Ascitic Interleukin 6 Is Associated with Poor Outcome and Spontaneous Bacterial Peritonitis: A Validation in Critically Ill Patients with Decompensated Cirrhosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Laboratory Analyses and Clinical Scores
2.3. Microbiological Analyses and Evidence of Infection
2.4. Data Collection
2.5. Statistical Analysis and Primary Endpoint
3. Results
3.1. Patients’ Characteristics and Laboratory Analyses
3.2. Levels of Ascitic Interleukin 6 in Infectious Diseases
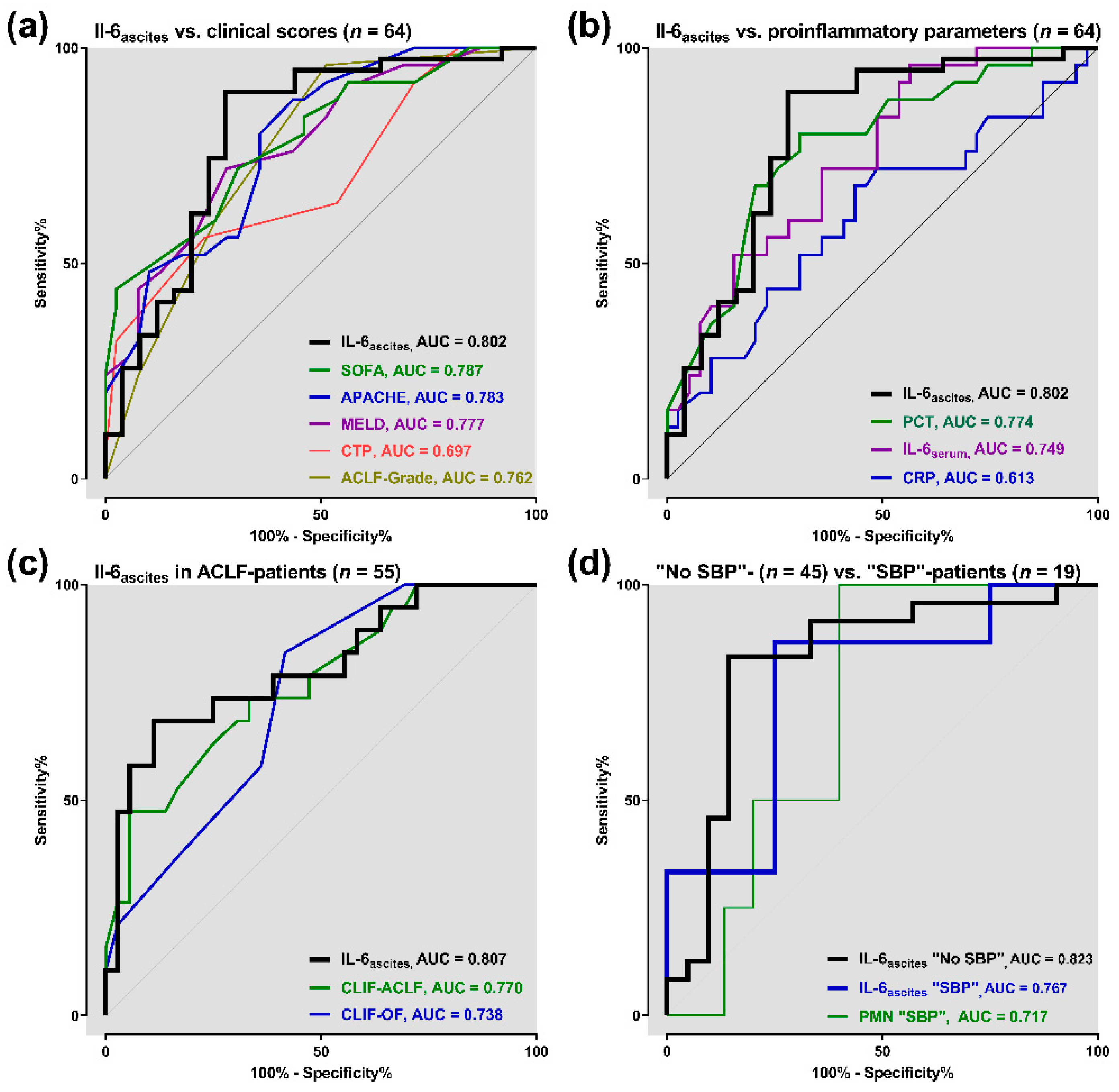
3.3. Prognostic Accuracy of Ascitic Interleukin 6
3.4. Correlation Analyses
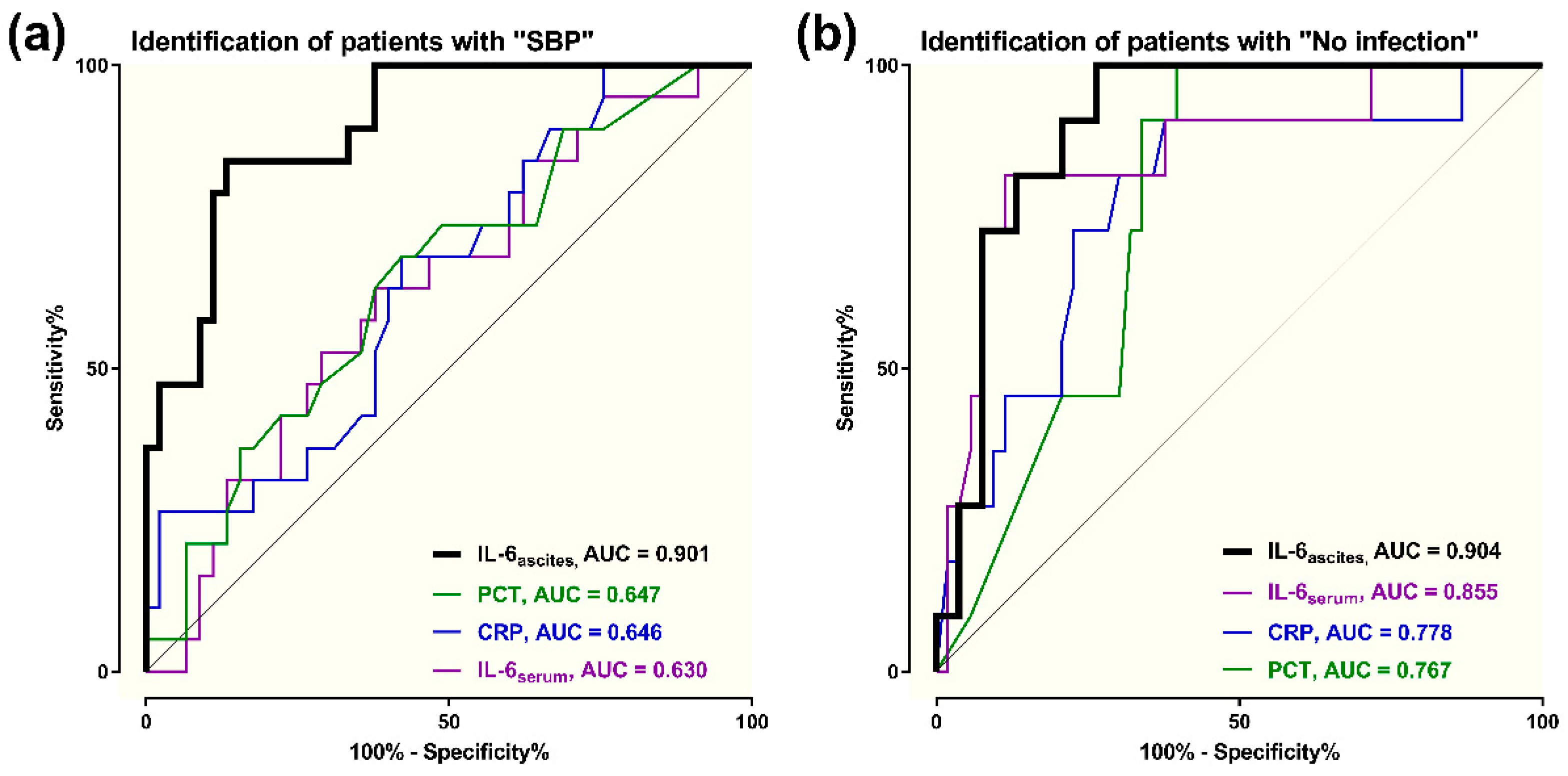
3.5. Diagnostic Accuracy of Ascitic Interleukin 6
3.6. Association of Ascitic Interleukin 6 with Renal Impairment
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ginès, P.; Quintero, E.; Arroyo, V.; Teres, J.; Bruguera, M.; Rimola, A.; Caballería, J.; Rodés, J.; Rozman, C. Compensated cirrhosis: Natural history and prognostic factors. Hepatology 1987, 7, 122–128. [Google Scholar] [CrossRef]
- Lévesque, É.; Saliba, F.; Ichai, P.; Samuel, D. Outcome of patients with cirrhosis requiring mechanical ventilation in ICU. J. Hepatol. 2014, 60, 570–578. [Google Scholar] [CrossRef]
- Bernardi, M.; Moreau, R.; Angeli, P.; Schnabl, B.; Arroyo, V. Mechanisms of decompensation and organ failure in cirrhosis: From peripheral arterial vasodilation to systemic inflammation hypothesis. J. Hepatol. 2015, 63, 1272–1284. [Google Scholar] [CrossRef]
- Malbrain, M.L.N.; Chiumello, D.; Pelosi, P.; Bihari, D.; Innes, R.; Ranieri, V.M.; Del Turco, M.; Wilmer, A.; Brienza, N.; Malcangi, V.; et al. Incidence and prognosis of intraabdominal hypertension in a mixed population of critically ill patients: A multiple-center epidemiological study. Crit. Care Med. 2005, 33, 315–322. [Google Scholar] [CrossRef]
- Fuhrmann, V.; Whitehouse, A.; Wendon, J. The ten tips to manage critically ill patients with acute-on-chronic liver failure. Intensiv. Care Med. 2018, 44, 1932–1935. [Google Scholar] [CrossRef]
- Moreau, R.; Jalan, R.; Ginès, P.; Pavesi, M.; Angeli, P.; Cordoba, J.; Durand, F.; Gustot, T.; Saliba, F.; Domenicali, M.; et al. Acute-on-Chronic Liver Failure Is a Distinct Syndrome That Develops in Patients With Acute Decompensation of Cirrhosis. Gastroenterology 2013, 144, 1426–1437. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J. Hepatol. 2018, 69, 406–460. [Google Scholar] [CrossRef] [Green Version]
- Fernández, J.; Navasa, M.; Gómez, J.; Colmenero, J.; Vila, J.; Arroyo, V.; Rodés, J.; Navasa, M. Bacterial infections in cirrhosis: Epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology 2002, 35, 140–148. [Google Scholar] [CrossRef]
- Garcia-Tsao, G. Bacterial infections in cirrhosis: Treatment and prophylaxis. J. Hepatol. 2005, 42, S85–S92. [Google Scholar] [CrossRef]
- Wong, F.; Bernardi, M.; Balk, R.; Christman, B.; Moreau, R.; Garcia-Tsao, G.; Patch, D.; Soriano, G.; Hoefs, J.; Navasa, M. Sepsis in cirrhosis: Report on the 7th meeting of the International Ascites Club. Gut 2005, 54, 718–725. [Google Scholar] [CrossRef] [Green Version]
- Bonnel, A.R.; Bunchorntavakul, C.; Reddy, K.R. Immune Dysfunction and Infections in Patients with Cirrhosis. Clin. Gastroenterol. Hepatol. 2011, 9, 727–738. [Google Scholar] [CrossRef]
- Albillos, A.; Lario, M.; Álvarez-Mon, M. Cirrhosis-associated immune dysfunction: Distinctive features and clinical relevance. J. Hepatol. 2014, 61, 1385–1396. [Google Scholar] [CrossRef] [Green Version]
- Shizuma, T. Spontaneous bacterial and fungal peritonitis in patients with liver cirrhosis: A literature review. World J. Hepatol. 2018, 10, 254–266. [Google Scholar] [CrossRef]
- Gentile, I.; Buonomo, A.R.; Scotto, R.; Zappulo, E.; Borgia, G. Infections worsen prognosis of patients with cirrhosis irrespective of the liver disease stage. Eur. J. Intern. Med. 2017, 46, e45–e47. [Google Scholar] [CrossRef]
- Alexopoulou, A.; Agiasotelli, D.; Vasilieva, L.E.; Dourakis, S.P. Bacterial translocation markers in liver cirrhosis. Ann. Gastroenterol. 2017, 30, 486–497. [Google Scholar] [CrossRef]
- Cervoni, J.-P.; Thévenot, T.; Weil, D.; Muel, E.; Barbot, O.; Sheppard, F.; Monnet, E.; Di Martino, V. C-Reactive protein predicts short-term mortality in patients with cirrhosis. J. Hepatol. 2012, 56, 1299–1304. [Google Scholar] [CrossRef]
- Papp, M.; Vitalis, Z.; Altorjay, I.; Tornai, I.; Udvardy, M.; Harsfalvi, J.; Vida, A.; Kappelmayer, J.; Lakatos, P.L.; Antal-Szalmas, P. Acute phase proteins in the diagnosis and prediction of cirrhosis associated bacterial infections. Liver Int. 2012, 32, 603–611. [Google Scholar] [CrossRef]
- Lazzarotto, C.; Ronsoni, M.F.; Fayad, L.; Nogueira, C.L.; Bazzo, M.L.; Narciso-Schiavon, J.L.; Schiavon, L.L.; Dantas-Corrêa, E.B. Acute phase proteins for the diagnosis of bacterial infection and prediction of mortality in acute complications of cirrhosis. Ann. Hepatol. 2013, 12, 431–439. [Google Scholar] [CrossRef]
- Akira, S.; Taga, T.; Kishimoto, T. Interleukin-6 in Biology and Medicine. Adv. Immunol. 1993, 54, 1–78. [Google Scholar] [CrossRef]
- Kishimoto, T. Interleukin-6: Discovery of a pleiotropic cytokine. Arthritis Res. Ther. 2006, 8, S2. [Google Scholar] [CrossRef] [Green Version]
- Harbarth, S.; Holeckova, K.; Froidevaux, C.; Pittet, D.; Ricou, B.; Grau, G.E.; Vadas, L.; Pugin, J.; Network, T.G.S. Diagnostic Value of Procalcitonin, Interleukin-6, and Interleukin-8 in Critically Ill Patients Admitted with Suspected Sepsis. Am. J. Respir. Crit. Care Med. 2001, 164, 396–402. [Google Scholar] [CrossRef] [Green Version]
- Jawa, R.S.; Anillo, S.; Huntoon, K.; Baumann, H.; Kulaylat, M. Analytic Review: Interleukin-6 in Surgery, Trauma, and Critical Care: Part I: Basic Science. J. Intensiv. Care Med. 2011, 26, 3–12. [Google Scholar] [CrossRef]
- Jawa, R.S.; Anillo, S.; Huntoon, K.; Baumann, H.; Kulaylat, M. Interleukin-6 in Surgery, Trauma, and Critical Care Part II: Clinical Implications. J. Intensiv. Care Med. 2010, 26, 73–87. [Google Scholar] [CrossRef]
- Viallon, A.; Zeni, F.; Pouzet, V.; Lambert, C.; Quenet, S.; Aubert, G.; Guyomarch, S.; Tardy, B.; Bertrand, J.-C. Serum and ascitic procalcitonin levels in cirrhotic patients with spontaneous bacterial peritonitis: Diagnostic value and relationship to pro-inflammatory cytokines. Intensiv. Care Med. 2000, 26, 1082–1088. [Google Scholar] [CrossRef]
- Suliman, M.A.; Khalil, F.M.; Alkindi, S.S.; Pathare, A.V.; Almadhani, A.A.; Soliman, N.A. Tumor necrosis factor-α and interleukin-6 in cirrhotic patients with spontaneous bacterial peritonitis. World J. Gastrointest. Pathophysiol. 2012, 3, 92–98. [Google Scholar] [CrossRef]
- Rimola, A.; Garcia-Tsao, G.; Navasa, M.; Piddock, L.J.; Planas, R.; Bernard, B.; Inadomi, J.M. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: A consensus document. J. Hepatol. 2000, 32, 142–153. [Google Scholar] [CrossRef]
- Moore, K.P.; Aithal, G.P. Guidelines on the management of ascites in cirrhosis. Gut 2006, 55 (Suppl. S6), vi1–vi12. [Google Scholar] [CrossRef] [Green Version]
- Jalan, R.; Saliba, F.; Pavesi, M.; Amorós, A.; Moreau, R.; Ginès, P.; Lévesque, É.; Durand, F.; Angeli, P.; Caraceni, P.; et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J. Hepatol. 2014, 61, 1038–1047. [Google Scholar] [CrossRef]
- Mayr, U.; Karsten, E.; Lahmer, T.; Rasch, S.; Thies, P.; Henschel, B.; Fischer, G.; Schmid, R.M.; Huber, W. Impact of large volume paracentesis on respiratory parameters including transpulmonary pressure and on transpulmonary thermodilution derived hemodynamics: A prospective study. PLoS ONE 2018, 13, e0193654. [Google Scholar] [CrossRef] [Green Version]
- Gines, A.; Fernandez-Esparrach, G.; Monescillo, A.; Vila, C.; Domenech, E.; Abecasis, R.; Angeli, P.; Ruiz-Del-Arbol, L.; Planas, R.; Sola, R.; et al. Randomized trial comparing albumin, dextran 70, and polygeline in cirrhotic patients with ascites treated by paracentesis. Gastroenterology 1996, 111, 1002–1010. [Google Scholar] [CrossRef]
- Pelletier, G.; Salmon, D.; Ink, O.; Hannoun, S.; Attali, P.; Buffet, C.; Etienne, J. Culture-negative neutrocytic ascites: A less severe variant of spontaneous bacterial peritonitis. J. Hepatol. 1990, 10, 327–331. [Google Scholar] [CrossRef]
- Chen, T.-A.; Tsao, Y.-C.; Chen, A.; Lo, G.-H.; Lin, C.-K.; Yu, H.-C.; Cheng, L.-C.; Hsu, P.-I.; Tsai, W.-L. Effect of intravenous albumin on endotoxin removal, cytokines, and nitric oxide production in patients with cirrhosis and spontaneous bacterial peritonitis. Scand. J. Gastroenterol. 2009, 44, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Nieto, J.C.; Sanchez, E.; Romero, C.; Roman, E.; Poca, M.; Guarner, C.; Juárez, C.; Soriano, G.; Vidal, S. Impaired innate immune response of leukocytes from ascitic fluid of patients with spontaneous bacterial peritonitis. J. Leukoc. Boil. 2015, 98, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.-Y.; Ke, Z.-Q.; Wang, M.; Li, Y. Procalcitonin and C-Reactive Protein in the Diagnosis and Prediction of Spontaneous Bacterial Peritonitis Associated With Chronic Severe Hepatitis B. Ann. Lab. Med. 2013, 33, 449–454. [Google Scholar] [CrossRef]
- Cekin, Y.; Cekin, A.H.; Duman, A.; Yilmaz, U.; Yeşil, B.; Yolcular, B.O. The Role of Serum Procalcitonin Levels in Predicting Ascitic Fluid Infection in Hospitalized Cirrhotic and Non-cirrhotic Patients. Int. J. Med. Sci. 2013, 10, 1367–1374. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Razik, A.; Mousa, N.; Elhammady, D.; Elhelaly, R.; Elzehery, R.; Elbaz, S.; Eissa, M.; El-Wakeel, N.; Eldars, W. Ascitic Fluid Calprotectin and Serum Procalcitonin as Accurate Diagnostic Markers for Spontaneous Bacterial Peritonitis. Gut Liver 2016, 10, 624–631. [Google Scholar] [CrossRef]
- Parsi, M.A.; Saadeh, S.N.; Zein, N.N.; Davis, G.L.; Lopez, R.; Boone, J.; Lepe, M.R.; Guo, L.; Ashfaq, M.; Klintmalm, G.; et al. Ascitic Fluid Lactoferrin for Diagnosis of Spontaneous Bacterial Peritonitis. Gastroenterology 2008, 135, 803–807. [Google Scholar] [CrossRef]
- Lee, S.S.; Min, H.J.; Choi, J.Y.; Cho, H.C.; Kim, J.; Lee, J.M.; Kim, H.J.; Ha, C.Y.; Kim, H.J.; Kim, T.H.; et al. Usefulness of ascitic fluid lactoferrin levels in patients with liver cirrhosis. BMC Gastroenterol. 2016, 16, 132. [Google Scholar] [CrossRef] [Green Version]
- Burri, E.; Schulte, F.; Muser, J.; Meier, R.; Beglinger, C. Measurement of calprotectin in ascitic fluid to identify elevated polymorphonuclear cell count. World J. Gastroenterol. 2013, 19, 2028–2036. [Google Scholar] [CrossRef]
- Koulaouzidis, A. Diagnosis of spontaneous bacterial peritonitis: An update on leucocyte esterase reagent strips. World J. Gastroenterol. 2011, 17, 1091–1094. [Google Scholar] [CrossRef]
- Dever, J.B.; Sheikh, M.Y. Review article: Spontaneous bacterial peritonitis—Bacteriology, diagnosis, treatment, risk factors and prevention. Aliment. Pharmacol. Ther. 2015, 41, 1116–1131. [Google Scholar] [CrossRef]
- Ximenes, R.O.; Farias, A.Q.; Neto, A.S.; Diniz, M.A.; Kubota, G.T.; Ivo, M.M.A.A.; Colacique, C.G.P.S.; D’Albuquerque, L.A.C.; Dias, R.D. Patients with cirrhosis in the ED: Early predictors of infection and mortality. Am. J. Emerg. Med. 2016, 34, 25–29. [Google Scholar] [CrossRef]
- Lin, K.-H.; Wang, F.-L.; Wu, M.-S.; Jiang, B.-Y.; Kao, W.-L.; Chao, H.-Y.; Wu, J.-Y.; Lee, C.-C. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection in patients with liver cirrhosis: A systematic review and meta-analysis. Diagn. Microbiol. Infect. Dis. 2014, 80, 72–78. [Google Scholar] [CrossRef]
- Navasa, M.; Follo, A.; Filella, X.; Jiménez, W.; Francitorra, A.; Planas, R.; Rimola, A.; Arroyo, V.; Rodés, J. Tumor necrosis factor and interleukin-6 in spontaneous bacterial peritonitis in cirrhosis: Relationship with the development of renal impairment and mortality. Hepatology 1998, 27, 1227–1232. [Google Scholar] [CrossRef]
- Zeni, F.; Tardy, B.; Vindimian, M.; Comtet, C.; Page, Y.; Cusey, I.; Bertrand, J.-C. High Levels of Tumor Necrosis Factor- and Interleukin-6 in the Ascitic Fluid of Cirrhotic Patients with Spontaneous Bacterial Peritonitis. Clin. Infect. Dis. 1993, 17, 218–223. [Google Scholar] [CrossRef]
- Wu, H.; Chen, L.; Sun, Y.; Meng, C.; Hou, W. The role of serum procalcitonin and C-reactive protein levels in predicting spontaneous bacterial peritonitis in patients with advanced liver cirrhosis. Pak. J. Med. Sci. 2016, 32, 1484–1488. [Google Scholar] [CrossRef]
- Na, S.H.; Kim, E.J.; Nam, E.Y.; Song, K.-H.; Choe, P.G.; Park, W.B.; Bang, J.H.; Kim, E.S.; Park, S.-W.; Bin Kim, H.; et al. Comparison of clinical characteristics and outcomes of spontaneous bacterial peritonitis and culture negative neutrocytic ascites. Scand. J. Gastroenterol. 2016, 52, 199–203. [Google Scholar] [CrossRef]
- Bal, C.K.; Bhatia, V.; Daman, R. Predictors of fifty days in-hospital mortality in patients with culture negative neutrocytic ascites. BMC Gastroenterol. 2017, 17, 64. [Google Scholar] [CrossRef] [Green Version]
- Connert, S.; Stremmel, W.; Elsing, C. Procalcitonin is a valid marker of infection in decompensated cirrhosis. Z. Für Gastroenterol. 2003, 41, 165–170. [Google Scholar] [CrossRef]
- Bunchorntavakul, C.; Chamroonkul, N.; Chavalitdhamrong, D. Bacterial infections in cirrhosis: A critical review and practical guidance. World J. Hepatol. 2016, 8, 307–321. [Google Scholar] [CrossRef]



| Variables | Result |
|---|---|
| Male sex, n/total (%) | 43/64 (67%) |
| Age, years | 61 (52–67) |
| Body weight, kg | 79 (65–87) |
| Body height, cm | 175 (167–180) |
| BMI | 25.7 (22.9–27.9) |
| APACHE II | 23 (17–28) |
| SOFA | 10 (8–15) |
| MELD | 27 (23–32) |
| CTP | 11 (10–12) |
| CTP C, n/total (%) | 55/64 (86%) |
| No ACLF—Grade 0, n/total (%) | 9/64 (14%) |
| ACLF, n/total (%) | 55/64 (86%) |
| ACLF Grade I, n/total (%) | 16/55 (29%) |
| ACLF Grade II, n/total (%) | 19/55 (35%) |
| ACLF Grade III, n/total (%) | 20/55 (36%) |
| CLIF-C OF, n = 55 | 11 (10–12) |
| CLIF-C ACLF, n = 55 | 55 (48–61) |
| Etiology of cirrhosis, n/total (%) | Alcoholic 48/64 (75%) |
| Viral 4/64 (6%) | |
| Autoimmune 2/64 (3%) | |
| Cryptogenic/NASH 10/64 (16%) (histological criteria of NASH 6/10) | |
| Primary admission diagnoses, n/total (%) | Sepsis 26/64 (41%) |
| Acute kidney failure/HRS 18/64 (28%) | |
| Hepatic encephalopathy 11/64 (17%) | |
| Gastrointestinal bleeding 9/64 (14%) | |
| Infection on ICU admission, n/total (%) | 53/64 (83%) |
| No infection on ICU admission, n/total (%) | 11/64 (17%) |
| Length of ICU stay, days | 13 (6–24) |
| 28-days mortality, n/total /%) | 28/64 (44%) |
| 3-month mortality, n/total (%) | 39/64 (61%) |
| ICU mortality, n/total (%) | 34/64 (53%) |
| Clinical cause of death, n/total (%) | Sepsis, Pneumonia 30/39 (77%) |
| Gastrointestinal bleeding 5/39 (13%) | |
| Cardiocirculatory failure 4/39 (10%) | |
| Dialysis during ICU, n /total (%) | 36/64 (56%) |
| Creatinine, mg/dL | 1.8 (1.4–2.7) |
| Bilirubin, mg/dL | 5.4 (2.0–14.9) |
| INR | 1.7 (1.5–2.1) |
| MAP, mmHg | 73 (68–81) |
| Use of vasopressors, n/total (%) | 31/64 (48%) |
| PaO2, mmHg | 87 (75–100) |
| FiO2, % | 30 (30–40) |
| Mechanical ventilation, n/total (%) | 24/64 (38%) |
| HE, n/total (%) | 34/64 (53%) |
| WBC, 109 cells/L | 10.7 (8.0–13.6) |
| Classification | Percentage (Fraction) | IL-6ascites, pg/mL | p-Value | |
|---|---|---|---|---|
| No infection | 17% (11/64) | 1031 (694–1713) | <0.001 | |
| Infection | 83% (53/64) | 8607 (4282–25,249) | ||
| No SBP | 70% (45/64) | 4275 (1169–8526) | <0.001 | |
| SBP | 30% (19/64) | 24,453 (12,329–63,836) | ||
| Community-acquired SBP | 32% (6/19) | 17,159 (10,703–39,539) | 0.323 | |
| Nosocomial SBP | 68% (13/19) | 38,679 (11,425–78,809) | ||
| CNNA | 47% (9/19) | 12,528 (6321–21,198) | 0.008 | |
| Culture-positive SBP | 53% (10/19) | 61,155 (28,003–84,643) | ||
| Single-infections | Urinary tract | 11% (7/53) | 2107 (1453–4123) | |
| Respiratory | 30% (19/53) | 7045 (2149–9419) | ||
| SBP | 11% (7/53) | 33,228 (12,329–63,839) | ||
| Bacteremia | 5% (3/3) | 9624 (1725–10,558) | ||
| Co- infections | Pneumonia + Urinary tract | 5% (3/53) | 6690 (4289–16,958) | |
| Pneumonia + SBP | 14% (9/53) | 16,375 (11,425–77,525) | ||
| Urinary tract + SBP | 6% (4/53) | 34,375 (8497–73,592) | ||
| Pneumonia + Bacteremia | 1% (1/53) | 9591 | ||
| Spearmans Coefficient rs | Linear Regression R2 | p-Value | |
|---|---|---|---|
| CRP, mg/dL | 0.453 | 0.055 | <0.001 |
| PCT, ng/mL | 0.445 | 0.019 | <0.001 |
| IL-6serum, pg/mL | 0.658 | 0.150 | <0.001 |
| APACHE-II | 0.494 | 0.236 | <0.001 |
| SOFA | 0.570 | 0.281 | <0.001 |
| MELD | 0.434 | 0.287 | <0.001 |
| CTP | 0.160 | 0.167 | 0.207 |
| ACLF-Grade | 0.375 | 0.108 | 0.002 |
| CLIF-OF | 0.330 | 0.234 | 0.014 |
| CLIF-ACLF | 0.381 | 0.202 | 0.004 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayr, U.; Lukas, M.; Elnegouly, M.; Vogt, C.; Bauer, U.; Ulrich, J.; Schmid, R.M.; Huber, W.; Lahmer, T. Ascitic Interleukin 6 Is Associated with Poor Outcome and Spontaneous Bacterial Peritonitis: A Validation in Critically Ill Patients with Decompensated Cirrhosis. J. Clin. Med. 2020, 9, 2865. https://doi.org/10.3390/jcm9092865
Mayr U, Lukas M, Elnegouly M, Vogt C, Bauer U, Ulrich J, Schmid RM, Huber W, Lahmer T. Ascitic Interleukin 6 Is Associated with Poor Outcome and Spontaneous Bacterial Peritonitis: A Validation in Critically Ill Patients with Decompensated Cirrhosis. Journal of Clinical Medicine. 2020; 9(9):2865. https://doi.org/10.3390/jcm9092865
Chicago/Turabian StyleMayr, Ulrich, Marina Lukas, Mayada Elnegouly, Christine Vogt, Ulrike Bauer, Joerg Ulrich, Roland M. Schmid, Wolfgang Huber, and Tobias Lahmer. 2020. "Ascitic Interleukin 6 Is Associated with Poor Outcome and Spontaneous Bacterial Peritonitis: A Validation in Critically Ill Patients with Decompensated Cirrhosis" Journal of Clinical Medicine 9, no. 9: 2865. https://doi.org/10.3390/jcm9092865
APA StyleMayr, U., Lukas, M., Elnegouly, M., Vogt, C., Bauer, U., Ulrich, J., Schmid, R. M., Huber, W., & Lahmer, T. (2020). Ascitic Interleukin 6 Is Associated with Poor Outcome and Spontaneous Bacterial Peritonitis: A Validation in Critically Ill Patients with Decompensated Cirrhosis. Journal of Clinical Medicine, 9(9), 2865. https://doi.org/10.3390/jcm9092865




