Association of the Qualitative Clock Drawing Test with Progression to Dementia in Non-Demented Older Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Neuropsychological Assessments
2.3. Clock Drawing Test
2.4. Dementia Diagnosis
2.5. Anti-Dementia Medications
2.6. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rasmussen, J.; Langerman, H. Alzheimer’s disease—Why we need early diagnosis. Degener. Neurol. Neuromuscul. Dis. 2019, 9, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Sommerlad, A. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Hameed, S.; Fuh, J.L.; Senanarong, V.; Ebenezer, E.G.M.; Looi, I.; Dominguez, J.C.; Park, K.W.; Karanam, A.K.; Simon, O. Role of fluid biomarkers and PET imaging in early diagnosis and its clinical implication in the management of Alzheimer’s disease. J. Alzheimers Dis. Rep. 2020, 4, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Shulman, K.I. Clock-drawing: Is it the ideal cognitive screening test? Int. J. Geriatr. Psychiatry 2000, 15, 548–561. [Google Scholar] [CrossRef]
- Pinto, E.; Peters, R. Literature review of the Clock Drawing Test as a tool for cognitive screening. Dement. Geriatr. Cogn. Disord. 2009, 27, 201–213. [Google Scholar] [CrossRef]
- Ehreke, L.; Luppa, M.; Luck, T.; Wiese, B.; Weyerer, S.; Eifflaender-Gorfer, S.; Weeg, D.; Olbrich, J.; Van Den Bussche, H.; Bachmann, C.; et al. Is the clock drawing test appropriate for screening for mild cognitive impairment?—Results of the German study on Ageing, Cognition and Dementia in Primary Care Patients (AgeCoDe). Dement. Geriatr. Cogn. Disord. 2009, 28, 365–372. [Google Scholar] [CrossRef]
- Kim, S.; Jahng, S.; Yu, K.H.; Lee, B.C.; Kang, Y. Usefulness of the clock drawing test as a cognitive screening instrument for mild cognitive impairment and mild dementia: An evaluation using three scoring systems. Dement. Neurocogn. Disord. 2018, 17, 100–109. [Google Scholar] [CrossRef]
- Amodeo, S.; Mainland, B.J.; Herrmann, N.; Shulman, K.I. The times they are a-changin’: Clock drawing and prediction of dementia. J. Geriatr. Psychiatry Neurol. 2015, 28, 145–155. [Google Scholar] [CrossRef]
- Spenciere, B.; Alves, H.; Charchat-Fichman, H. Scoring systems for the Clock Drawing Test: A historical review. Dement. Neuropsychol. 2017, 11, 6–14. [Google Scholar] [CrossRef]
- Cahn, D.A.; Salmon, D.P.; Monsch, A.U.; Butters, N.; Wiederholt, W.C.; Corey-Bloom, J.; Barrett-Connor, E. Screening for dementia of the Alzheimer type in the community: The utility of the Clock Drawing Test. Arch. Clin. Neuropsychol. 1996, 11, 529–539. [Google Scholar] [CrossRef]
- Yamamoto, S.; Mogi, N.; Umegaki, H.; Suzuki, Y.; Ando, F.; Shimokata, H.; Iguchi, A. The clock drawing test as a valid screening method for mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 2004, 18, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Ehreke, L.; Luppa, M.; König, H.H.; Villringer, A.; Riedel-Heller, S.G. Does the clock drawing test predict dementia? Results of the Leipzig longitudinal study of the aged (LEILA 75+). Dement. Geriatr. Cogn. Disord. 2011, 31, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.; Pinto, E.M. Predictive value of the Clock Drawing Test. A review of the literature. Dement. Geriatr. Cogn. Disord. 2008, 26, 351–355. [Google Scholar] [CrossRef]
- O’Rourke, N.; Tuokko, H.; Hayden, S.; Lynn Beattie, B. Early identification of dementia: Predictive validity of the clock test. Arch. Clin. Neuropsychol. 1997, 12, 257–267. [Google Scholar] [CrossRef][Green Version]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Aisen, P.S.; Petersen, R.C.; Donohue, M.C.; Gamst, A.; Raman, R.; Thomas, R.G.; Walter, S.; Trojanowski, J.Q.; Shaw, L.M.; Beckett, L.A.; et al. Clinical core of the Alzheimer’s disease neuroimaging initiative: Progress and plans. Alzheimers Dement. 2010, 6, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Mohs, R.C.; Cohen, L. Alzheimer’s Disease Assessment Scale (ADAS). Psychopharmacol. Bull. 1988, 24, 627–628. [Google Scholar]
- Wechsler, D. Wechsler Memory Scale—Revised Manual; The Psychological Corporation: San Antonio, TX, USA, 1987. [Google Scholar]
- Wechsler, D. Wechsler Adult Intelligence Scale, 3rd ed.; The Psychological Corporation Limited: London, UK, 1997. [Google Scholar]
- Spreen, O.; Strauss, E. A Compendium of Neuropsychological Tests: Administration, Norms and Commentary, 2nd ed.; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Stroop, J.R. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935, 18, 643–662. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Petersen, R.C.; Doody, R.; Kurz, A.; Mohs, R.C.; Morris, J.C.; Rabins, P.V.; Ritchie, K.; Rossor, M.; Thal, L.; Winblad, B. Current concepts in mild cognitive impairment. Arch. Neurol. 2001, 58, 1985–1992. [Google Scholar] [CrossRef]
- Nakashima, H.; Umegaki, H.; Makino, T.; Kato, K.; Abe, S.; Suzuki, Y.; Kuzuya, M. Neuroanatomical correlates of error types on the Clock Drawing Test in Alzheimer’s disease patients. Geriatr. Gerontol. Int. 2016, 16, 777–784. [Google Scholar] [CrossRef]
- Freedman, M.; Leach, L.; Kaplan, E.; Winocur, G.; Shulman, K.L.; Delis, C.D. Clock Drawing: A Neuropsychological Analysis; Oxford University Press: New York, NY, USA, 1994. [Google Scholar]
- Katzman, R.; Jackson, J.E. Alzheimer disease: Basic and clinical advances. J. Am. Geriatr. Soc. 1991, 39, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Rouleau, I.; Salmon, D.P.; Butters, N. Longitudinal analysis of clock drawing in Alzheimer’s disease patients. Brain Cogn. 1996, 31, 17–34. [Google Scholar] [CrossRef] [PubMed][Green Version]
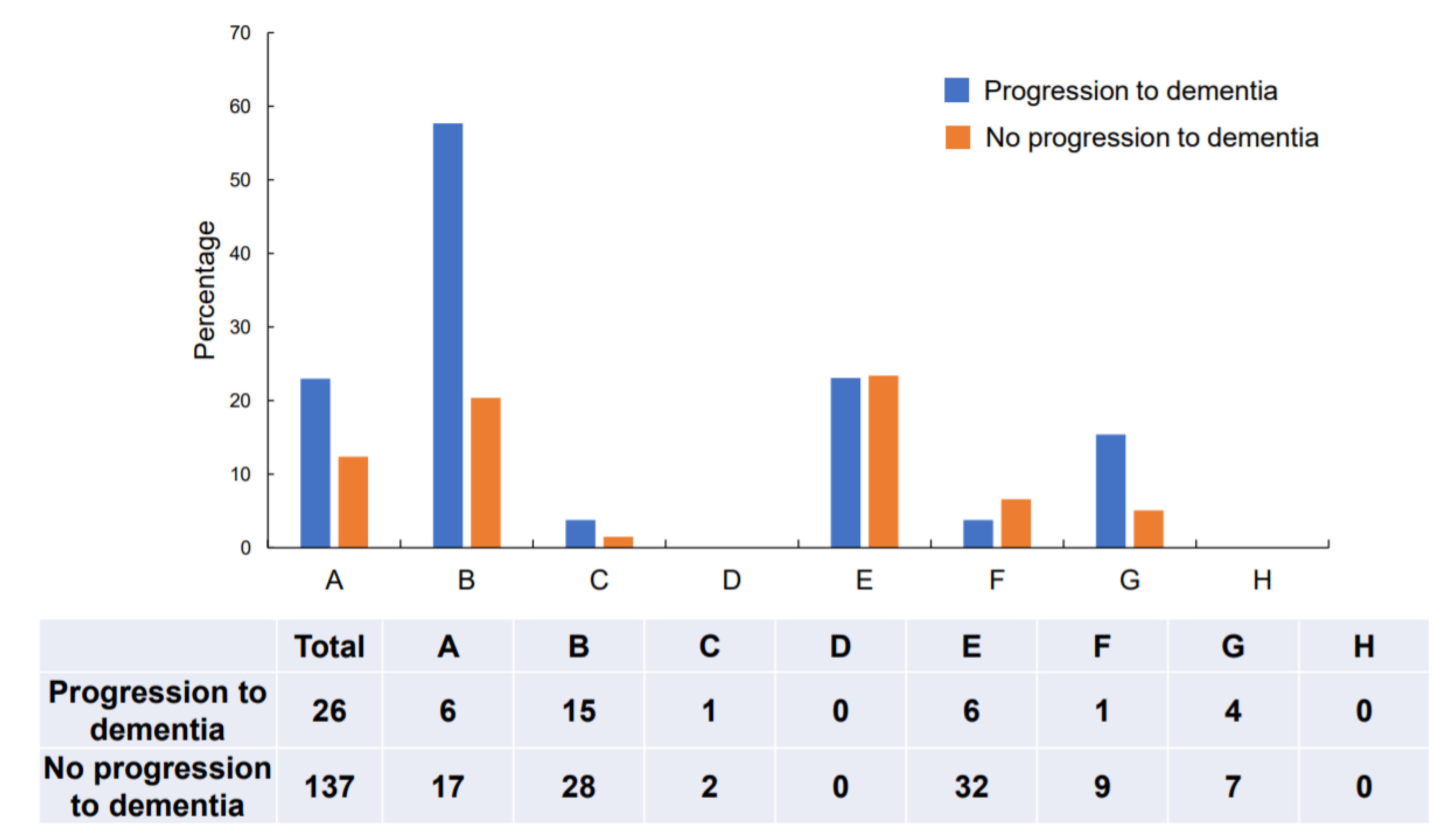
| All Participants (n = 163) | Progression to Dementia (n = 26) | No Progression to Dementia (n = 137) | p-Value | |
|---|---|---|---|---|
| Age, mean ± SD | 76.1 ± 7.1 | 79.0 ± 5.2 | 75.5 ± 7.3 | 0.005 |
| Men, n (%) | 67 (41.1) | 9 (34.6) | 58 (42.3) | 0.520 |
| Education years | 12.9 ± 2.8 | 12.9 ± 3.4 | 12.9 ± 2.6 | 0.938 |
| MMSE, mean ± SD | 27.4 ± 1.8 | 26.1 ± 1.6 | 27.6 ± 1.7 | <0.001 |
| CDT, mean ± SD | 7.1 ± 2.1 | 5.6 ± 2.5 | 7.4 ± 1.9 | <0.001 |
| Anti-dementia medications, n (%) | 25 (18.4) | 5 (19.2) | 20 (14.6) | 0.227 |
| Odds (95% CI) Model 1 | p-Value | Odds (95% CI) Model 2 | p-Value | Odds (95% CI) Model 3 | p-Value | |
|---|---|---|---|---|---|---|
| Age | 1.071 (0.967–1.119) | 0.187 | 1.088 (0.968–1.141) | 0.240 | 1.062 (0.960–1.174) | 0.244 |
| Sex | 0.702 (0.191–2.571) | 0.593 | 0.632 (0.159–2.507) | 0.514 | 0.811 (0.238–2.760) | 0.737 |
| Education | 1.285 (1.015–1.627) | 0.037 | 1.302 (1.017–1.666) | 0.036 | 1.222 (0.983–1.520) | 0.071 |
| Anti-dementia medications | 2.394 (0.605–9.458) | 0.214 | 2.723 (0.658–11.269) | 0.167 | 2.319 (0.607–80853) | 0.218 |
| MMSE | 0.640 (0.419–0.976) | 0.038 | 0.594 (0.397–0.889) | 0.011 | 0.533 (0.359–0.792) | 0.001 |
| CDT | 0.750 (0.569–0.987) | 0.040 | – | – | – | – |
| Conceptual deficits | – | – | 6.342 (1.825–22.046) | 0.004 | – | – |
| Planning deficits | – | – | – | – | 1.345 (0.382–4.742) | 0.645 |
| MMSE Score | Conceptual Deficits | Progression to Dementia | No Progression to Dementia |
| 24 ≤ MMSE ≤ 27 | No | 6 | 46 |
| Yes | 13 | 13 | |
| MMSE ≥ 28 | No | 5 | 62 |
| Yes | 2 | 15 | |
| Conceptual Deficits | Progression to Dementia | No Progression to Dementia | |
| 24 ≤ MMSE ≤ 26 | No | 4 | 27 |
| Yes | 11 | 10 | |
| MMSE ≥ 27 | No | 7 | 81 |
| Yes | 4 | 18 |
| Total | Without Conceptual Deficits | With Conceptual Deficits | p-Value | |
|---|---|---|---|---|
| Participants, n | 163 | 120 | 43 | |
| Age, mean ± SD | 76.1 ± 7.1 | 75.7 ± 7.4 | 77.0 ± 6.4 | 0.297 |
| Men, n (%) | 67 (41.1) | 49 (40.8) | 18 (41.9) | 0.906 |
| Years of education | 12.9 ± 2.8 | 13.1 ± 2.7 | 12.5 ± 3.0 | 0.297 |
| CDT | 7.1 (± 2.1) | 8 (± 1.2) | 4.4 (± 1.6) | <0.01 |
| Logical memory I | 15.3 (± 7.3) | 15.9 (± 7.3) | 13.7 (± 7.1) | 0.10 |
| Logical memory II | 9.1 (± 7.3) | 9.6 (± 7.4) | 7.7 (± 7.2) | 0.14 |
| Word recall immediate | 6.1 (± 1.7) | 6.3 (± 1.7) | 5.5 (± 1.7) | 0.01 |
| Word recall delayed | 5.5 (± 2.8) | 5.8 (± 2.9) | 4.7 (± 2.3) | 0.01 |
| Verbal fluency category | 15.6 (± 4.6) | 15.7 (± 4.6) | 15.1 (± 4.6) | 0.46 |
| Verbal fluency initial letter | 9.3 (± 3.8) | 9.6 (± 3.9) | 8.7 (± 3.4) | 0.17 |
| Digit span | 13.2 (± 3.3) | 13.4 (± 3.4) | 12.6 (± 2.9) | 0.17 |
| Digit symbol | 41.7 (± 12.3) | 42.5 (± 12.6) | 39.2 (± 11.4) | 0.14 |
| Stroop | 16.9 (± 13.7) | 17.2 (± 14.8) | 16.6 (± 10.0) | 0.81 |
| TMT-A | 63 (± 26.5) | 61.5 (± 27.3) | 68.1 (± 23.8) | 0.18 |
| TMT-B | 162.6 (± 82.0) | 161.9 (± 86.3) | 166.3 (± 68.8) | 0.78 |
| ADAS-J Cog | 8.3 (± 4.9) | 7.6 (± 3.9) | 10.3 (± 6.8) | 0.02 |
| MMSE | 27.4 (± 1.8) | 27.6 (± 1.7) | 26.8 (± 1.9) | <0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umegaki, H.; Suzuki, Y.; Yamada, Y.; Komiya, H.; Watanabe, K.; Nagae, M.; Kuzuya, M. Association of the Qualitative Clock Drawing Test with Progression to Dementia in Non-Demented Older Adults. J. Clin. Med. 2020, 9, 2850. https://doi.org/10.3390/jcm9092850
Umegaki H, Suzuki Y, Yamada Y, Komiya H, Watanabe K, Nagae M, Kuzuya M. Association of the Qualitative Clock Drawing Test with Progression to Dementia in Non-Demented Older Adults. Journal of Clinical Medicine. 2020; 9(9):2850. https://doi.org/10.3390/jcm9092850
Chicago/Turabian StyleUmegaki, Hiroyuki, Yusuke Suzuki, Yosuke Yamada, Hitoshi Komiya, Kazuhisa Watanabe, Masaaki Nagae, and Masafumi Kuzuya. 2020. "Association of the Qualitative Clock Drawing Test with Progression to Dementia in Non-Demented Older Adults" Journal of Clinical Medicine 9, no. 9: 2850. https://doi.org/10.3390/jcm9092850
APA StyleUmegaki, H., Suzuki, Y., Yamada, Y., Komiya, H., Watanabe, K., Nagae, M., & Kuzuya, M. (2020). Association of the Qualitative Clock Drawing Test with Progression to Dementia in Non-Demented Older Adults. Journal of Clinical Medicine, 9(9), 2850. https://doi.org/10.3390/jcm9092850





