NGAL Correlates with Femoral and Carotid Plaque Volume Assessed by Sonographic 3D Plaque Volumetry
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Ultrasound Examination
2.3. Statistical Analysis
3. Results
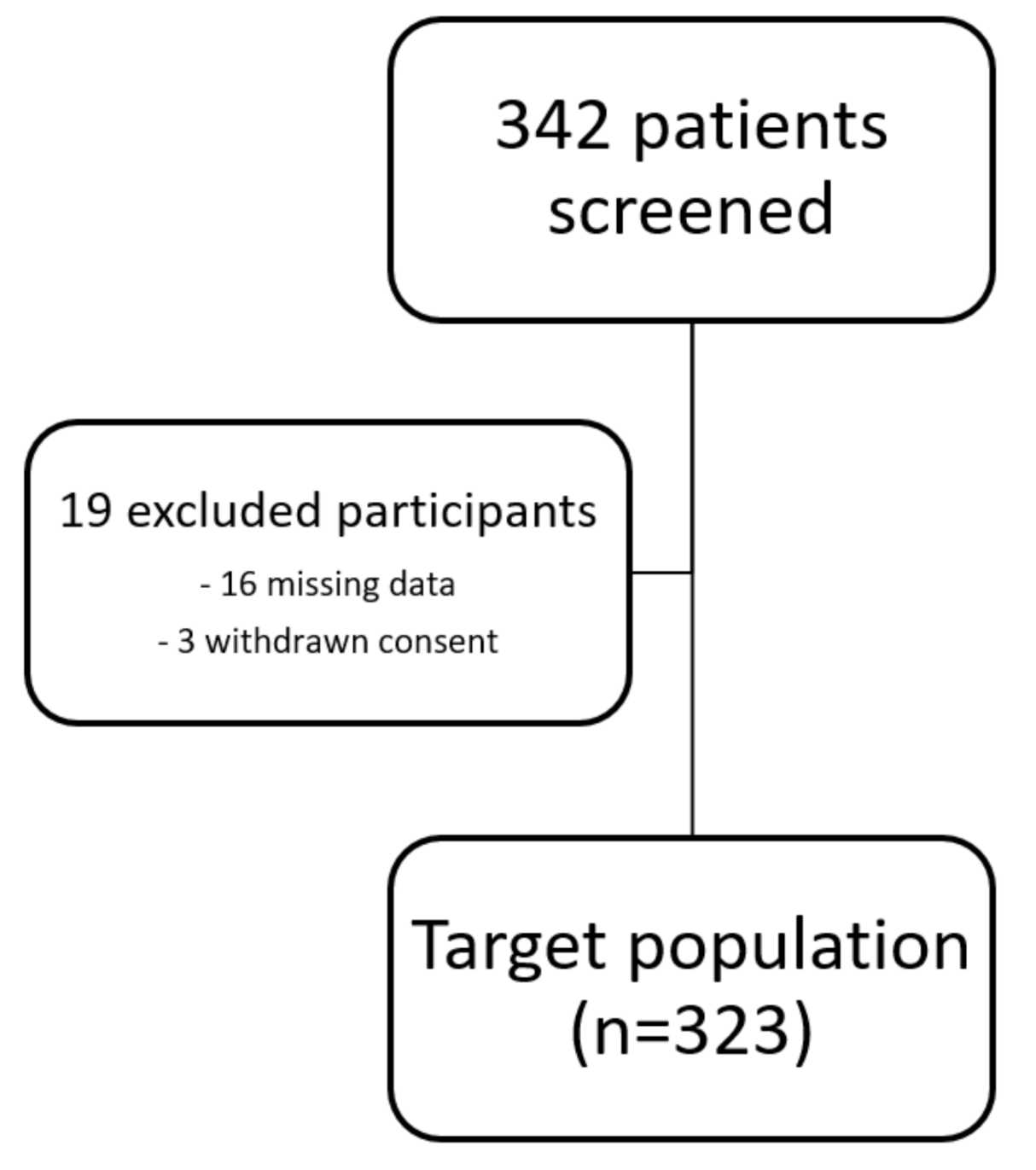
3.1. Study Population
3.2. Plaque Volume, Intima Media Thickness, Ankle Brachial Index, and Pulse Wave Velocity
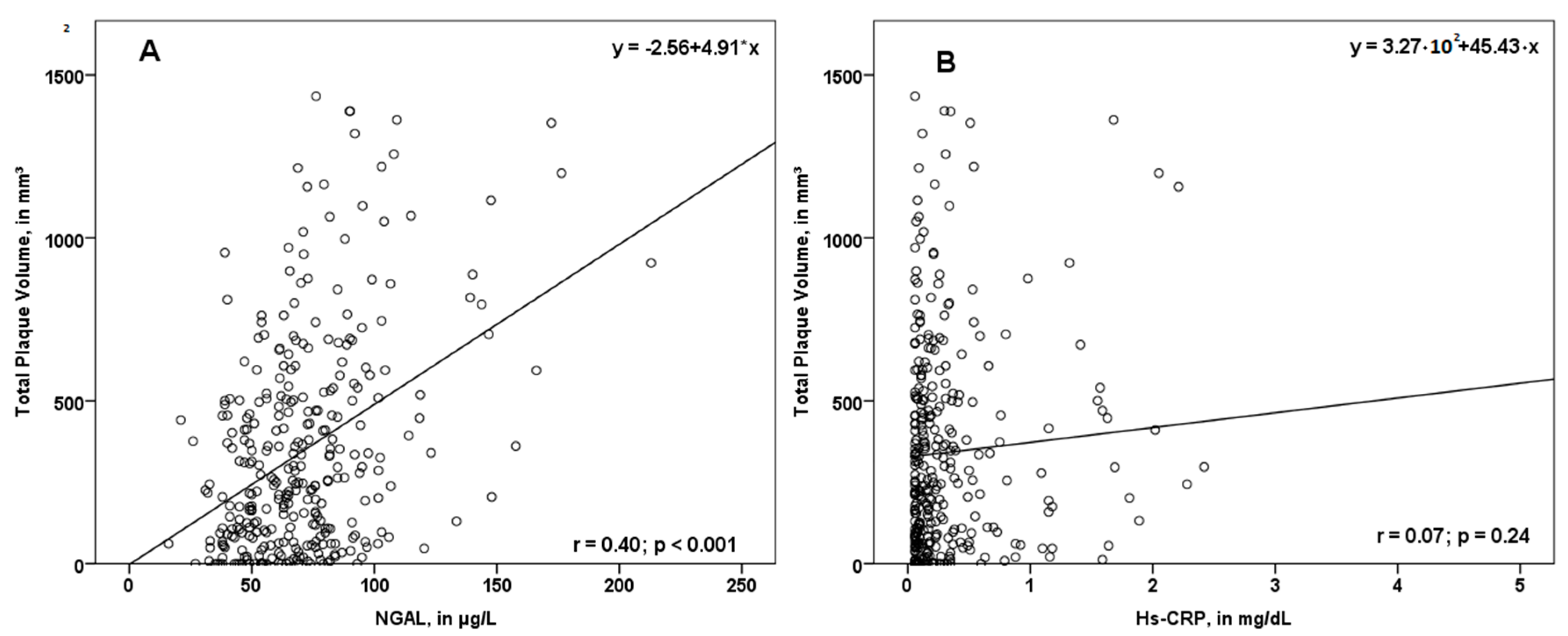
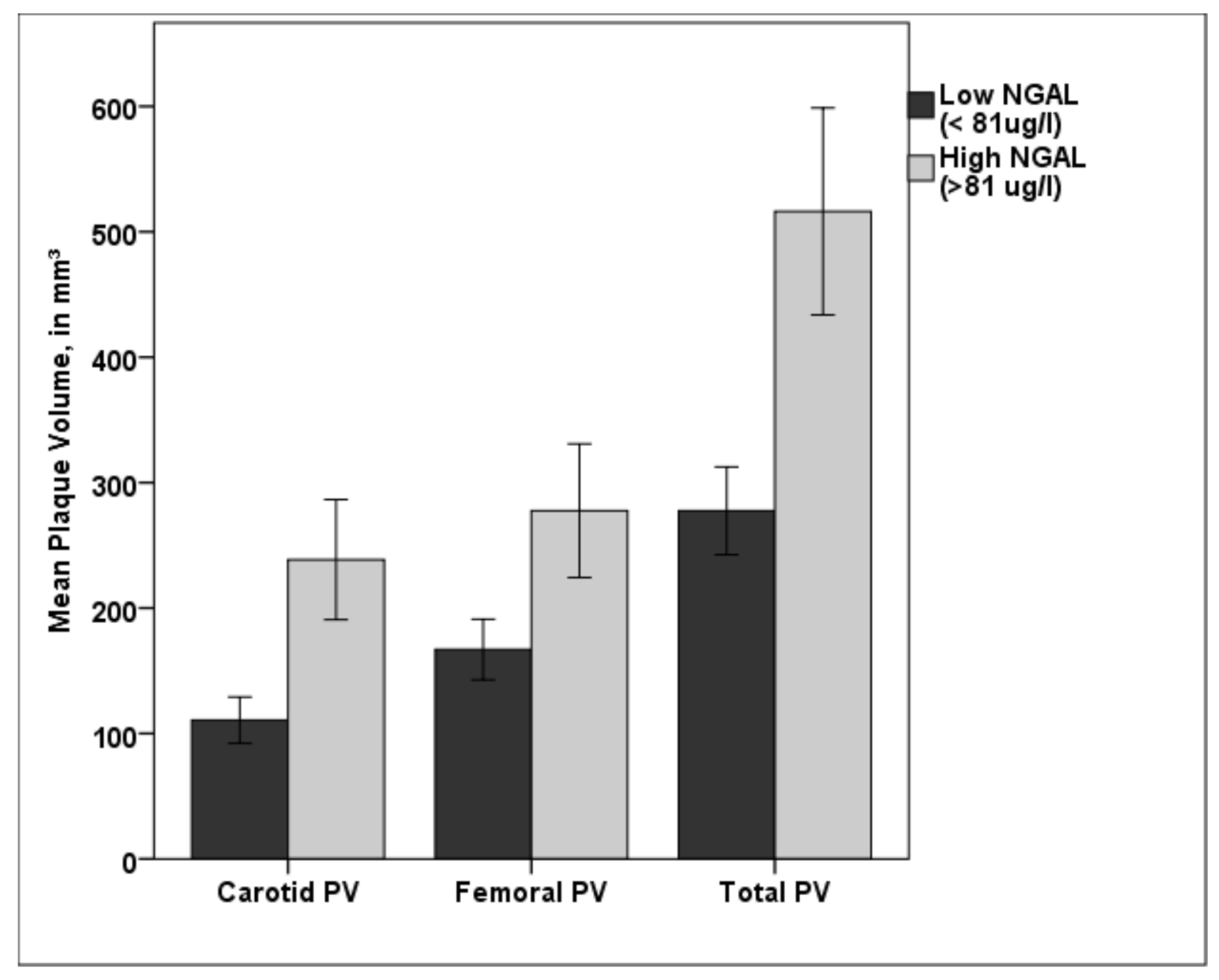
3.3. Associations between NGAL and PV
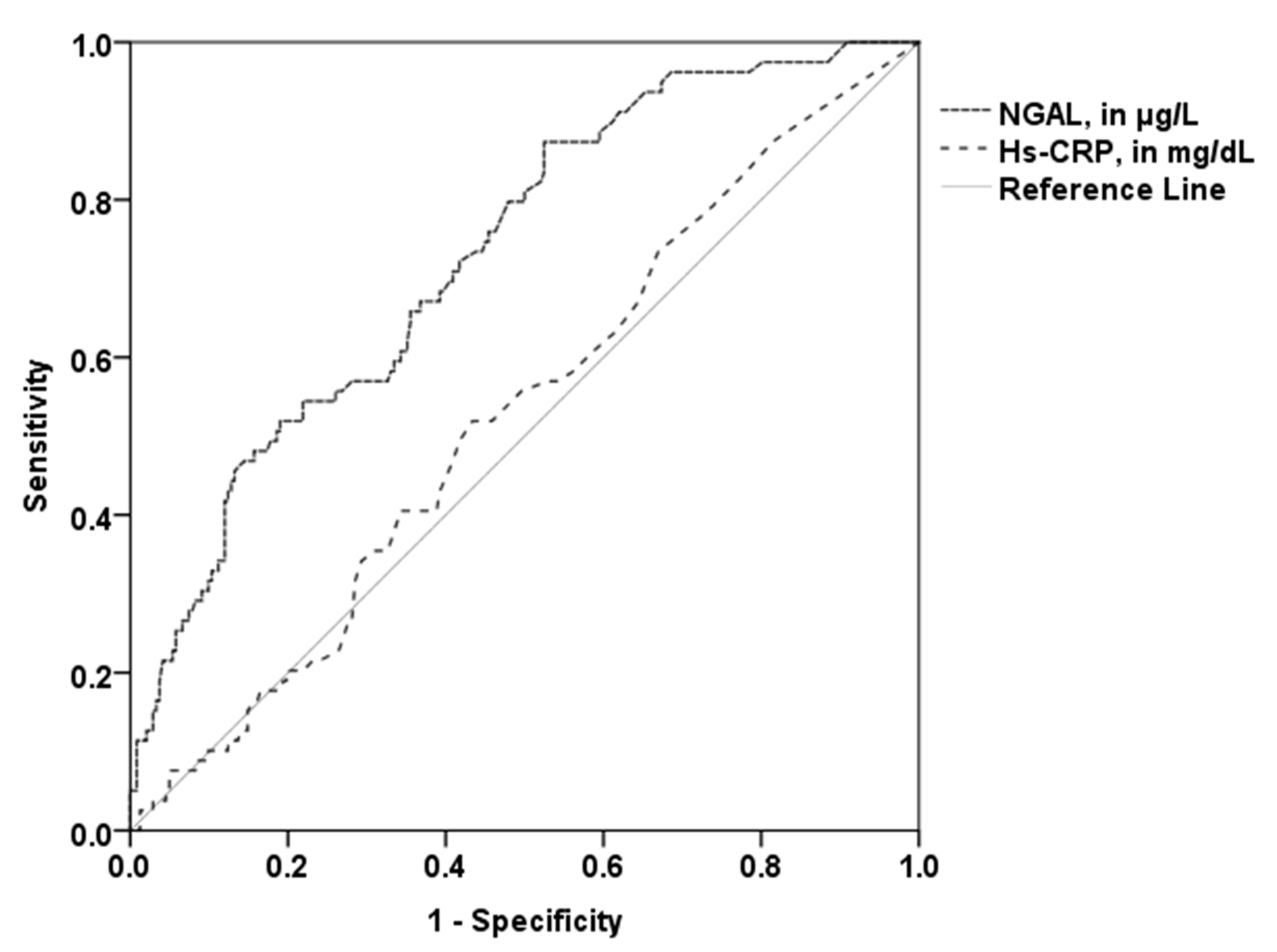
3.4. Utility of NGAL for the Prediction of Higher PV
3.5. Multivariate Analysis
4. Discussion
Limitations
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABI | ankle brachial index |
| AUC | area under the curve |
| BMI | body mass index |
| BP | blood pressure |
| CVD | cardiovascular disease |
| CVRF | cardiovascular risk factor |
| CVRFs | cardiovascular risk factors |
| eGFR | estimated glomerular filtration rate |
| HDL | high density lipoprotein |
| hs-CRP | high sensitivity C reactive protein |
| IMT | intima media thickness |
| LDL | low density lipoprotein |
| NGAL | neutrophil gelatinase-associated lipocalin |
| PV | plaque volume |
| SD | standard deviation |
References
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corra, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, L.; Bainton, D.F.; Sengelov, H.; Borregaard, N. Identification of neutrophil gelatinase-associated lipocalin as a novel matrix protein of specific granules in human neutrophils. Blood 1994, 83, 799–807. [Google Scholar] [CrossRef]
- Sivalingam, Z.; Larsen, S.B.; Grove, E.L.; Hvas, A.M.; Kristensen, S.D.; Magnusson, N.E. Neutrophil gelatinase-associated lipocalin as a risk marker in cardiovascular disease. Clin. Chem. Lab. Med. 2017, 56, 5–18. [Google Scholar] [CrossRef]
- Hemdahl, A.L.; Gabrielsen, A.; Zhu, C.; Eriksson, P.; Hedin, U.; Kastrup, J.; Thoren, P.; Hansson, G.K. Expression of neutrophil gelatinase-associated lipocalin in atherosclerosis and myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 136–142. [Google Scholar] [CrossRef]
- Yndestad, A.; Landro, L.; Ueland, T.; Dahl, C.P.; Flo, T.H.; Vinge, L.E.; Espevik, T.; Froland, S.S.; Husberg, C.; Christensen, G.; et al. Increased systemic and myocardial expression of neutrophil gelatinase-associated lipocalin in clinical and experimental heart failure. Eur. Heart J. 2009, 30, 1229–1236. [Google Scholar] [CrossRef]
- Soylu, K.; Aksan, G.; Nar, G.; Ozdemir, M.; Gulel, O.; Inci, S.; Aksakal, A.; Soylu, A.I.; Yilmaz, O. Serum neutrophil gelatinase-associated lipocalin levels are correlated with the complexity and the severity of atherosclerosis in acute coronary syndrome. Anatol. J. Cardiol. 2015, 15, 450–455. [Google Scholar] [CrossRef]
- Zykov, M.V.; Kashtalap, V.V.; Bykova, I.S.; Hryachkova, O.N.; Kalaeva, V.V.; Shafranskaya, K.S.; Karetnikova, V.N.; Barbarash, O.L. Clinical and Prognostic Value of Serum Neutrophil Gelatinase-Associated Lipocalin in Patients With ST-Segment Elevation Myocardial Infarction. Kardiologiia 2016, 56, 24–29. [Google Scholar] [CrossRef]
- Zografos, T.; Haliassos, A.; Korovesis, S.; Giazitzoglou, E.; Voridis, E.; Katritsis, D. Association of neutrophil gelatinase-associated lipocalin with the severity of coronary artery disease. Am. J. Cardiol. 2009, 104, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Sivalingam, Z.; Erik Magnusson, N.; Grove, E.L.; Hvas, A.M.; Dalby Kristensen, S.; Bojet Larsen, S. Neutrophil gelatinase-associated lipocalin (NGAL) and cardiovascular events in patients with stable coronary artery disease. Scand. J. Clin. Lab. Investig. 2018, 78, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Tarin, C.; Fernandez-Garcia, C.E.; Burillo, E.; Pastor-Vargas, C.; Llamas-Granda, P.; Castejon, B.; Ramos-Mozo, P.; Torres-Fonseca, M.M.; Berger, T.; Mak, T.W.; et al. Lipocalin-2 deficiency or blockade protects against aortic abdominal aneurysm development in mice. Cardiovasc. Res. 2016, 111, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lam, K.S.; Kraegen, E.W.; Sweeney, G.; Zhang, J.; Tso, A.W.; Chow, W.S.; Wat, N.M.; Xu, J.Y.; Hoo, R.L.; et al. Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin. Chem. 2007, 53, 34–41. [Google Scholar] [CrossRef]
- Eilenberg, W.; Stojkovic, S.; Piechota-Polanczyk, A.; Kaider, A.; Kozakowski, N.; Weninger, W.J.; Nanobachvili, J.; Wojta, J.; Huk, I.; Demyanets, S.; et al. Neutrophil gelatinase associated lipocalin (NGAL) is elevated in type 2 diabetics with carotid artery stenosis and reduced under metformin treatment. Cardiovasc. Diabetol. 2017, 16, 98. [Google Scholar] [CrossRef]
- Eilenberg, W.; Stojkovic, S.; Kaider, A.; Kozakowski, N.; Domenig, C.M.; Burghuber, C.; Nanobachvili, J.; Huber, K.; Klinger, M.; Neumayer, C.; et al. NGAL and MMP-9/NGAL as biomarkers of plaque vulnerability and targets of statins in patients with carotid atherosclerosis. Clin. Chem. Lab. Med. 2017, 56, 147–156. [Google Scholar] [CrossRef]
- Eilenberg, W.; Stojkovic, S.; Piechota-Polanczyk, A.; Kaun, C.; Rauscher, S.; Groger, M.; Klinger, M.; Wojta, J.; Neumayer, C.; Huk, I.; et al. Neutrophil Gelatinase-Associated Lipocalin (NGAL) is Associated with Symptomatic Carotid Atherosclerosis and Drives Pro-inflammatory State In Vitro. Eur. J. Vasc. Endovasc. Surg. 2016, 51, 623–631. [Google Scholar] [CrossRef]
- Noflatscher, M.; Schreinlechner, M.; Sommer, P.; Kerschbaum, J.; Berggren, K.; Theurl, M.; Kirchmair, R.; Marschang, P. Influence of Traditional Cardiovascular Risk Factors on Carotid and Femoral Atherosclerotic Plaque Volume as Measured by Three-Dimensional Ultrasound. J. Clin. Med. 2018, 8, 32. [Google Scholar] [CrossRef]
- Touboul, P.J.; Hennerici, M.G.; Meairs, S.; Adams, H.; Amarenco, P.; Bornstein, N.; Csiba, L.; Desvarieux, M.; Ebrahim, S.; Hernandez Hernandez, R.; et al. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc. Dis. 2012, 34, 290–296. [Google Scholar] [CrossRef]
- Hajian-Tilaki, K. Receiver Operating Characteristic (ROC) Curve Analysis for Medical Diagnostic Test Evaluation. Caspian J. Intern. Med. 2013, 4, 627–635. [Google Scholar]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Ankle Brachial Index, C.; Fowkes, F.G.; Murray, G.D.; Butcher, I.; Heald, C.L.; Lee, R.J.; Chambless, L.E.; Folsom, A.R.; Hirsch, A.T.; Dramaix, M.; et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: A meta-analysis. JAMA 2008, 300, 197–208. [Google Scholar] [CrossRef]
- Criqui, M.H.; McClelland, R.L.; McDermott, M.M.; Allison, M.A.; Blumenthal, R.S.; Aboyans, V.; Ix, J.H.; Burke, G.L.; Liu, K.; Shea, S. The ankle-brachial index and incident cardiovascular events in the MESA (Multi-Ethnic Study of Atherosclerosis). J. Am. Coll. Cardiol. 2010, 56, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, B.; Massberg, S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 2013, 13, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Eilenberg, W.; Stojkovic, S.; Kaider, A.; Piechota-Polanczyk, A.; Nanobachvili, J.; Domenig, C.M.; Wojta, J.; Huk, I.; Demyanets, S.; Neumayer, C. Neutrophil Gelatinase Associated Lipocalin (NGAL) for Identification of Unstable Plaques in Patients with Asymptomatic Carotid Stenosis. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto, A.M., Jr.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 2008, 359, 2195–2207. [Google Scholar] [CrossRef]
- Piechota-Polanczyk, A.; Demyanets, S.; Mittlboeck, M.; Hofmann, M.; Domenig, C.M.; Neumayer, C.; Wojta, J.; Klinger, M.; Nanobachvili, J.; Huk, I. The Influence of Simvastatin on NGAL, Matrix Metalloproteinases and Their Tissue Inhibitors in Human Intraluminal Thrombus and Abdominal Aortic Aneurysm Tissue. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 549–555. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schmidt-Ott, K.M.; Mori, K.; Li, J.Y.; Kalandadze, A.; Cohen, D.J.; Devarajan, P.; Barasch, J. Dual action of neutrophil gelatinase-associated lipocalin. J. Am. Soc. Nephrol. 2007, 18, 407–413. [Google Scholar] [CrossRef]
- Fernandez-Ortiz, A.; Jimenez-Borreguero, L.J.; Penalvo, J.L.; Ordovas, J.M.; Mocoroa, A.; Fernandez-Friera, L.; Laclaustra, M.; Garcia, L.; Molina, J.; Mendiguren, J.M.; et al. The Progression and Early detection of Subclinical Atherosclerosis (PESA) study: Rationale and design. Am. Heart J. 2013, 166, 990–998. [Google Scholar] [CrossRef]
- Sillesen, H.; Muntendam, P.; Adourian, A.; Entrekin, R.; Garcia, M.; Falk, E.; Fuster, V. Carotid plaque burden as a measure of subclinical atherosclerosis: Comparison with other tests for subclinical arterial disease in the High Risk Plaque BioImage study. JACC Cardiovasc. Imaging 2012, 5, 681–689. [Google Scholar] [CrossRef]
| Study Population (n = 323) | Low Total PV (n = 243) | High Total PV (n = 80) | p-Value | |
|---|---|---|---|---|
| Age, years | 64.2 (±9.0) | 62.7 (±8.9) | 68.6 (±7.7) | <0.001 |
| Female, n (%) | 144 (44.6) | 122 (50.2) | 22 (27.5) | <0.001 |
| CVRF, n | 2.0 (1.0) | 2.0 (1.0) | 3.0 (2.0) | 0.002 |
| Hypertension, n (%) | 210 (65.0) | 139 (57.2) | 71 (88.8) | <0.001 |
| Hyperlipidemia, n (%) | 283 (87.6) | 211 (86.8) | 72 (90.0) | 0.45 |
| Diabetes mellitus, n (%) | 42 (13.0) | 27 (11.1) | 15 (18.8) | 0.078 |
| Current smoker, n (%) | 74 (22.9) | 49 (20.2) | 25 (31.3) | 0.041 |
| Family history for CVD, n (%) | 82 (25.4) | 67 (27.6) | 15 (18.8) | 0.12 |
| Established CVD, n (%) | 114 (35.3) | 64 (26.3) | 50 (62.5) | <0.001 |
| Coronary heart disease, n (%) | 90 (27.9) | 49 (20.2) | 41 (51.2) | <0.001 |
| Peripheral artery disease, n (%) | 23 (7.1) | 8 (3.3) | 15 (18.8) | <0.001 |
| Cerebrovascular disease, n (%) | 26 (8.0) | 16. (6.6) | 10 (12.5) | 0.092 |
| hs-CRP, mg/dL | 0.17 (0.3) | 0.16 (0.3) | 0.19 (0.3) | 0.448 |
| NGAL, µg/L | 67.0 (30.1) | 63.0 (28.2) | 81.9 (33.2) | <0.001 |
| Total cholesterol, mg/dL | 195.0 (61.8) | 201.0 (61.5) | 175.0 (45.0) | <0.001 |
| LDL cholesterol, mg/dL | 116.0 (53.0) | 122.0 (55.0) | 104.0 (42.0) | <0.001 |
| HDL cholesterol, mg/dL | 58.0 (25.0) | 61.0 (26.0) | 52.0 (21.0) | 0.001 |
| Triglycerides, mg/dL | 129.0 (88.8) | 126.0 (80.5) | 138.0 (107.0) | 0.08 |
| eGFR, mL/min/1.73m2 | 76.2 (±15.8) | 76.6 (21.6) | 73.8 (24.7) | 0.061 |
| BMI, kg/m2 | 25.4 (4.8) | 25.3 (5.0) | 25.6 (4.2) | 0.46 |
| Systolic BP, mmHg | 120.5 (26.5) | 117.5 (24.5) | 126.3 (34.3) | 0.003 |
| Antihypertensive medication, n (%) | 186 (57.6) | 137 (56.4) | 49 (61.3) | 0.44 |
| Statins or other lipid lowering medication, n (%) | 178 (55.1) | 132 (54.3) | 46 (57.5) | 0.62 |
| High-intensity Statins, (n%) | 62 (19.2) | 41 (16.9) | 21 (26.3) | 0.07 |
| Moderate-intensity Statins, (n%) | 101 (31.3) | 67 (27.6) | 34 (42.5) | 0.012 |
| Low-intensity Statins, (n%) | 6 (1.9) | 5 (2.1) | 1 (1.3) | 0.64 |
| Antiplatelet medication, n (%) | 142 (44.0) | 101 (41.6) | 41 (51.2) | 0.13 |
| Antidiabetic medication, n (%) | 35 (10.8) | 22 (9.1) | 13 (16.3) | 0.07 |
| Insulin, n (%) | 8 (2.5) | 7 (2.9) | 1 (1.3) | 0.42 |
| Metformin therapy, n (%) | 27 (8.4) | 16 (6.6) | 11 (13.8) | 0.045 |
| Measured Parameters * | Study Population (n = 323) | Low Total PV (n = 243) | High Total PV (n = 80) | p-Value |
|---|---|---|---|---|
| Total PV, mm3 | 250.0 (426.0) | 163.0 (263.0) | 732.5 (363.0) | <0.001 |
| Carotid PV, mm3 | 76.0 (211.0) | 39.0 (115.0) | 351.0 (356.8) | <0.001 |
| Femoral PV, mm3 | 144.0 (253.0) | 75.0 (189.0) | 463.0 (326.8) | <0.001 |
| Carotid IMT, mm | 0.72 (0.19) | 0.71 (0.18) | 0.79 (0.15) | <0.001 |
| Ankle-brachial index | 0.91 (0.14) | 0.92 (0.13) | 0.89 (0.14) | 0.004 |
| Pulse wave velocity, m/s | 6.27 (2.42) | 5.90 (2.28) | 6.52 (2.67) | 0.029 |
| Parameter | b | p |
|---|---|---|
| Total Plaque Volume (cPV) | ||
| Age | 0.40 | <0.001 |
| NGAL | 0.28 | <0.001 |
| Established CVD | 0.24 | <0.001 |
| Male Gender | 0.21 | <0.001 |
| Smoking | 0.18 | <0.001 |
| GFR | 0.11 | 0.042 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schreinlechner, M.; Noflatscher, M.; Lener, D.; Bauer, A.; Kirchmair, R.; Marschang, P.; Theurl, M. NGAL Correlates with Femoral and Carotid Plaque Volume Assessed by Sonographic 3D Plaque Volumetry. J. Clin. Med. 2020, 9, 2811. https://doi.org/10.3390/jcm9092811
Schreinlechner M, Noflatscher M, Lener D, Bauer A, Kirchmair R, Marschang P, Theurl M. NGAL Correlates with Femoral and Carotid Plaque Volume Assessed by Sonographic 3D Plaque Volumetry. Journal of Clinical Medicine. 2020; 9(9):2811. https://doi.org/10.3390/jcm9092811
Chicago/Turabian StyleSchreinlechner, Michael, Maria Noflatscher, Daniela Lener, Axel Bauer, Rudolf Kirchmair, Peter Marschang, and Markus Theurl. 2020. "NGAL Correlates with Femoral and Carotid Plaque Volume Assessed by Sonographic 3D Plaque Volumetry" Journal of Clinical Medicine 9, no. 9: 2811. https://doi.org/10.3390/jcm9092811
APA StyleSchreinlechner, M., Noflatscher, M., Lener, D., Bauer, A., Kirchmair, R., Marschang, P., & Theurl, M. (2020). NGAL Correlates with Femoral and Carotid Plaque Volume Assessed by Sonographic 3D Plaque Volumetry. Journal of Clinical Medicine, 9(9), 2811. https://doi.org/10.3390/jcm9092811





