Impact of PD-L1 Scores and Changes on Clinical Outcome in Rectal Cancer Patients Undergoing Neoadjuvant Chemoradiotherapy
Abstract
1. Introduction
2. Experimental Section
2.1. Patients
2.2. Immunohistochemistry
2.3. Microsatellite Status
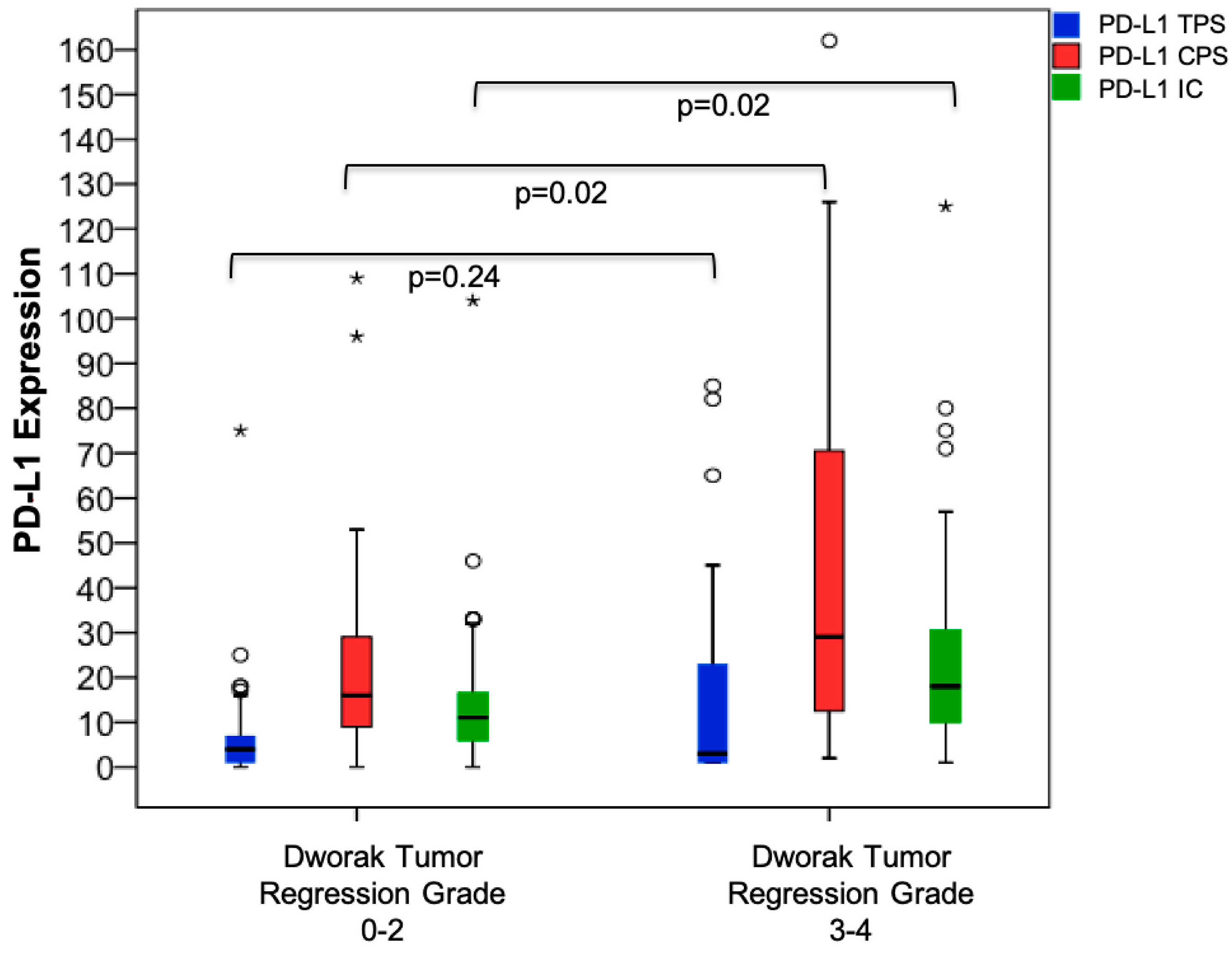
2.4. Tumor Regression Grade
2.5. Statistical Analysis
3. Results
3.1. Patient and Treatment Characteristics
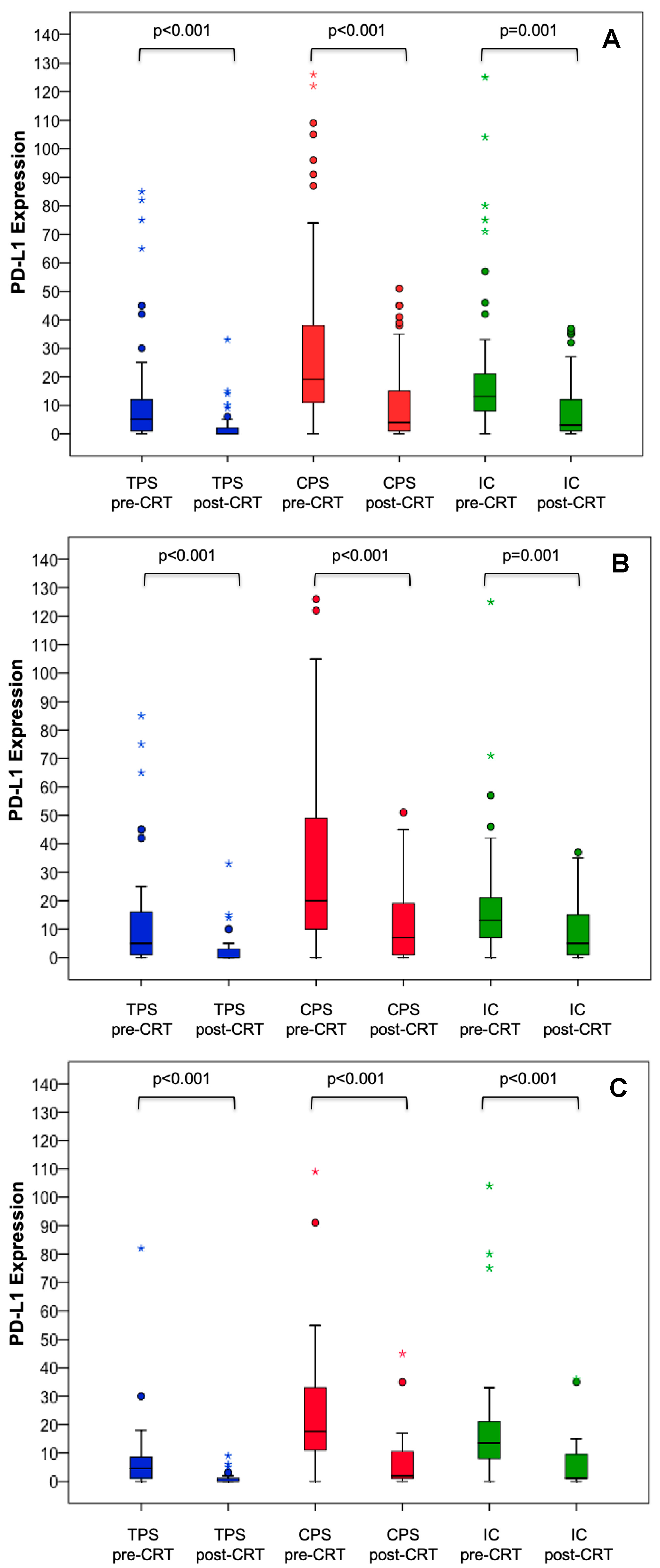
3.2. PD-L1 Expression Prior to Neoadjuvant CRT and PD-L1 Changes after Completion of CRT
3.3. PD-L1 Expression Prior to Neoadjuvant CRT and Association with Surrogate Endpoints
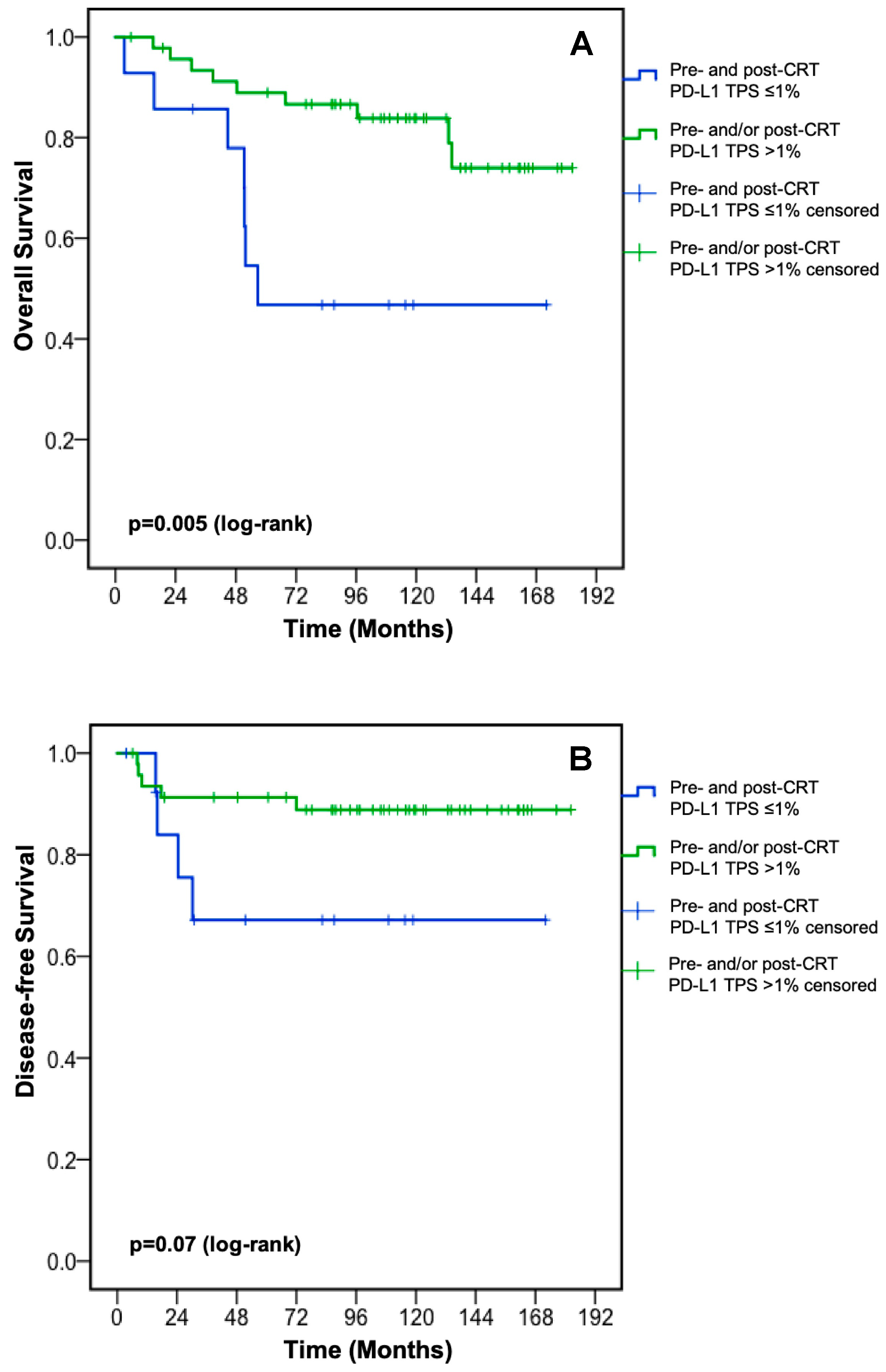
3.4. PD-L1 Expression and Clinical Outcome
3.4.1. PD-L1 Expression Prior to Neoadjuvant CRT
3.4.2. PD-L1 Changes after Completion of CRT
3.5. Univariate and Multivariate Analysis for DFS and OS
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Appendix A
| DFS | OS | |||
|---|---|---|---|---|
| PD-L1 Expression Prior to CRT (n) | Median (months) | p-Value (log-rank) | Median (months) | p-Value (log-rank) |
| TPS | ||||
| <1% (4) ≥1% (61) | 24.5 NR | 0.04 | 56.7 NR | 0.21 |
| ≤5% (39) >5% (26) | NR NR | 0.83 | NR NR | 0.16 |
| ≤10% (48) >10% (17) | NR NR | 0.27 | NR NR | 0.11 |
| ≤20% (56) >20% (9) | NR NR | 0.20 | NR NR | 0.27 |
| ≤50% (61) >50% (4) | NR NR | 0.41 | NR NR | 0.23 |
| CPS | ||||
| <1 (2) ≥1 (63) | 24.5 NR | 0.17 | 44.7 NR | 0.32 |
| ≤10 (15) >10 (50) | NR NR | 0.34 | NR NR | 0.58 |
| ≤20 (34) >20 (31) | NR NR | 0.85 | 134.2 NR | 0.05 |
| ≤50 (52) >50 (13) | NR NR | 0.97 | NR NR | 0.24 |
| IC | ||||
| <1% (2) ≥1% (63) | 24.5 NR | 0.17 | 44.7 NR | 0.32 |
| ≤5% (11) >5% (54) | NR NR | 0.60 | 132.9 NR | 0.47 |
| ≤10% (23) >10% (42) | NR NR | 0.87 | NR NR | 0.66 |
| ≤20% (46) >20% (19) | NR NR | 0.64 | NR NR | 0.18 |
| ≤50% (59) >50% (6) | NR NR | 0.88 | NR NR | 0.43 |
| Parameter | PD-L1 TPS ≤1% N = 17 (%) | PD-L1 TPS >1% N = 48 (%) | p-Value |
|---|---|---|---|
| Age | 0.755 | ||
| ≤65 years | 11 (65) | 29 (60) | |
| >65 years | 6 (35) | 19 (40) | |
| Sex | 0.368 | ||
| female | 4 (23) | 17 (35) | |
| male | 13 (77) | 31 (65) | |
| cTNM stage | 0.559 | ||
| I | 0 (0) | 1 (2) | |
| II | 9 (53) | 19 (40) | |
| III | 8 (47) | 28 (58) | |
| cT stage | 0.662 | ||
| T2 | 1 (6) | 1 (2) | |
| T3 | 14 (82) | 43 (90) | |
| T4 | 2 (12) | 4 (8) | |
| cN stage | 0.422 | ||
| N- | 9 (53) | 20 (42) | |
| N+ | 8 (47) | 28 (58) | |
| Histologic grade | 0.230 | ||
| I | 1 (6) | 0 (0) | |
| II | 14 (82) | 42 (91) | |
| III | 2 (12) | 4 (9) | |
| NA | 0 | 2 | |
| Lymphovascular invasion | 0.657 | ||
| no | 11 (85) | 30 (79) | |
| yes | 2 (15) | 8 (21) | |
| NA | 4 | 10 | |
| Venous invasion | 0.069 | ||
| no | 10 (83) | 38 (97) | |
| yes | 2 (17) | 1 (3) | |
| NA | 5 | 9 |
References
- National Comprehensive Cancer Network. Rectal Cancer (Version 3.2019). Available online: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf (accessed on 20 October 2019).
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rödel, C.; Cervantes, A.; Arnold, D. ESMO Guidelines Committee Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29 (Suppl. 4). [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.S.; Kim, S.Y.; Lee, J.S.; Nam, B.-H.; Kim, K.-P.; Kim, J.E.; Park, Y.S.; Park, J.O.; Baek, J.Y.; Kim, T.-Y.; et al. Oxaliplatin-Based Adjuvant Chemotherapy for Rectal Cancer After Preoperative Chemoradiotherapy (ADORE): Long-Term Results of a Randomized Controlled Trial. J. Clin. Oncol. 2019, 37, 3111–3123. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazieres, J.; Hermes, B.; Şenler, F.Ç.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Dómine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Schachter, J.; Long, G.; Arance, A.; Grob, J.-J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulières, D.; Melichar, B.; et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef]
- Balar, A.V.; Castellano, D.; O’Donnell, P.H.; Grivas, P.; Vuky, J.; Powles, T.; Plimack, E.R.; Hahn, N.M.; De Wit, R.; Pang, L.; et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2017, 18, 1483–1492. [Google Scholar] [CrossRef]
- Balar, A.V.; Galsky, M.D.; E Rosenberg, J.; Powles, T.; Petrylak, D.P.; Bellmunt, J.; Loriot, Y.; Necchi, A.; Hoffman-Censits, J.; Perez-Gracia, J.L.; et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet 2017, 389, 67–76. [Google Scholar] [CrossRef]
- Motzer, R.J.; Tannir, N.; McDermott, D.F.; Frontera, O.A.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthelemy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.-A.; Wright, G.S.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; De Castro, G.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Overman, M.J.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.-J.; Gelsomino, F.; Aglietta, M.; Morse, M.A.; Van Cutsem, E.; McDermott, R.; Hill, A.; et al. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair–Deficient/Microsatellite Instability–High Metastatic Colorectal Cancer. J. Clin. Oncol. 2018, 36, 773–779. [Google Scholar] [CrossRef]
- Andre, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.J.A.; Smith, D.M.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab versus chemotherapy for microsatellite instability-high/mismatch repair deficient metastatic colorectal cancer: The phase 3 KEYNOTE-177 Study. J. Clin. Oncol. 2020, 38. [Google Scholar] [CrossRef]
- Sinicrope, F.A.; Ou, F.-S.; Zemla, T.; Nixon, A.B.; Mody, K.; Levasseur, A.; Dueck, A.C.; Dhanarajan, A.R.; Lieu, C.H.; Cohen, D.J.; et al. Randomized trial of standard chemotherapy alone or combined with atezolizumab as adjuvant therapy for patients with stage III colon cancer and deficient mismatch repair (ATOMIC, Alliance A021502). J. Clin. Oncol. 2019, 37, e15169. [Google Scholar] [CrossRef]
- Saigusa, S.; Toiyama, Y.; Tanaka, K.; Inoue, Y.; Mori, K.; Ide, S.; Imaoka, H.; Kawamura, M.; Mohri, Y.; Kusunoki, M. Implication of programmed cell death ligand 1 expression in tumor recurrence and prognosis in rectal cancer with neoadjuvant chemoradiotherapy. Int. J. Clin. Oncol. 2016, 21, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Hecht, M.; Büttner-Herold, M.; Erlenbach-Wünsch, K.; Haderlein, M.; Croner, R.; Grützmann, R.; Hartmann, A.; Fietkau, R.; Distel, L.V. PD-L1 is upregulated by radiochemotherapy in rectal adenocarcinoma patients and associated with a favourable prognosis. Eur. J. Cancer 2016, 65, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.-F.; Huang, C.-Y.; Ke, T.-W.; Chen, T.-W.; Lan, Y.-C.; You, Y.-S.; Chen, W.T.-L.; Chao, K.C. Upregulation of tumor PD-L1 by neoadjuvant chemoradiotherapy (neoCRT) confers improved survival in patients with lymph node metastasis of locally advanced rectal cancers. Cancer Immunol. Immunother. 2018, 68, 283–296. [Google Scholar] [CrossRef]
- Ogura, A.; Akiyoshi, T.; Yamamoto, N.; Kawachi, H.; Ishikawa, Y.; Mori, S.; Oba, K.; Nagino, M.; Fukunaga, Y.; Ueno, M. Pattern of programmed cell death-ligand 1 expression and CD8-positive T-cell infiltration before and after chemoradiotherapy in rectal cancer. Eur. J. Cancer 2018, 91, 11–20. [Google Scholar] [CrossRef]
- Chen, T.-W.; Huang, K.C.-Y.; Chiang, S.-F.; Chen, W.T.-L.; Ke, T.-W.; Chao, K.C. Prognostic relevance of programmed cell death-ligand 1 expression and CD8+ TILs in rectal cancer patients before and after neoadjuvant chemoradiotherapy. J. Cancer Res. Clin. Oncol. 2019, 145, 1043–1053. [Google Scholar] [CrossRef]
- Yothers, G.; George, T.J.; Petrelli, N.J.; O’Connell, M.J.; Beart, R.W.; Allegra, C.J.; Roh, M.S.; Lopa, S.H.; Colangelo, L.H.; Sharif, S.; et al. Neoadjuvant rectal cancer (RC) score to predict survival: Potential surrogate endpoint for early phase trials. J. Clin. Oncol. 2014, 32, 3533. [Google Scholar] [CrossRef]
- Roach, C.; Zhang, N.; Corigliano, E.; Jansson, M.; Toland, G.; Ponto, G.; Dolled-Filhart, M.; Emancipator, K.; Stanforth, D.; Kulangara, K. Development of a Companion Diagnostic PD-L1 Immunohistochemistry Assay for Pembrolizumab Therapy in Non–Small-cell Lung Cancer. Appl. Immunohistochem. Mol. Morphol. 2016, 24, 392–397. [Google Scholar] [CrossRef]
- Kulangara, K.; Zhang, N.; Corigliano, E.; Guerrero, L.; Waldroup, S.; Jaiswal, D.; Malinka, J.; Shah, S.; Hanks, D.; Wang, J.; et al. Clinical Utility of the Combined Positive Score for Programmed Death Ligand-1 Expression and the Approval of Pembrolizumab for Treatment of Gastric Cancer. Arch. Pathol. Lab. Med. 2019, 143, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Vennapusa, B.; Baker, B.; Kowanetz, M.; Boone, J.; Menzl, I.; Bruey, J.-M.; Fine, G.; Mariathasan, S.; McCaffery, I.; Mocci, S.; et al. Development of a PD-L1 Complementary Diagnostic Immunohistochemistry Assay (SP142) for Atezolizumab. Appl. Immunohistochem. Mol. Morphol. 2019, 27, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Dworak, O.; Keilholz, L.; Hoffmann, A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int. J. Color. Dis. 1997, 12, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crino, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef]
- Droeser, R.A.; Hirt, C.; Viehl, C.T.; Frey, D.M.; Nebiker, C.A.; Huber, X.; Zlobec, I.; Eppenberger-Castori, S.; Tzankov, A.; Rosso, R.; et al. Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur. J. Cancer 2013, 49, 2233–2242. [Google Scholar] [CrossRef]
- Wang, H.B.; Yao, H.; Li, C.S.; Liang, L.X.; Zhang, Y.; Chen, Y.X.; Fang, J.-Y.; Xu, J. Rise of PD-L1 expression during metastasis of colorectal cancer: Implications for immunotherapy. J. Dig. Dis. 2017, 18, 574–581. [Google Scholar] [CrossRef]
- Gatalica, Z.; Snyder, C.; Maney, T.; Ghazalpour, A.; Holterman, D.A.; Xiao, N.; Overberg, P.; Rose, I.; Basu, G.D.; Vranic, S.; et al. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol. Biomarkers Prev. 2014, 23, 2965–2970. [Google Scholar] [CrossRef]
- Li, Y.; He, M.; Zhou, Y.; Yang, C.; Wei, S.; Bian, X.; Christopher, O.; Xie, L. The Prognostic and Clinicopathological Roles of PD-L1 Expression in Colorectal Cancer: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2019, 10, 139. [Google Scholar] [CrossRef]
- Yoshino, T.; Bando, H.; Tsukada, Y.; Inamori, K.; Yuki, S.; Komatsu, Y.; Homma, S.; Uemura, M.; Kato, T.; Kotani, D.; et al. Voltage: Investigator-initiated clinical trial of nivolumab monotherapy and subsequent radical surgery following preoperative chemoradiotherapy in patients with microsatellite stable locally advanced rectal cancer. J. Clin. Oncol. 2019, 37, 3606. [Google Scholar] [CrossRef]
- Eng, C.; Kim, T.W.; Bendell, J.; Argilés, G.; Tebbutt, N.C.; Di Bartolomeo, M.; Falcone, A.; Fakih, M.; Kozloff, M.; Segal, N.H.; et al. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): A multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2019, 20, 849–861. [Google Scholar] [CrossRef]
- Allen, E.; Jabouille, A.; Rivera, L.B.; Lodewijckx, I.; Missiaen, R.; Steri, V.; Feyen, K.; Tawney, J.; Hanahan, D.; Michael, I.P.; et al. Combined antiangiogenic and anti–PD-L1 therapy stimulates tumor immunity through HEV formation. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Rodel, C.; Liersch, T.; Becker, H.; Fietkau, R.; Hohenberger, W.; Hothorn, T.; Graeven, U.; Arnold, D.; Lang-Welzenbach, M.; Raab, H.-R.; et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: Initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. 2012, 13, 679–687. [Google Scholar] [CrossRef]
- Deng, Y.; Chi, P.; Lan, P.; Wang, L.; Chen, W.; Cui, L.; Chen, D.; Cao, J.; Wei, H.; Peng, X.; et al. Modified FOLFOX6 With or Without Radiation Versus Fluorouracil and Leucovorin With Radiation in Neoadjuvant Treatment of Locally Advanced Rectal Cancer: Initial Results of the Chinese FOWARC Multicenter, Open-Label, Randomized Three-Arm Phase III Trial. J. Clin. Oncol. 2016, 34, 3300–3307. [Google Scholar] [CrossRef]
- Aschele, C.; Cionini, L.; Lonardi, S.; Pinto, C.; Cordio, S.; Rosati, G.; Artale, S.; Tagliagambe, A.; Ambrosini, G.; Rosetti, P.; et al. Primary Tumor Response to Preoperative Chemoradiation With or Without Oxaliplatin in Locally Advanced Rectal Cancer: Pathologic Results of the STAR-01 Randomized Phase III Trial. J. Clin. Oncol. 2011, 29, 2773–2780. [Google Scholar] [CrossRef]
- Rödel, C.; Graeven, U.; Fietkau, R.; Hohenberger, W.; Hothorn, T.; Arnold, D.; Hofheinz, R.; Ghadimi, M.; Wolff, H.; Lang-Welzenbach, M.; et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): Final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015, 16, 979–989. [Google Scholar] [CrossRef]
- El Sissy, C.; Kirilovsky, A.; Eynde, M.V.D.; Mușină, A.-M.; Anitei, M.-G.; Romero, A.M.S.; Marliot, F.; Haicheur, N.; Junca, A.; Doyen, J.; et al. A diagnostic biopsy-adapted immunoscore predicts response to neoadjuvant treatment and selects patients with rectal cancer eligible for a watch-and-wait strategy. Clin. Cancer Res. 2020. [Google Scholar] [CrossRef]

| Parameter | N = 72 (%) |
|---|---|
| Age | |
| ≤65 years | 44 (61) |
| >65 years | 28 (39) |
| Sex | |
| female | 23 (32) |
| male | 49 (68) |
| ypN stage | |
| N- | 48 (67) |
| N+ | 24 (33) |
| cTNM stage | |
| I | 1 (1) |
| II | 29 (40) |
| III | 37 (52) |
| IV | 5 (7) |
| Histologic grade | |
| I | 1 (1) |
| II | 61 (87) |
| III | 8 (12) |
| NA | 2 |
| Dworak tumor regression grade | |
| 0 | 3 (4) |
| I | 16 (22) |
| II | 25 (35) |
| III | 20 (28) |
| IV | 8 (11) |
| Microsatellite status | |
| MSS | 56 (98) |
| MSI | 1 (2) |
| NA | 15 |
| CRT backbone | |
| 5-FU or capecitabine | 29 (40) |
| 5-FU + oxaliplatin or capecitabine + oxaliplatin | 43 (60) |
| NAR score | |
| low | 11 (15) |
| intermediate | 36 (50) |
| high | 25 (35) |
| Adjuvant chemotherapy | |
| yes | 36 (52) |
| no | 33 (48) |
| NA | 3 |
| UVA | MVA | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Sex male (n = 45) female (n = 22) | 1.86 (0.39–8.95) | 0.44 | − | − |
| Age >65 (n = 25) ≤65 (n = 42) | 0.56 (0.12–2.69) | 0.47 | − | − |
| ypN stage N+ (n = 20) N− (n = 47) | 1.21 (0.30–4.84) | 0.79 | − | − |
| Dworak TRG 3–4 (n = 28) 0–2 (n = 39) | 0.74 (0.18–2.95) | 0.67 | − | − |
| Histologic grade 3 (n = 7) 1 + 2 (n = 58) | 0.20 (0.002–17.98) | 0.49 | − | − |
| Adjuvant chemotherapy yes (n = 35) no (n = 30) | 0.88 (0.22–3.52) | 0.86 | − | − |
| PD-L1 TPS pre-CRT >1% (n = 48) ≤1% (n = 17) | 0.40 (0.11–1.50) | 0.18 | − | − |
| NAR score intermediate/high (n = 56) low (n = 11) | 27.20 (0.02–43,658.20) | 0.38 | − | − |
| UVA | MVA | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Sex male (n = 45) female (n = 22) | 2.24 (0.74–6.76) | 0.15 | - | - |
| Age >65 (n = 25) ≤65 (n = 42) | 3.12 (1.25–7.82) | 0.02 | 4.09 (1.54–10.86) | 0.005 |
| ypN stage N+ (n = 20) N− (n = 47) | 1.11 (0.42–2.91) | 0.84 | - | - |
| Dworak TRG 3–4 (n = 28) 0–2 (n = 39) | 1.10 (0.44–2.75) | 0.84 | - | - |
| Histologic grade 3 (n = 7) 1 + 2 (n = 58) | 0.67 (0.25–1.84) | 0.44 | - | - |
| Adjuvant chemotherapy yes (n = 35) no (n = 30) | 0.72 (0.28–1.83) | 0.49 | - | - |
| PD-L1 TPS pre-CRT >1% (n = 48) ≤1% (n = 17) | 0.36 (0.14–0.91) | 0.03 | 0.29 (0.11–0.76) | 0.01 |
| NAR score intermediate/high (n = 56) low (n = 11) | 1.89 (0.44–8.17) | 0.40 | − | − |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huemer, F.; Klieser, E.; Neureiter, D.; Schlintl, V.; Rinnerthaler, G.; Pagès, F.; Kirilovsky, A.; El Sissy, C.; Iglseder, W.; Singhartinger, F.; et al. Impact of PD-L1 Scores and Changes on Clinical Outcome in Rectal Cancer Patients Undergoing Neoadjuvant Chemoradiotherapy. J. Clin. Med. 2020, 9, 2775. https://doi.org/10.3390/jcm9092775
Huemer F, Klieser E, Neureiter D, Schlintl V, Rinnerthaler G, Pagès F, Kirilovsky A, El Sissy C, Iglseder W, Singhartinger F, et al. Impact of PD-L1 Scores and Changes on Clinical Outcome in Rectal Cancer Patients Undergoing Neoadjuvant Chemoradiotherapy. Journal of Clinical Medicine. 2020; 9(9):2775. https://doi.org/10.3390/jcm9092775
Chicago/Turabian StyleHuemer, Florian, Eckhard Klieser, Daniel Neureiter, Verena Schlintl, Gabriel Rinnerthaler, Franck Pagès, Amos Kirilovsky, Carine El Sissy, Wolfgang Iglseder, Franz Singhartinger, and et al. 2020. "Impact of PD-L1 Scores and Changes on Clinical Outcome in Rectal Cancer Patients Undergoing Neoadjuvant Chemoradiotherapy" Journal of Clinical Medicine 9, no. 9: 2775. https://doi.org/10.3390/jcm9092775
APA StyleHuemer, F., Klieser, E., Neureiter, D., Schlintl, V., Rinnerthaler, G., Pagès, F., Kirilovsky, A., El Sissy, C., Iglseder, W., Singhartinger, F., Jäger, T., Dinnewitzer, A., Zaborsky, N., Steiner, M., Greil, R., & Weiss, L. (2020). Impact of PD-L1 Scores and Changes on Clinical Outcome in Rectal Cancer Patients Undergoing Neoadjuvant Chemoradiotherapy. Journal of Clinical Medicine, 9(9), 2775. https://doi.org/10.3390/jcm9092775







