal plane knee laxity on hip and knee neuromechanics during drop landings. Am. J. Sports Med. 2009, 37, 1821–1830. [Google Scholar] [CrossRef] [Green Version]
- Salci, Y.; Kentel, B.B.; Heycan, C.; Akin, S.; Korkusuz, F. Comparison of landing maneuvers between male and female college volleyball players. Clin. Biomech. 2004, 19, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Quatman, C.E.; Ford, K.R.; Myer, G.D.; Hewett, T.E. Maturation leads to gender differences in landing force and vertical jump performance. Am. J. Sports Med. 2006, 34, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, R.J.; Kulas, A.S.; Perrin, D.H.; Riemann, B.L.; Shultz, S.J. Sex differences in lower extremity biomechanics during single leg landings. Clin. Biomech. 2007, 22, 681–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norcross, M.F.; Blackburn, J.T.; Goerger, B.M.; Padua, D.A. The association between lower extremity energy absorption and biomechanical factors related to anterior cruciate ligament injury. Clin. Biomech. 2010, 25, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, R.T.; Shultz, S.J.; Schmitz, R.J.; Perrin, D.H. Triple-hop distance as a valid predictor of lower limb strength and power. J. Athl. Train. 2008, 43, 144–151. [Google Scholar] [CrossRef] [Green Version]
- Bodor, M. Quadriceps protects the anterior cruciate ligament. J. Orthop. Res. 2001, 19, 629–633. [Google Scholar] [CrossRef]
- Logerstedt, D.; Di Stasi, S.; Grindem, H.; Lynch, A.; Eitzen, I.; Engebretsen, L.; Risberg, M.A.; Axe, M.J.; Snyder-Mackler, L. Self-reported knee function can identify athletes who fail return-to-activity criteria up to 1 year after anterior cruciate ligament reconstruction: A Delaware-Oslo ACL cohort study. J. Orthop. Sports Phys. Ther. 2014, 44, 914–923. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, L.C.; Paterno, M.V.; Ford, K.R.; Myer, G.D.; Hewett, T.E. Strength asymmetry and landing mechanics at return to sport after anterior cruciate ligament reconstruction. Med. Sci. Sports Exerc. 2015, 47, 1426–1434. [Google Scholar] [CrossRef] [Green Version]
- Myklebust, G.; Engebretsen, L.; Brækken, I.H.; Skjølberg, A.; Olsen, O.E.; Bahr, R. Prevention of anterior cruciate ligament injuries in female team handball players: A prospective intervention study over three seasons. Clin. J. Sports Med. 2003, 13, 71–78. [Google Scholar] [CrossRef]
- Tate, J.J.; Milner, C.E.; Fairbrother, J.T.; Zhang, S. The effects of a home-based instructional program aimed at improving frontal plane knee biomechanics during a jump landing task. J. Orthop. Sports Phys. Ther. 2013, 43, 486–494. [Google Scholar] [CrossRef]
- Greska, E.K.; Cortes, N.; Van Lunen, B.L.; Onate, J.A. A feedback inclusive neuromuscular training program alters frontal plane kinematics. J. Strength Cond. Res. 2012, 26, 1609–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hewett, T.E.; Lindenfeld, T.N.; Riccobene, J.V.; Noyes, F.R. The effect of neuromuscular training on the incidence of knee injury in female athletes: A prospective study. Am. J. Sports Med. 1999, 27, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Mandelbaum, B.R.; Silvers, H.J.; Watanbe, D.S.; Knarr, J.F.; Thomas, S.D.; Griffin, L.Y.; Kirkendall, D.T.; Garrett, W., Jr. Effectiveness of a neuromuscular and proprioceptive training program in preventing anterior cruciate ligament injuries in female athletes: 2-year follow-up. Am. J. Sports Med. 2005, 33, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Sturnick, D.R.; Vacek, P.M.; DeSarno, M.J.; Gardner-Morse, M.G.; Tourville, T.W.; Slauterbeck, J.R.; Johnson, R.J.; Shultz, S.J.; Beynnon, B.D. Combined anatomic factors predicting risk of anterior cruciate ligament injury for males and females. Am. J. Sports Med. 2015, 43, 839–847. [Google Scholar] [CrossRef]
- Quatman, C.E.; Hewett, T.E. The anterior cruciate ligament injury controversy: Is “valgus collapse” a sex-specific mechanism? Br. J. Sports Med. 2009, 43, 328–335. [Google Scholar] [CrossRef]
- Boden, B.P.; Dean, G.S.; Feagin, J.A., Jr.; Garrett, W.E., Jr. Mechanisms of anterior cruciate ligament injury. Orthopedics 2000, 23, 573–578. [Google Scholar] [CrossRef]
- Olsen, O.-E.; Myklebust, G.; Engebretsen, L.; Bahr, R. Injury mechanisms for anterior cruciate ligament injuries in team handball: A systematic video analysis. Am. J. Sports Med. 2004, 32, 1002–1012. [Google Scholar] [CrossRef]
- Bendjaballah, M.; Shirazi-Adl, A.; Zukor, D. Finite element analysis of human knee joint in varus-valgus. Clin. Biomech. 1997, 12, 139–148. [Google Scholar] [CrossRef]
- Chaudhari, A.M.; Andriacchi, T.P. The mechanical consequences of dynamic frontal plane limb alignment for non-contact ACL injury. J. Biomech. 2006, 39, 330–338. [Google Scholar] [CrossRef]
- Liederbach, M.; Dilgen, F.E.; Rose, D.J. Incidence of anterior cruciate ligament injuries among elite ballet and modern dancers. Am. J. Sports Med. 2008, 36, 1779–1788. [Google Scholar] [CrossRef]
- Zazulak, B.T.; Hewett, T.E.; Reeves, N.P.; Goldberg, B.; Cholewicki, J. Deficits in neuromuscular control of the trunk predict knee injury risk: A prospective biomechanical-epidemiologic study. Am. J. Sports Med. 2007, 35, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Malloy, P.; Morgan, A.; Meinerz, C.; Geiser, C.; Kipp, K. The association of dorsiflexion flexibility on knee kinematics and kinetics during a drop vertical jump in healthy female athletes. Knee Surg. Sports Traumatol. Arthrosc. 2014. [Google Scholar] [CrossRef] [PubMed]
- Wahlstedt, C.; Rasmussen-Barr, E. Anterior cruciate ligament injury and ankle dorsiflexion. Knee Surg. Sports Traumatol. Arthrosc. 2014. [Google Scholar] [CrossRef] [PubMed]
- Myer, G.D.; Ford, K.R.; Khoury, J.; Succop, P.; Hewett, T.E. Biomechanics laboratory-based prediction algorithm to identify female athletes with high knee loads that increase risk of ACL injury. Br. J. Sports Med. 2011, 45, 245–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, K.; Driban, J.B.; Sitler, M.R.; Cattano, N.M.; Hootman, J.M. Five-year clinical outcomes of a randomized trial of anterior cruciate ligament treatment strategies: An evidence-based practice paper. J. Athl. Train. 2014, 50, 110–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arendt, E.A.; Agel, J.; Dick, R. Anterior cruciate ligament injury patterns among collegiate men and women. J. Athl. Train. 1999, 34, 86–92. [Google Scholar] [PubMed]
- Park, S.-K.; Stefanshyn, D.; Ramage, B.; Hart, D.; Ronsky, J. Relationship between knee joint laxity and knee joint mechanics during the menstrual cycle. Br. J. Sports Med. 2009, 43, 174–179. [Google Scholar] [CrossRef] [Green Version]
- Wojtys, E.M.; Huston, L.J.; Lindenfeld, T.N.; Hewett, T.E.; Greenfield, M.L.V.H. Association between the menstrual cycle and anterior cruciate ligament injuries in female athletes. Am. J. Sports Med. 1998, 26, 614–619. [Google Scholar] [CrossRef]
- Wojtys, E.M.; Huston, L.J.; Boynton, M.D.; Spindler, K.P.; Lindenfeld, T.N. The effect of the menstrual cycle on anterior cruciate ligament injuries in women as determined by hormone levels. Am. J. Sports Med. 2002, 30, 182–188. [Google Scholar] [CrossRef]
- Myklebust, G.; Maehlum, S.; Holm, I.; Bahr, R. A prospective cohort study of anterior cruciate ligament injuries in elite Norwegian team handball. Scand. J. Med. Sci. Sports 1998, 8, 149–153. [Google Scholar] [CrossRef]
- Slauterbeck, J.R.; Fuzie, S.F.; Smith, M.P.; Clark, R.J.; Xu, K.T.; Starch, D.W.; Hardy, D.M. The menstrual cycle, sex hormones, and anterior cruciate ligament injury. J. Athl. Train. 2002, 37, 275. [Google Scholar] [PubMed]
- Pollard, C.D.; Braun, B.; Hamill, J. Influence of gender, estrogen and exercise on anterior knee laxity. Clin. Biomech. (Bristol, Avon.) 2006, 21, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Beynnon, B.D.; Johnson, R.J.; Braun, S.; Sargent, M.; Bernstein, I.M.; Skelly, J.M.; Vacek, P.M. The relationship between menstrual cycle phase and anterior cruciate ligament injury: A case-control study of recreational alpine skiers. Am. J. Sports Med. 2005, 34, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.; Harter, R.A.; Hayes, B.T.; Wojtys, E.M.; Murtaugh, P. The interrelationships among sex hormone concentrations, motoneuron excitability, and anterior tibial displacement in women and men. J. Athl. Train. 2008, 43, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Carcia, C.R.; Shultz, S.J.; Granata, K.P.; Gansneder, B.M.; Perrin, D.H. Knee ligament behavior following a controlled loading protocol does not differ by menstrual cycle day. Clin. Biomech. 2004, 19, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Adachi, N.; Nawata, K.; Maeta, M.; Kurozawa, Y. Relationship of the menstrual cycle phase to anterior cruciate ligament injuries in teenaged female athletes. Arch. Orthop. Trauma Surg. 2007, 128, 473–478. [Google Scholar] [CrossRef]
- Belanger, M.J.; Moore, D.C.; Crisco, J.J., III; Fadale, P.D.; Hulstyn, M.J.; Ehrlich, M.G. Knee laxity does not vary with the menstrual cycle, before or after exercise. Am. J. Sports Med. 2004, 32, 1150–1157. [Google Scholar] [CrossRef]
- Karageanes, S.J.; Blackburn, K.; Vangelos, Z.A. The association of the menstrual cycle with the laxity of the anterior cruciate ligament in adolescent female athletes. Clin. J. Sports Med. 2000, 10, 162–168. [Google Scholar] [CrossRef]
- Blackburn, J.T.; Padua, D.A. Influence of trunk flexion on hip and knee joint kinematics during a controlled drop landing. Clin. Biomech. 2008, 23, 313–319. [Google Scholar] [CrossRef]
- Lephart, S.M.; Ferris, C.M.; Riemann, B.L.; Myers, J.B.; Fu, F.H. Gender differences in strength and lower extremity kinematics during landing. Clin. Orthop. Relat. Res. 2002, 401, 162–169. [Google Scholar] [CrossRef]
- Padua, D.A.; Marshall, S.W.; Boling, M.C.; Thigpen, C.A.; Garrett, W.E.; Beutler, A.I. The Landing Error Scoring System (LESS) is a valid and reliable clinical assessment tool of jump-landing biomechanics: The JUMP-ACL study. Am. J. Sports Med. 2009, 37, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Padua, D.A.; DiStefano, L.J.; Beutler, A.I.; de la Motte, S.J.; DiStefano, M.J.; Marshall, S.W. The Landing Error Scoring System as a Screening Tool for an Anterior Cruciate Ligament Injury–Prevention Program in Elite-Youth Soccer Athletes. J. Athl. Train. 2015, 50, 589–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pappas, E.; Hagins, M.; Sheikhzadeh, A.; Nordin, M.; Rose, D. Biomechanical differences between unilateral and bilateral landings from a jump: Gender differences. Clin. J. Sports Med. 2007, 17, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Yeow, C.H.; Lee, P.V.S.; Goh, J.C.H. An investigation of lower extremity energy dissipation strategies during single-leg and double-leg landing based on sagittal and frontal plane biomechanics. Hum. Mov. Sci. 2011, 30, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.; Steele, J.R.; Cook, J.L.; Purdam, C.R.; McGhee, D.E. Lower limb movement symmetry cannot be assumed when investigating the stop-jump landing. Med. Sci. Sports Exerc. 2011, 44, 1123–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerulli, G.; Benoit, D.L.; Lamontagne, M.; Caraffa, A.; Liti, A. In vivo anterior cruciate ligament strain behaviour during a rapid deceleration movement: Case report. Knee Surg. Sports Traumatol. Arthrosc. 2003, 11, 307–311. [Google Scholar] [CrossRef]
- Koga, H.; Nakamae, A.; Shima, Y.; Iwasa, J.; Myklebust, G.; Engebretsen, L.; Bahr, R.; Krosshaug, T. Mechanisms for noncontact anterior cruciate ligament injuries: Knee joint kinematics in 10 injury situations from female team handball and basketball. Am. J. Sports Med. 2010, 38, 2218–2225. [Google Scholar] [CrossRef]
- Lephart, S.M.; Abt, J.P.; Ferris, C.M.; Sell, T.C.; Nagai, T.; Myers, J.B.; Irrgang, J.J. Neuromuscular and biomechanical characteristic changes in high school athletes: A plyometric versus basic resistance program. Br. J. Sports Med. 2005, 39, 932–938. [Google Scholar] [CrossRef] [Green Version]
- Frontera, W.R.; Hughes, V.A.; Lutz, K.J.; Evans, W.J. A cross-sectional study of muscle strength and mass in 45-to 78-yr-old men and women. J. App. Physiol. 1991, 71, 644–650. [Google Scholar] [CrossRef]
- Lindle, R.S.; Metter, E.J.; Lynch, N.A.; Fleg, J.L.; Fozard, J.L.; Tobin, J.; Roy, T.A.; Hurley, B.F. Age and gender comparisons of muscle strength in 654 women and men aged 20–93 yr. J. App. Physiol. 1997, 83, 1581–1587. [Google Scholar] [CrossRef] [Green Version]
- Arendt, E.; Dick, R. Knee injury patterns among men and women in collegiate basketball and soccer NCAA data and review of literature. Am. J. Sports Med. 1995, 23, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Gwinn, D.E.; Wilckens, J.H.; McDevitt, E.R.; Ross, G.; Kao, T.-C. The relative incidence of anterior cruciate ligament injury in men and women at the United States Naval Academy. Am. J. Sports Med. 2000, 28, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Hootman, J.M.; Dick, R.; Agel, J. Epidemiology of collegiate injuries for 15 sports: Summary and recommendations for injury prevention initiatives. J. Athl. Train. 2007, 42, 311–319. [Google Scholar] [PubMed]
- Gray, J.; Taunton, J.; McKenzie, D.; Clement, D.; McConkey, J.; Davidson, R. A survey of injuries to the anterior cruciate ligament of the knee in female basketball players. Int. J. Sports Med. 1985, 6, 314–316. [Google Scholar] [CrossRef] [Green Version]
- Teyhen, D.S.; Shaffer, S.W.; Lorenson, C.L.; Halfpap, J.P.; Donofry, D.F.; Walker, M.J.; Dugan, J.L.; Childs, J.D. The Functional Movement Screen: A reliability study. J. Orthop. Sports Phys. Ther. 2012, 42, 530–540. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, A.-D.; Shultz, S.J.; Schmitz, R.J.; Luecht, R.M.; Perrin, D.H. A preliminary multifactorial approach describing the relationships among lower extremity alignment, hip muscle activation, and lower extremity joint excursion. J. Athl. Train. 2011, 46, 246. [Google Scholar] [CrossRef]
- Moul, J.L. Differences in selected predictors of anterior cruciate ligament tears between male and female NCAA Division I Collegiate Basketball Players. J. Athl. Train. 1998, 33, 118–121. [Google Scholar]
- Woodford-Rogers, B.; Cyphert, L.; Denegar, C.R. Risk factors for anterior cruciate ligament injury in high school and college athletes. J. Athl. Train. 1994, 29, 343–346. [Google Scholar]
- Shultz, S.J.; Schmitz, R.J.; Nguyen, A.-D.; Levine, B.J. Joint laxity is related to lower extremity energetics during a drop jump landing. Med. Sci. Sports Exerc. 2010, 42, 771–780. [Google Scholar] [CrossRef] [Green Version]
| Female Mean ± SD | Male Mean ± SD | Sex Difference Significance | |
|---|---|---|---|
| Age (years) | 20.667 ± 1.461 | 21.571 ± 1.287 | p = 0.039 * |
| Height (cm) | 65.524 ± 1.874 | 70.702 ± 2.363 | p < 0.001 * |
| Mass (kg) | 64.190 ± 9.059 | 82.202 ± 7.606 | p < 0.001 * |
| LEFS Score | 79.524 ± 1.250 | 79.143 ± 1.558 | p = 0.387 |
| Beighton Score | 2.095 ± 1.640 | 0.476 ± 0.750 | p < 0.001 * |
| Self-reported days active in past six months | 4.762 ± 1.221 | 4.667 ± 1.133 | p = 0.795 |
| Variable | Female Mean ± SD | Male Mean ± SD | Sex Difference Significance |
|---|---|---|---|
| FFM | 47.487 ± 3.684 kg * | 72.297 ± 5.835 kg | p < 0.001 * |
| DPROM | 17.444 ± 5.015° * | 13.460 ± 7.359° | p = 0.047 * |
| MK | 946.761 ± 159.423 Watts | 1412.310 ± 225.437 Watts | p < 0.001 * |
| SLTH | 429.825 ± 42.660 cm * | 539.175 ± 53.724 | p < 0.001 * |
| HipLR | 145.623 ± 27.041 N * | 206.078 ± 34.486 N | p < 0.001 * |
| H:Q | 0.828 ± 0.137 | 0.767 ± 0.092 | p = 0.097 |
| nGRFz | 4.463 ± 0.896 | 4.061 ± 0.935 | p = 0.163 |
| nGRFy | −3.801 ± 0.910 FFM * | −2.816 ± 0.989 FFM | p = 0.002 * |
| nGRFz | SLTH | MK | DPROM | H:Q | FFM | HipLR | |
|---|---|---|---|---|---|---|---|
| nGRFz | r = 1.00 | r = −0.399 * | r = −0.336 * | r = −0.335 * | r = 0.309 * | r = −0.258 | r = −0.186 |
| p = 0.009 | p = 0.030 | p = 0.030 | p = 0.047 | p = 0.098 | p = 0.238 | ||
| SLTH | r = 1.00 | r = 0.752 * | r = −0.126 | r = −0.407 * | r = 0.694 * | r = 0.511 * | |
| p < 0.001 | p = 0.427 | p = 0.008 | p < 0.001 | p < 0.001 | |||
| MK | r = 1.00 | r = −0.204 | r = −0.417 * | r = 0.753 * | r = 0.657 * | ||
| p = 0.195 | p = 0.006 | p < 0.001 | p < 0.001 | ||||
| DPROM | r = 1.00 | r = −0.221 | r = −0.295 | r = −0.302 | |||
| p = 0.159 | p = 0.058 | p = 0.052 | |||||
| H:Q | r = 1.00 | r = −0.285 | r = −0.316 * | ||||
| p = 0.068 | p = 0.042 | ||||||
| FFM | r = 1.00 | r = 0.711 * | |||||
| p < 0.001 | |||||||
| HipLR | r = 1.00 |
| nGRFy | H:Q | FFM | MK | SLTH | HipLR | DPROM | |
|---|---|---|---|---|---|---|---|
| nGRFy | r = 1.00 | r = −0.530 * | r = 0.528 * | r = 0.521 * | r = 0.459 * | r = 0.400 * | r = 0.228 |
| p < 0.001 | p < 0.001 | p < 0.001 | p = 0.002 | p = 0.009 | p = 0.147 |
| nGRFz | nGRFy | FFM | DPROM | MK | SLTH | H:Q | HipLR | |
|---|---|---|---|---|---|---|---|---|
| Sex | rpb = −0.219 p = 0.163 | rpb = 0.472 * p = 0.002 | rpb = 0.934 * p < 0.001 | rpb = −0.308 * p = 0.047 | rpb = 0.774 * p < 0.001 | rpb = 0.756 * p < 0.001 | rpb = 0.259 p = 0.097 | rpb = 0.707 * p < 0.001 |
| ODS | rpb = −0.267 p = 0.087 | rpb = 0.095 p = 0.548 | rpb = −0.125 p = 0.429 | rpb = 0.473 * p = 0.002 | rpb = 0.075 p = 0.635 | rpb = 0.095 p = 0.549 | rpb = −0.064 p = 0.688 | rpb = −0.095 p = 0.549 |
| ODS–Fail | ODS–Pass | ||
|---|---|---|---|
| Female | Number | 7 | 14 |
| Percent | 0.333 | 0.666 | |
| Male | Number | 10 | 11 |
| Percent | 0.476 | 0.524 |
| Variable | Coefficient | Error | T | p | Model Adjusted R2 | Model p |
|---|---|---|---|---|---|---|
| Constant | 7.868 | 0.916 | 8.589 | <0.001 | 0.274 | 0.001 |
| SLTH | −0.006 | 0.002 | −3.340 | 0.002 | ||
| DPROM | −0.055 | 0.019 | −2.918 | 0.006 |
| Variable | Coefficient | Error | T | p | Model Adjusted R2 | Model p |
|---|---|---|---|---|---|---|
| Constant | −4.394 | 1.373 | −3.200 | 0.003 | 0.476 | <0.001 |
| H:Q | −2.579 | 1.070 | −2.410 | 0.021 | ||
| FFM | 0.041 | 0.010 | 4.197 | <0.001 | ||
| DPROM | 0.041 | 0.020 | 2.060 | 0.046 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cacolice, P.A.; Carcia, C.R.; Scibek, J.S.; Phelps, A.L. Ground Reaction Forces Are Predicted with Functional and Clinical Tests in Healthy Collegiate Students. J. Clin. Med. 2020, 9, 2907. https://doi.org/10.3390/jcm9092907
Cacolice PA, Carcia CR, Scibek JS, Phelps AL. Ground Reaction Forces Are Predicted with Functional and Clinical Tests in Healthy Collegiate Students. Journal of Clinical Medicine. 2020; 9(9):2907. https://doi.org/10.3390/jcm9092907
Chicago/Turabian StyleCacolice, Paul A., Christopher R. Carcia, Jason S. Scibek, and Amy L. Phelps. 2020. "Ground Reaction Forces Are Predicted with Functional and Clinical Tests in Healthy Collegiate Students" Journal of Clinical Medicine 9, no. 9: 2907. https://doi.org/10.3390/jcm9092907
APA StyleCacolice, P. A., Carcia, C. R., Scibek, J. S., & Phelps, A. L. (2020). Ground Reaction Forces Are Predicted with Functional and Clinical Tests in Healthy Collegiate Students. Journal of Clinical Medicine, 9(9), 2907. https://doi.org/10.3390/jcm9092907






